Method for preparing lipidosome coated with protein drugs
A technology of liposomes and lipid substances, applied in the directions of liposome delivery, peptide/protein components, pharmaceutical formulations, etc., can solve the problems of waste of raw materials, inability to reflect the advantages of liposome preparations, and inability to achieve effective doses, etc. , to achieve the effect of increasing the application value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
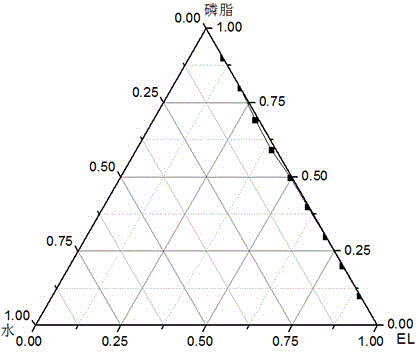
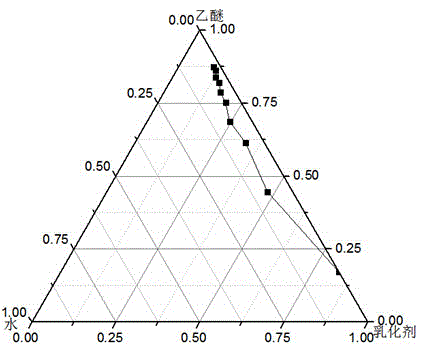
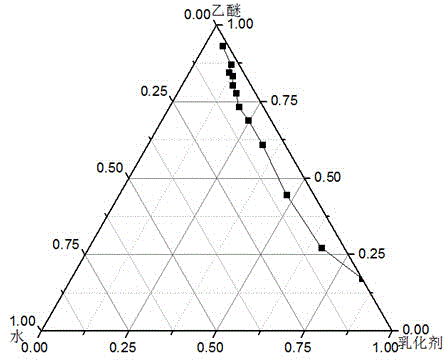
[0031] This embodiment is to adopt the method proposed by the present invention to prepare bovine serum albumin nanoliposomes. Step a: Preparation of microemulsion phase. Weigh 12mg of phosphatidylcholine, add 5ml of ether, and evaporate under reduced pressure to form a film. Add 5ml of diethyl ether to redissolve the membrane, add 8mg of Cremophor EL, and add dropwise 0.2ml of bovine serum albumin aqueous solution with a concentration of 2mg / ml under magnetic stirring until light blue opalescence, and the microemulsion phase is obtained. The ratio of oil phase, water phase and surfactant is optimized by pseudo ternary phase diagram: using Cremophor EL and phospholipid as mixed emulsifier according to Km is 9:1, 8:2, 7:3, 6:4, 5: 5. Mix 4:6, 3:7, 2:8, 1:9 evenly, ether is the oil phase, add distilled water drop by drop under magnetic stirring, observe the phenomenon of the system from turbid to clear or from clear to turbid, and record the critical The percentages of each co...
Embodiment 2
[0035] Step a: Preparation of microemulsion phase. Weigh 24 mg of phosphatidylcholine, add dichloromethane, and evaporate under reduced pressure to form a film. Add 9ml of diethyl ether to redissolve the membrane, add 16mg of Cremophor EL, add dropwise 0.6ml of 1mg / ml bovine serum albumin aqueous solution under magnetic stirring until light blue opalescence, and obtain the microemulsion phase.
[0036] Step b: Preparation of micellar phase. Weigh 30 mg of phosphatidylcholine, 5 mg of cholesterol, and 5 mg of cholesterol succinate, add an appropriate amount of dichloromethane, and evaporate under reduced pressure to form a film. Add 4ml of distilled water and 0.5m of propylene glycol to hydrate at 50°C to obtain the micellar phase;
[0037] Step c: the micellar phase was added to the microemulsion phase, and the probe was sonicated in an ice bath for 10 minutes, then the ether was removed by rotary evaporation at 30° C., and the remaining ether in the system was further remov...
Embodiment 3
[0039] Preparation of ovalbumin nanoliposomes. Step a: Preparation of microemulsion phase. Weigh 14 mg of phosphatidylcholine, add 5 ml of dichloromethane, and evaporate under reduced pressure to form a film. Add 5ml of diethyl ether to redissolve the membrane, add 406mg of Cremophor RH, and add dropwise 0.5ml of ovalbumin phosphate buffer with a concentration of 3mg / ml under magnetic stirring until light blue opalescence is obtained to obtain the microemulsion phase.
[0040] Step b: Preparation of micellar phase. Weigh 42 mg of distearoylphosphatidylethanolamine, 5 mg of positively charged lipid carbamoyl cholesterol, and 5 mg of cholesterol succinate, add appropriate amount of dichloromethane, and evaporate under reduced pressure to form a film. Add 2ml of phosphate buffer and 1ml of ethanol to hydrate at 60°C to obtain the micellar phase;
[0041] Step c: According to step c in Example 1, the average particle size of ovalbumin nanoliposomes was determined to be 68.68nm,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com