Lornoxicam freeze-dried injection and preparation method thereof
A technology of freeze-dried powder injection and lornoxicam, which is applied in the direction of freeze-dried delivery, anti-inflammatory agent, powder delivery, etc. To avoid problems such as poor product resolubility, achieve the effect of low difficulty in rejecting waste, good freeze-drying and forming, and uniform and stable quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
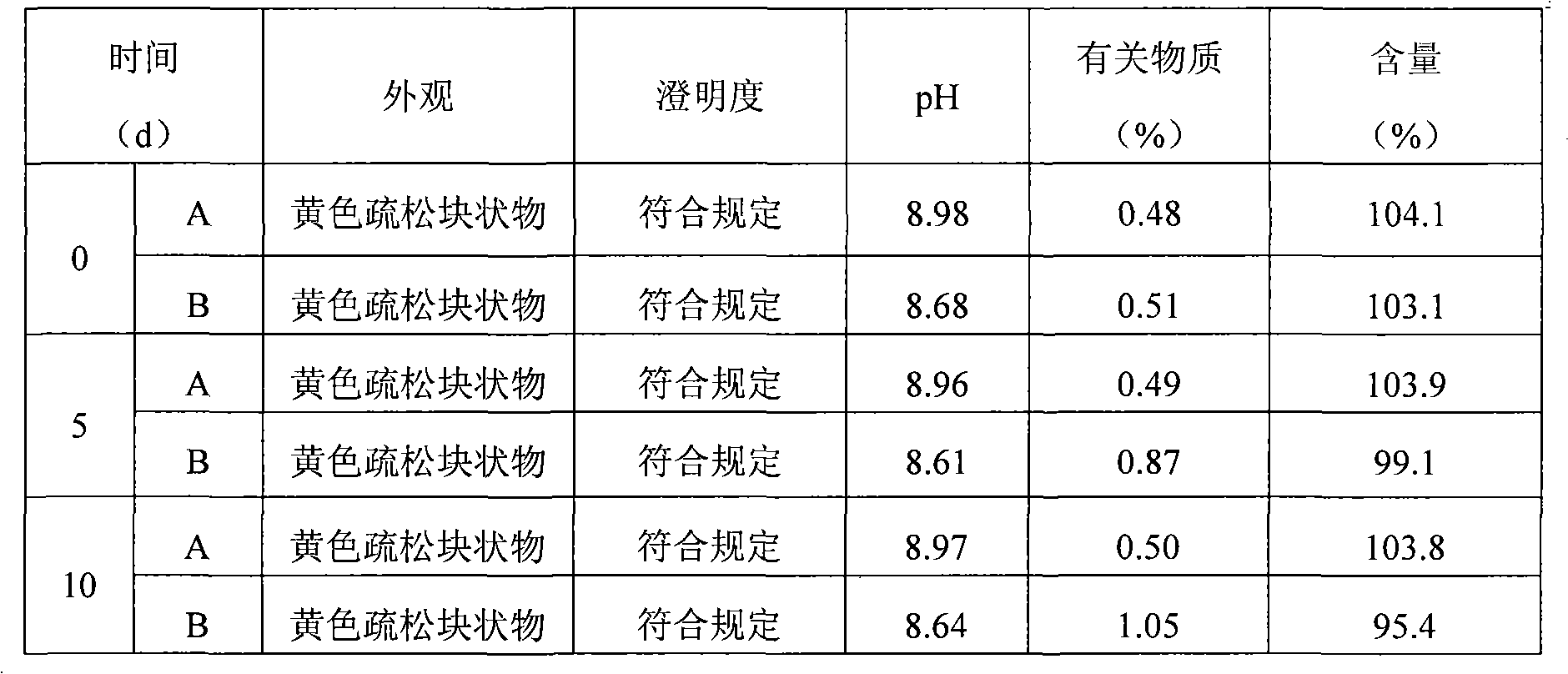
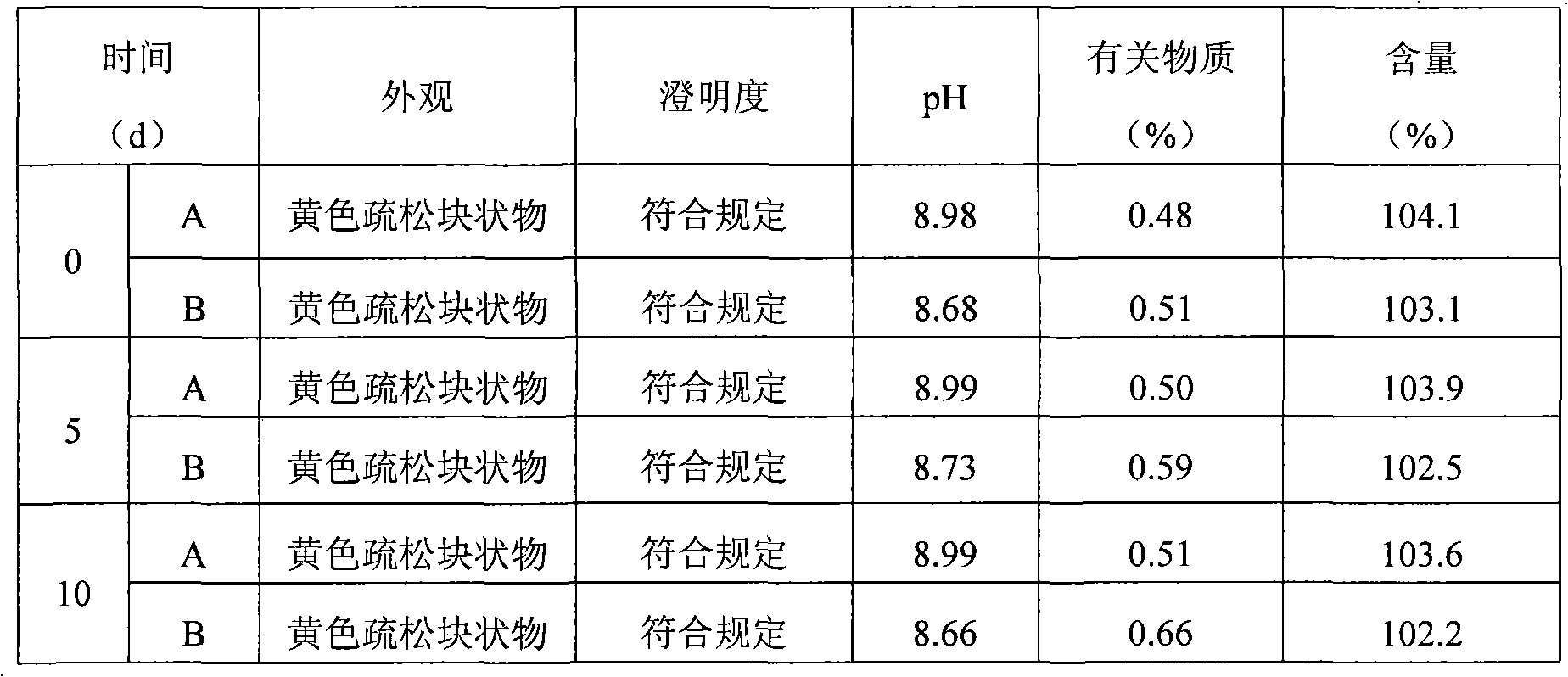
Image
Examples
Embodiment 1
[0062] (1) Prescription:
[0063] Lornoxicam 8.6g
[0064] Mannitol 50.0g
[0065] Tromethamine 12.0g
[0066] EDTA0.2g
[0067] Activated carbon 1.0g
[0068] Water for injection 2000ml
[0069] 0.5mol / l sodium hydroxide solution appropriate amount (pH regulator)
[0070] 0.5mol / l hydrochloric acid solution appropriate amount (pH regulator)
[0071]
[0072] Makes 1000 bottles
[0073] (2) Preparation process:
[0074] 1) Take mannitol, tromethamine and EDTA in the liquid preparation tank, add water for injection to 80% preparation amount, then add lornoxicam, when adding lornoxicam, the solution temperature in the liquid preparation tank is controlled at 4°C ;
[0075] 2) After adding lornoxicam, adjust the pH value to 10.5 with sodium hydroxide solution while stirring, and then adjust the pH value to 8.5 with hydrochloric acid solution to obtain a clear solution;
[0076] 3) Add activated carbon to the clear solution,...
Embodiment 2
[0079] (1) Prescription:
[0080] Lornoxicam 8.6g
[0081] Mannitol 30.1g
[0082] Tromethamine 12.0g
[0083] EDTA0.129g
[0084] Activated carbon 1.0g
[0085] Water for injection 2000ml
[0086] 0.5mol / l sodium hydroxide solution appropriate amount (pH regulator)
[0087] 0.5mol / l hydrochloric acid solution appropriate amount (pH regulator)
[0088]
[0089] Makes 1000 bottles
[0090] (2) Preparation process:
[0091] 1) Take mannitol, tromethamine and EDTA in the liquid preparation tank, add water for injection to 80% preparation amount, then add lornoxicam, when adding lornoxicam, the solution temperature in the liquid preparation tank is controlled at 2°C ;
[0092] 2) After the addition of lornoxicam is completed, adjust the pH value to 11.5 with sodium hydroxide solution while stirring, and then adjust the pH value to 9.5 with hydrochloric acid solution to obtain a clear solution;
[0093] 3) Add activated car...
Embodiment 3
[0096] (1) Prescription:
[0097] Lornoxicam 8.6g
[0098] Mannitol 73.1g
[0099] Tromethamine 12.0g
[0100] EDTA0.215g
[0101] Activated carbon 1.0g
[0102] Water for injection 2000ml
[0103] 0.5mol / l sodium hydroxide solution appropriate amount (pH regulator)
[0104] 0.5mol / l hydrochloric acid solution appropriate amount (pH regulator)
[0105]
[0106] Makes 1000 bottles
[0107] (2) Preparation process:
[0108] 1) Take mannitol, tromethamine and EDTA in the liquid preparation tank, add water for injection to 80% preparation amount, then add lornoxicam, and control the solution temperature in the liquid preparation tank at 6°C when adding lornoxicam ;
[0109] 2) After the addition of lornoxicam is completed, adjust the pH value to 11 with sodium hydroxide solution while stirring, and then adjust the pH value to 9.0 with hydrochloric acid solution to obtain a clear solution;
[0110] 3) Add activated carbon to th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com