Dantrolene sodium freeze-dried powder injection for injection and preparation method thereof
A technology for freeze-dried powder injection and dantrolene sodium, applied in the field of medicine, can solve the problems of poor resolubility of dantrolene sodium freeze-dried powder for injection, poor water solubility of dantrolene sodium, low bioavailability and the like, and achieves considerable Economic and social benefits, short administration time, and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
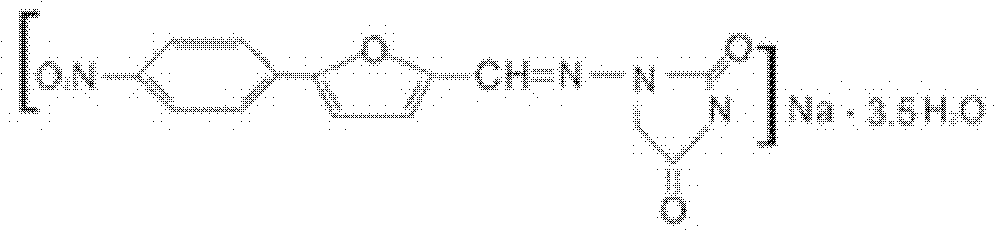
[0083] [embodiment 1] the preparation of dantrolene sodium crystal
[0084] 1) Dissolving dantrolene sodium in a propylene glycol solvent to make a concentration of 10% dantrolene sodium solution is placed in a reactor in a constant temperature water bath with a temperature of 5° C.;
[0085] 2) Turn on the agitator, add the dantrolene sodium solution into water at a rotating speed of 2000r / min, stir for 18min to form a slurry, wherein the volume ratio of the dantrolene sodium solution to water is 1:20;
[0086] 3) The obtained slurry was left to stand for 3 hours, vacuum filtered and dried to obtain dantrolene sodium crystals.
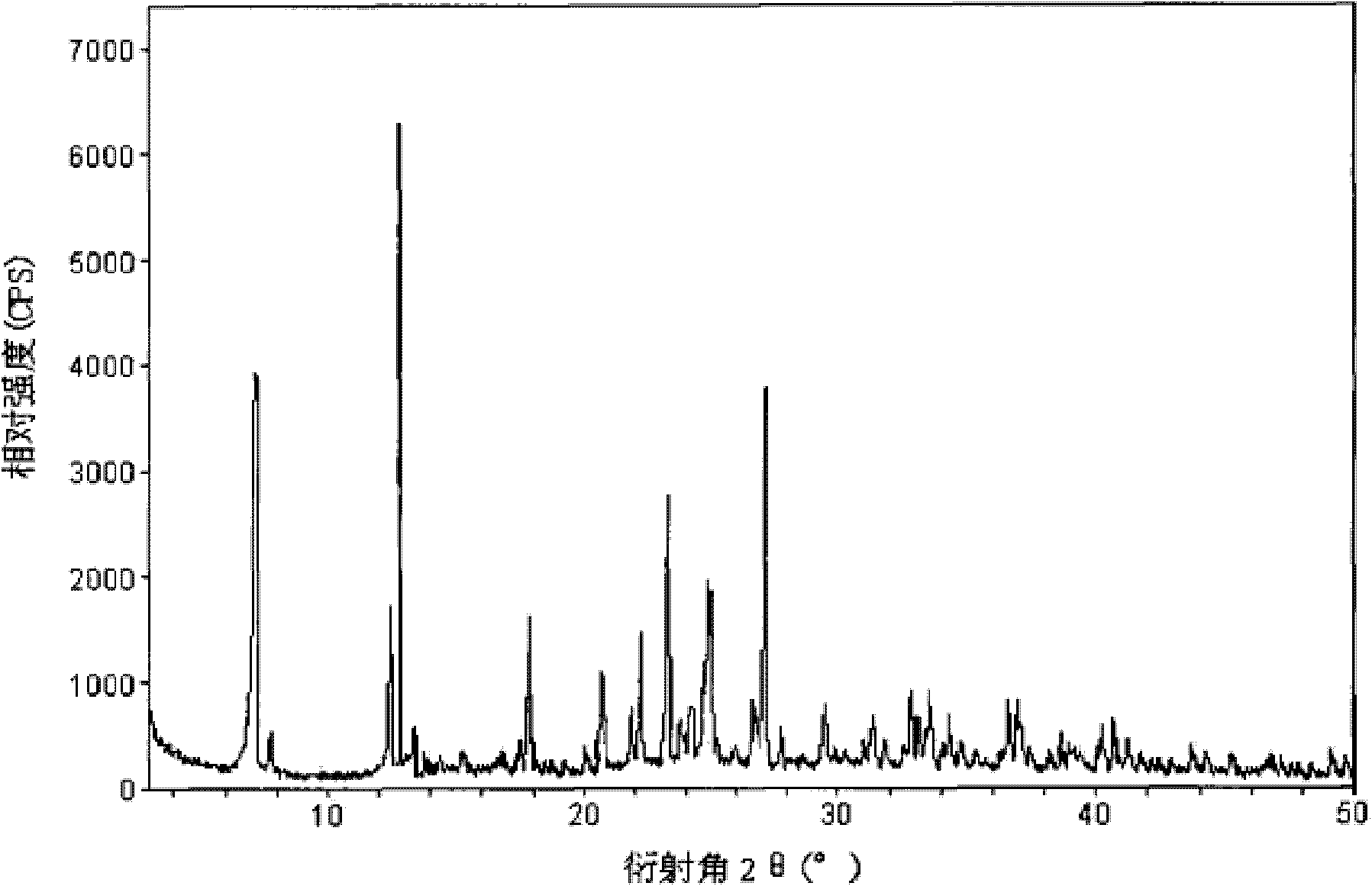
[0087] According to FT-IR analysis, the molecular structure of dantrolene sodium did not change before and after ultra-micronization;
[0088] The X-ray powder diffraction spectrogram (XRD spectrogram) that uses Cu-K α ray measurement to the prepared dantrolene sodium crystal is shown in figure 1 , whose characteristic peaks are displayed at 2θ of 7...
Embodiment 1
[0093] [Preparation Example 1] Dantrolene Sodium Freeze-Dried Powder for Injection
[0094] Prescription: per 1000 prescriptions
[0095]
[0096] Preparation Process:
[0097] 1) take the dantrolene sodium and lactose of prescription quantity;
[0098] 2) Add 6000ml of water for injection and prescribed amount of dantrolene sodium into the liquid preparation tank, then add the prescribed amount of lactose, and fully stir to dissolve;
[0099] 3) adjust the pH value of the solution to 9.7 with 1mol / L sodium bicarbonate;
[0100] 4) Add water for injection to make up to 8000ml, and mix well;
[0101] 5) Add 0.03% activated carbon to the clear solution, stir and absorb for 30 minutes, then use a 0.45 μm microporous membrane filter to decarbonize, filter through a 0.45 μm primary sterile filter, and filter through a 0.45 μm secondary terminal sterile filter. In the liquid bottle, take the above-mentioned medicinal liquid to measure the content and pH value of the semi-fini...
Embodiment 2
[0107] [Preparation Example 2] Dantrolene Sodium Freeze-Dried Powder for Injection
[0108] Prescription: per 1000 prescriptions
[0109]
[0110] Preparation process: the specific operation steps are the same as in Example 1, and the difference from Example 1 is that the freeze-drying in step 6) is:
[0111] Pre-freezing stage: reduce the temperature of the shelf to -48°C at a rate of 0.70°C / min, and after the temperature of the subpackaged filtrate product drops to -35°C, continue to keep warm for 4 hours, and the temperature of the cold hydrazine drops rapidly below -50°C. Open the well valve of the box and evacuate until the vacuum in the box reaches about 10pa;
[0112] Primary drying stage: Slowly raise the shelf temperature to -2°C at a rate of 0.30°C / min, keep warm, and keep warm for 4 hours after the ice crystals of the product in the primary drying completely disappear;
[0113] Secondary drying stage: raise the temperature of the shelf to 17°C at a rate of 0.53...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com