Hydrophilic amide polymer self-assembly particle drug release system suitable for hydrophobic drugs and preparation method thereof
A technology of amide polymers and hydrophobic drugs, which can be used in pharmaceutical formulations, medical preparations containing active ingredients, and medical preparations containing active ingredients. and adverse reactions, increase the cost of preparations, etc., to achieve good freeze-drying redispersibility, improve bioavailability, and low price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
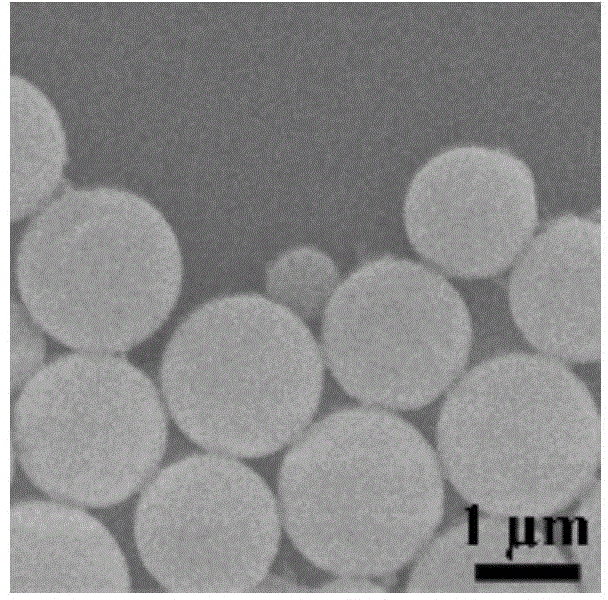
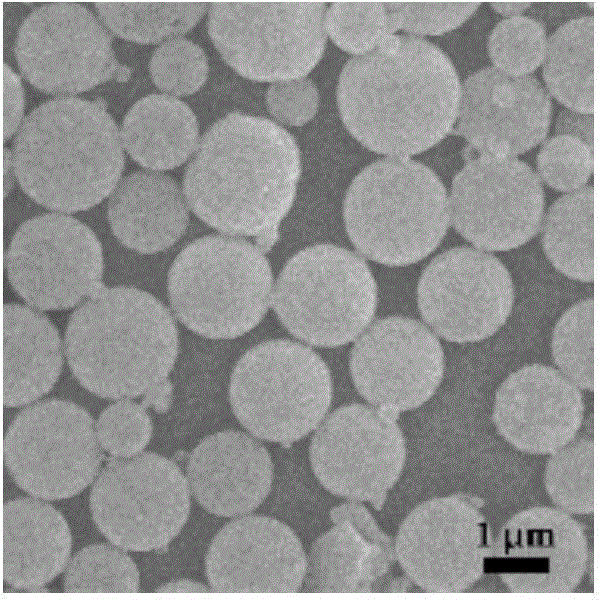
Image
Examples
Embodiment 1
[0035] Dissolve poly(N-methacrylamide) with a molecular weight of 5000Da, flurbiprofen and cyclosporin A in 1ml methanol, where the polymer concentration is 10mg / ml and the flurbiprofen concentration is 2.5mg / ml The concentration of cyclosporin A is 5mg / ml; then the solution is placed in a dialysis bag with a molecular weight cut-off of 3500Da, and the solution is dialyzed in deionized water under magnetic stirring at a temperature of 10℃, and the deionized water is replaced every 0.5 hour interval; 6 hours The product is collected by centrifugation and freeze-dried to obtain assembly particles. The drug loading amount of cyclosporin A in the resulting assembly particles was 25.8%.
Embodiment 2
[0037] Dissolve poly(N-ethylacrylamide) with a molecular weight of 10000Da, indomethacin and rapamycin in 1ml ethanol, where the polymer concentration is 10mg / ml and the indomethacin concentration is 10mg / ml. The concentration of rapamycin is 15mg / ml; then the solution is placed in a dialysis bag with a molecular weight cut-off of 5000Da, and the solution is dialyzed in deionized water under magnetic stirring at a temperature of 20℃, and the deionized water is replaced every 3 hours; after 12 hours The product is collected by centrifugation and freeze-dried to obtain assembly particles. The drug loading amount of rapamycin in the resulting assembly particles was 30.2%.
Embodiment 3
[0039] The poly(N-n-propylacrylamide) with a molecular weight of 15000Da, sulindac and tamsulolimus were dissolved in 2ml of acetone, the polymer concentration was 8mg / ml, the sulindac concentration was 20mg / ml, The concentration of Rolimus is 30mg / ml; then the solution is placed in a dialysis bag with a molecular weight cut-off of 10000Da, and the solution is dialyzed in deionized water under magnetic stirring at a temperature of 15°C. The deionized water is replaced every 3 hours; centrifuged after 24 hours The product is collected and freeze-dried to obtain assembly particles. The drug loading amount of tamsulolimus in the resulting assembly particles was 45.9%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com