Patents
Literature
33results about How to "Freeze-drying time is short" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
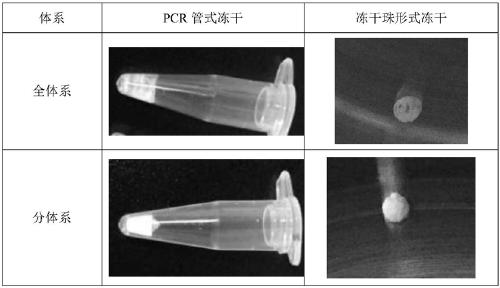
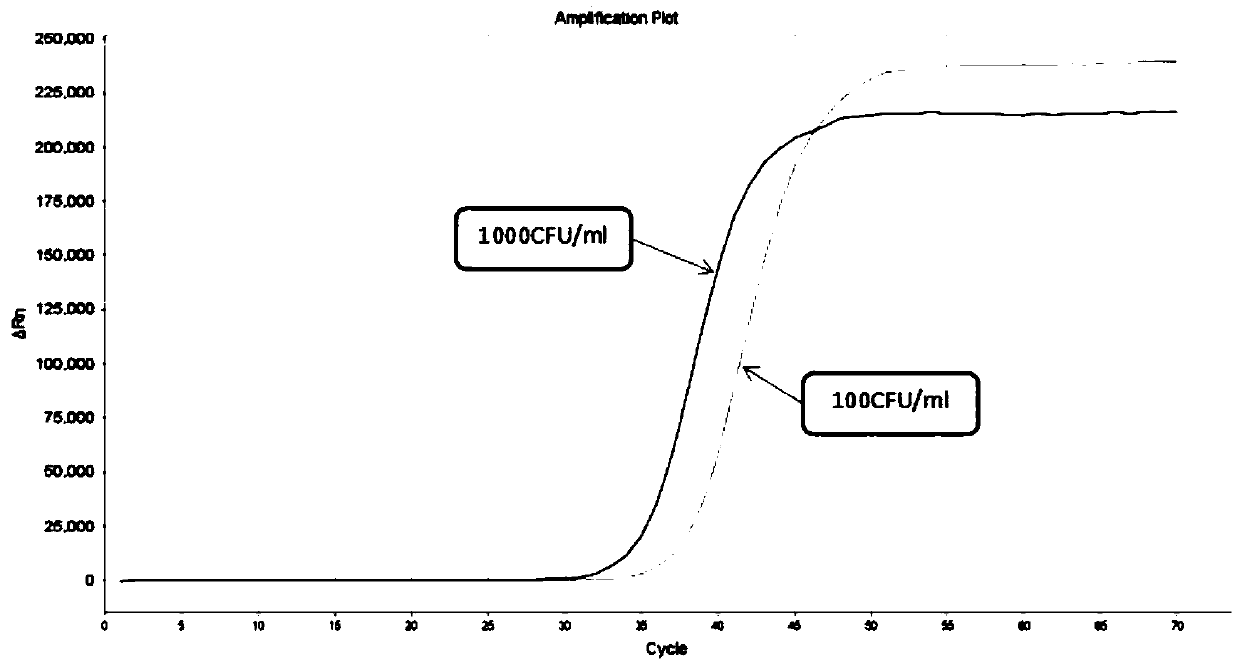
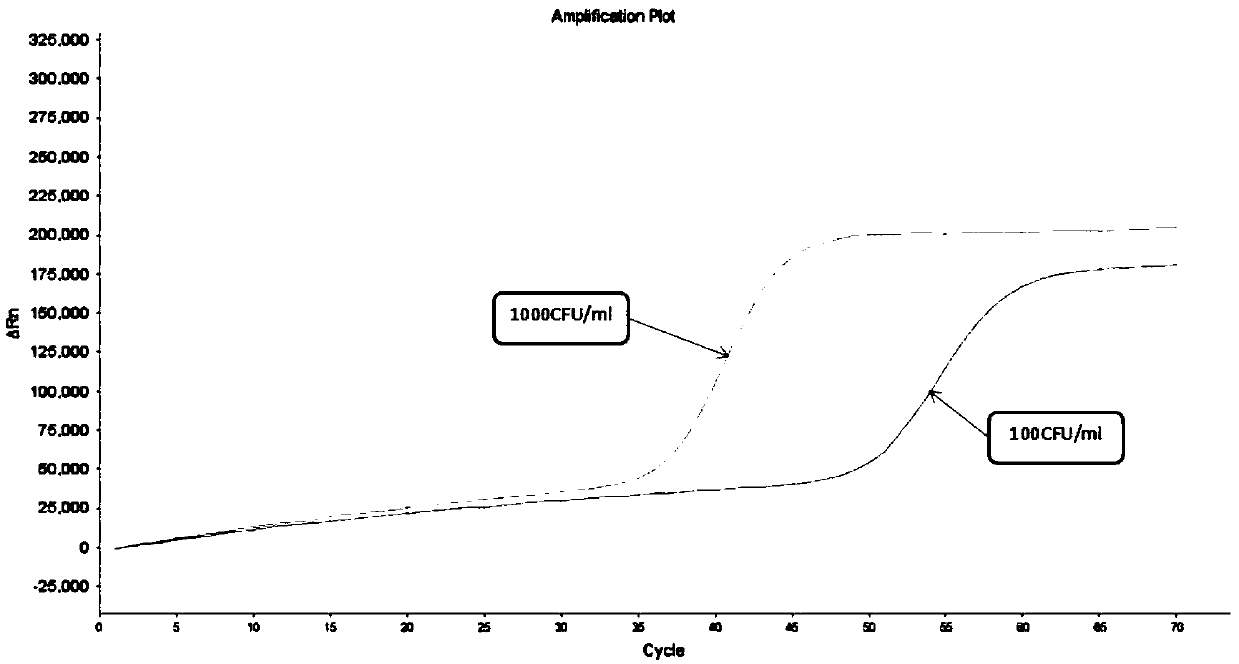
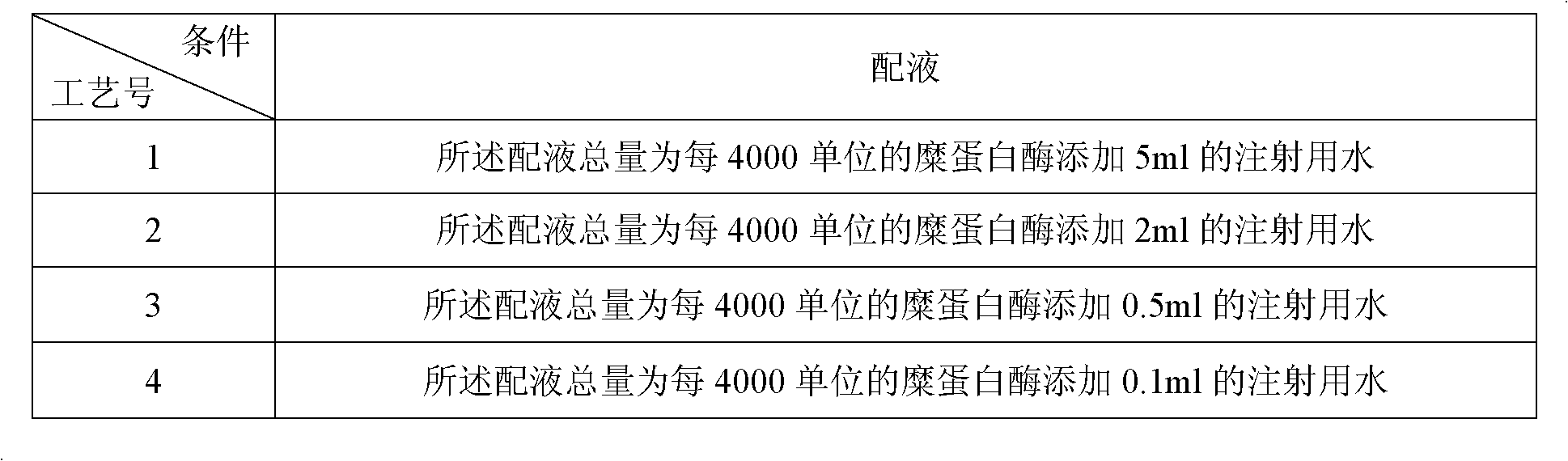
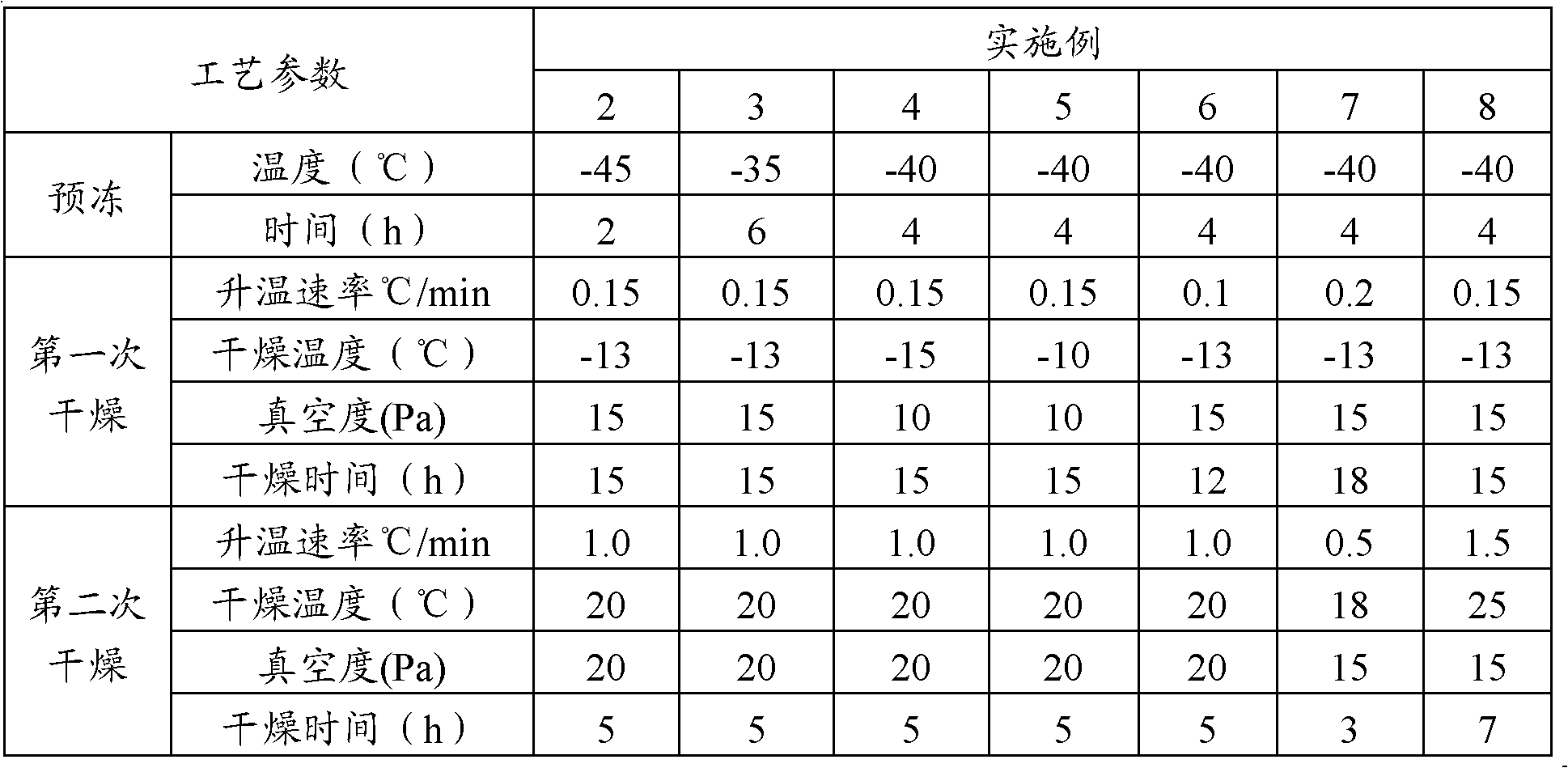
Freeze-drying protection system required for nucleic acid amplification reagent and preparation method of freeze-drying protection system
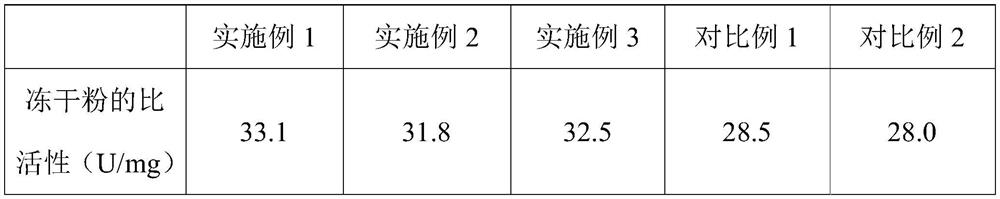
InactiveCN109593834AKeep aliveNo biohazardMicrobiological testing/measurementFreeze thawingFreeze-drying
The invention belongs to the technical field of medicine preparation, and particularly relates to a freeze-drying protection system required for a nucleic acid amplification reagent and a preparationmethod of the freeze-drying protection system. The freeze-drying protection system comprises the nucleic acid amplification reagent and a freeze-drying protection additive, wherein the nucleic acid amplification reagent is a reagent used for LAMP reaction amplification, the freeze-drying protection additive is one or a compound of the following reagents: polyethylene glycol, mannitol, polyvinylpyrrolidone, glucan, trehalose, sucrose, bovine serum albumin, collagen, threonine and glycine, and the concentration of the weight-to-volume ratio of the freeze-drying protection additive and the amplification reaction reagent is 3% to 25%. The freeze-drying protection additive used in the invention has the advantages of small volume, short freeze-drying time, high efficiency and low energy consumption, and the freeze-drying protection system can be directly used for gene chip experiments, does not cause repeated freeze-thaw and waste of reagents, and can effectively ensure the activity of active substances in the freeze-drying process.
Owner:百康芯(天津)生物科技有限公司
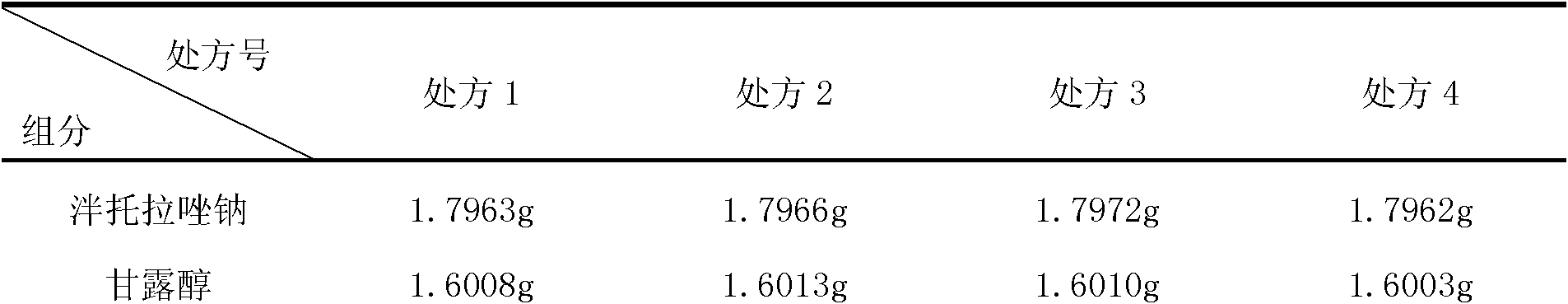
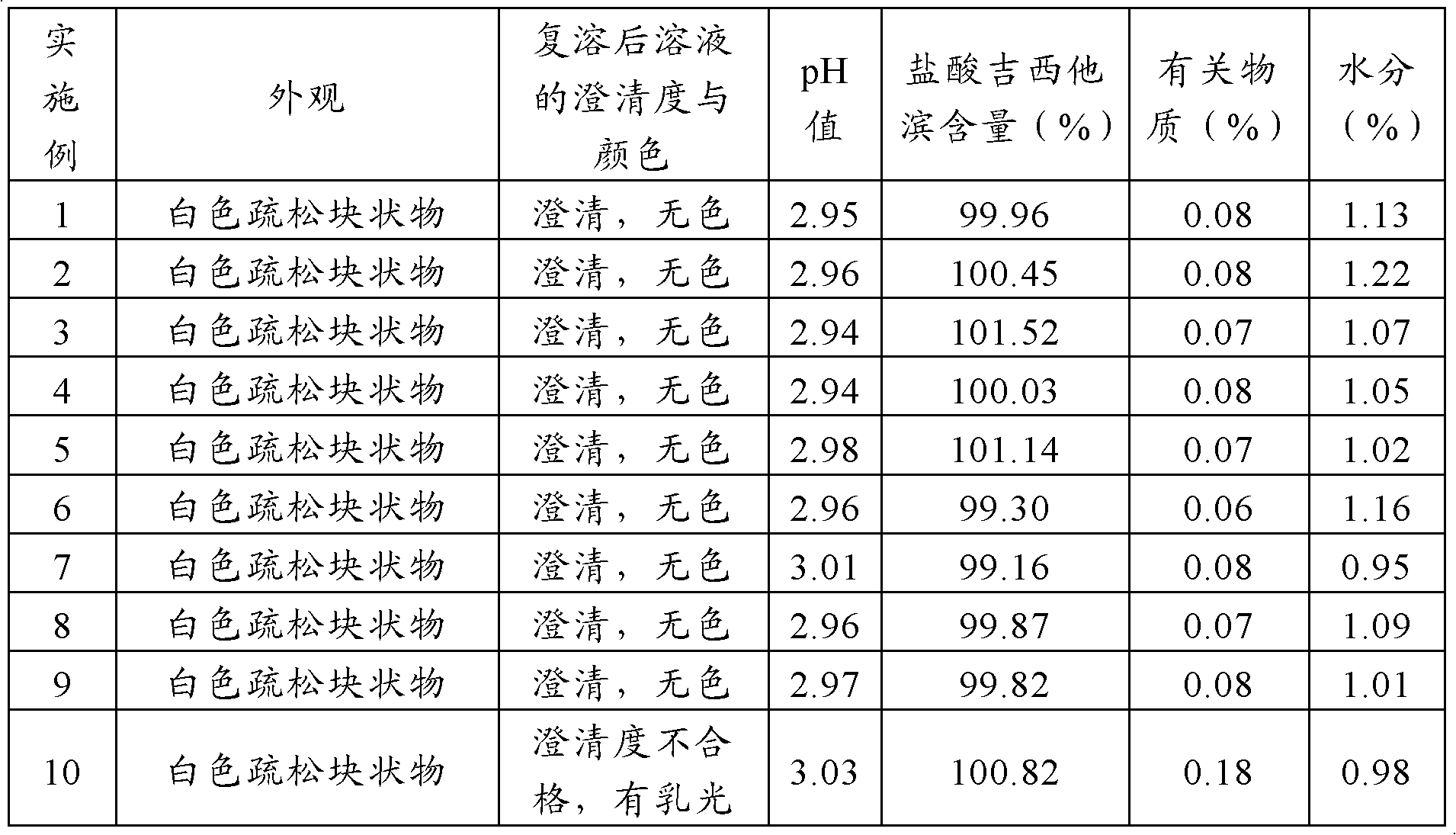
Pantoprazole sodium freeze-dried powder injection and preparation method thereof
ActiveCN102085190AShorten the secondary drying timeGood lookingOrganic active ingredientsPowder deliveryCLARITYFreeze-drying
The invention relates to a pantoprazole sodium freeze-dried powder injection and a preparation method thereof. The powder injection is prepared from pantoprazole sodium and mannitol, wherein the consumption ratio of the pantoprazole sodium to the mannitol is (1:0.8)-(1:1.6), and the PH value is 10.5-11.0. In the invention, by lowering the pre-freezing temperature, properly lowering the freezing temperature, maintaining the lowered freezing temperature for a proper time, properly shortening two-stage drying time and carrying out other adjustment processes, good appearance and quality of the product can be kept under the condition that the content of the mannitol is low, the processes are reliable and feasible, and the effect is obvious. The prepared product has low content of related substances and has controllable quality, and the freeze-dried product has good clarity and formability after being redissolved.
Owner:HAINAN JINRUI PHARMA CO LTD
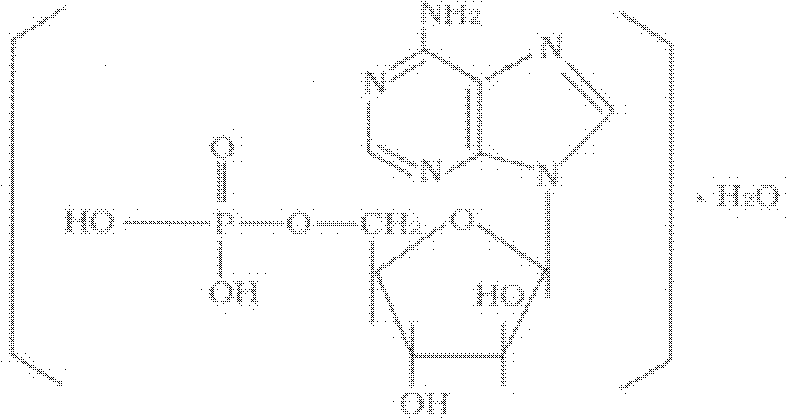
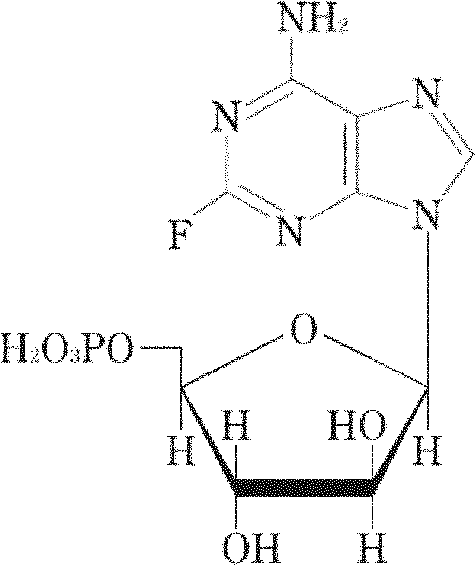
Vidarabine monophosphate freeze-dried powder injection and preparation method thereof
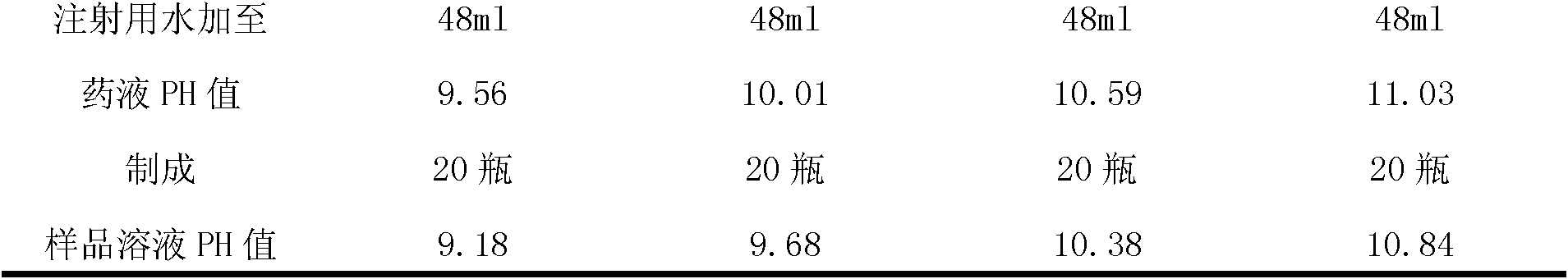
ActiveCN102379853AGood formabilityHigh clarityPowder deliveryOrganic active ingredientsFreeze-dryingPhosphoric acid
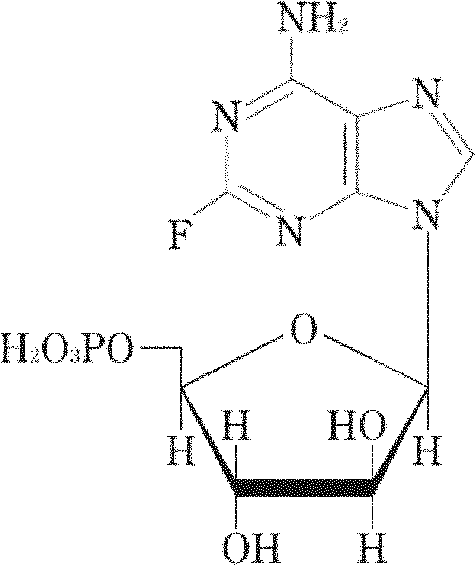
The invention relates to a vidarabine monophosphate freeze-dried powder injection and a preparation method thereof, and the vidarabine monophosphate freeze-dried powder injection is prepared by freeze-drying vidarabine monophosphate, sodium hydroxide solution and water for injection, wherein the sodium hydroxide solution is used as a pH regulator, and the sodium hydroxide solution is used until the pH value of liquid medicine is 7.0-7.5. The vidarabine monophosphate freeze-dried powder injection provided by the invention is few in types of auxiliary materials, low in using amount, good in forming property of a freeze-dried product, good in re-dissolving property, clear in appearance of the solution before freezing, good in clarity after being freeze-dried, low in content of impurities, good in stability and controllable in quality, and can reduce the potential safety hazard of the vidarabine monophosphate freeze-dried powder injection, and improve the efficacy of the vidarabine monophosphate freeze-dried powder injection.
Owner:HAINAN JINRUI PHARMA CO LTD
Dexrazoxane freezing-dried powder injection and preparation method thereof
InactiveCN101143134AFix stability issuesFreeze-dried wellOrganic active ingredientsPowder deliveryMicropore FilterFreeze-drying
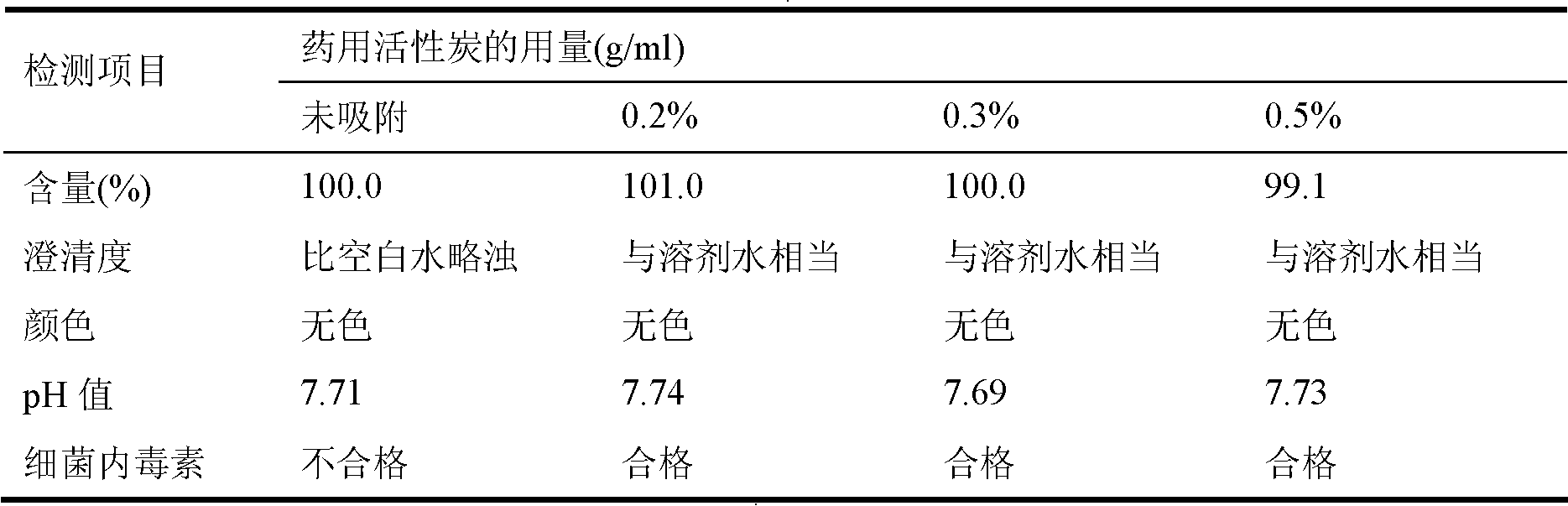
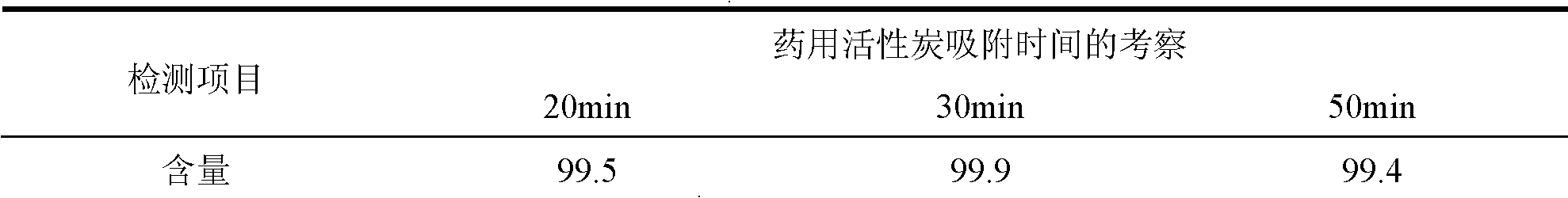
The invention relates to a dexrazoxane freeze-dried powder injection and the preparation method. The preparation is used for resisting the cardiac toxicity which is induced by the cumulate quantity of adriamycin. The dexrazoxane freeze-dried powder injection contains the active components of the dexrazoxane and hydrochloric acid, the weight proportion of which is 1 to 0.05 to 0.5, and the preferential proportion is 1 to 0.2 to 0.5. The preparation method is that the hydrochloric acid is put into an aseptic vessel; the water for injecting is added till 80 percent of the preparation quantity, and the temperature is reduced and kept at 2 to 6 DEG C; the dexrazoxane is added to be mixed, and the hydrochloric acid of 1.0mol / L is dripped slowly into the solution to be solved while mixing the solution; the water for injecting is added till the full quantity; active carbon of 0.3 percent is added for absorbing for thirty minutes, and then the solution is decarbonized; after the medium body content is mensurated as being eligible, the solution is filtered by a 0.22 micron-micropore filtering filer; the filtrate is filled into a 25ml cillin bottle according to the filling quantity of 10ml each bottle, and the bottles are partially plugged by buna plugs and filled onto a plate to be sent into a freeze-drying box; a temperature probe is inserted, and the box door is closed; the filtrate is warmed, sublimed and dried be stages; nitrogen is puffed; the plugs are pressed; the filtrate is taken out from the box for rolling the openings, detecting the quality and packaging and the preparation can be obtained.
Owner:JIANGSU AOSAIKANG PHARMA CO LTD
Method for preparing recombinant human insulin crystal
InactiveCN105753966AUniform crystal formLarge particlesPeptide preparation methodsInsulinsOrganic solventHazardous substance
The invention provides a method for preparing recombinant human insulin crystal, and belongs to the technical field of protein crystallization. According to the method, zinc ions are subjected to three times of crystallization, the obtained insulin crystal is large in particle size and good in homogeneity, phenols or organic solvents used in normal methods are not used in the method, so that residues of harmful substances in the product are avoided and the quality of the insulin crystal is improved; moreover, the method is stable in preparation process and beneficial to industrial production.
Owner:TONGHUA DONGBAO PHARMA
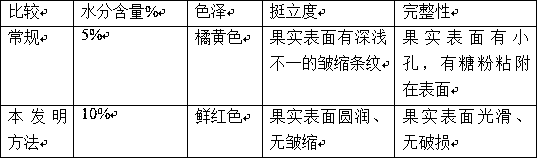
Processing method of freeze-dried fresh fructus lycii
The invention discloses a processing method of freeze-dried fresh fructus lycii. The freeze-dried fresh fructus lycii is obtained by enabling fresh fructus lycii to be at a frozen state in a freeze-drying process all the time through a freeze-drying technology so as to realize sublimation and dehydration purposes under the conditions that any additive is not added, the fructus lycii does not need to be dewaxed and an initial state of the fresh fructus lycii is kept, and packaging without the need of nitrogen gas. The method disclosed by the invention comprises the following steps: treating raw materials, washing and draining, quickly freezing, freezing in vacuum and drying, and packaging a finished product. The processing method disclosed by the invention has the beneficial effects that (1) an initial natural state of the fresh fructus lycii is kept, and nutrient components and active substances are kept completely; color and luster, apparent shapes and re-watered appearance and taste keeping basically consistent with those of fresh fruits; (2) long-time storage of the fresh fructus lycii is realized and the shelf life is prolonged; the sublimation and freeze-drying time of moisture is relatively short, the production cost is reduced and the energy consumption is greatly reduced; (3) any additive is not added so that the freeze-dried fresh fructus lycii is safe and reliable to eat and the product has long-time stability in marketing sale.
Owner:青铜峡市青绿食品有限公司
Fludarabine phosphate freeze-dried powder injection and preparation method thereof
ActiveCN102091046AThe appearance is plump and does not shrinkUniform colorOrganic active ingredientsPowder deliveryFreeze-dryingCLARITY
The invention belongs to the technical field of medicines, and relates to a fludarabine phosphate freeze-dried powder injection and a preparation method thereof. The fludarabine phosphate freeze-dried powder injection consists of fludarabine phosphate and mannitol in a mass ratio of 1:(0.6-1.2). The method for preparing the fludarabine phosphate freeze-dried powder injection comprises the following steps of: adding water for injection into a liquid preparing tank, adding the fludarabine phosphate in a prescription amount, adding alkali, stirring until the mixture is dissolved completely; and adding the mannitol, regulating the pH value, adding active carbon for decolorizing, filtering for decolorizing, performing fine filtering by using a filter membrane, subpackaging, and performing freeze drying. The fludarabine phosphate freeze-dried powder injection has excellent formability, clarified appearance of solution to be frozen, high redissolution performance of freeze-dried products, high clarity after redissolution, low impurity content and moisture content, high stability and controllable quality.
Owner:HAINAN JINRUI PHARMA
Dantrolene sodium freeze-dried powder injection for injection and preparation method thereof
ActiveCN102302461AImprove toleranceDosing time is shortPowder deliveryOrganic active ingredientsForeign matterCLARITY
The invention belongs to the technical field of medicines, and particularly relates to a dantrolene sodium freeze-dried powder injection for injection and a preparation method thereof. The freeze-dried powder injection is prepared by freeze drying dantrolene sodium, lactobiose, a pH regulator and water for injection, wherein a mass ratio of the dantrolene sodium to the lactobiose is (1:0.5) to (1:2.5). The preparation method comprises the following steps of: adding the dantrolene sodium to the lactobiose in a prescription amount into the water for the injection, stirring, regulating the pH value to 9.0 to 10.5 by using the pH regulator, adding active carbon for injection, removing a heat source, decolorizing, filtering for decarburizing, performing fine filtering by using a filter membrane, packaging in separate bags, freezing and drying. The dantrolene sodium freeze-dried powder injection is studied on the basis of a freeze-dried process, namely the dantrolene sodium freeze-dried powder injection is cooled, heated by a small margin and is cooled again, so that the moisture of the freeze-dried product is reduced, and the freeze-dried product is high in redissolution and visible foreign matters and does not have insoluble granules. The dantrolene sodium freeze-dried powder injection for the injection is high in resolubility, clarity and stability, and low impurity content.
Owner:HAINAN JINRUI PHARMA
Chymotrypsin composition freeze-dried powder and preparation method thereof
ActiveCN102860988AFull and uniform appearanceGood resolubilityPowder deliverySenses disorderPorositySolubility
The invention relates to a chymotrypsin preparation, particularly relates to chymotrypsin composition freeze-dried powder and a preparation method thereof. The hymotrypsin composition freeze-dried powder comprises chymotrypsin, dextran 20 and sorbitol, wherein the ratio of chymotrypsin to dextran 20 to sorbitol is 1 million units: 1-1.8g: 0.05-0.6g. The average porosity of the hymotrypsin composition freeze-dried powder is 85-98%. The invention further relates to a preparation method of the hymotrypsin composition freeze-dried powder. The hymotrypsin composition freeze-dried powder disclosed herein has the advantages of full and uniform appearance, high porosity, good re-solubility, high stability, simple prescription, low proportion of pharmaceutic adjuvants, low side effect to human body, safety and reliability. According to the invention, the preparation technology of the hymotrypsin composition freeze-dried powder is further improved, the temperature, liquid volume, and freeze-drying process are improved, and sterilization is conducted twice.
Owner:HAINAN HERUI PHARMA
Processing method and application of slightly lyophilized Chinese wolfberries
ActiveCN108041153AReduce microbial contentReduce pesticide residuesFruit and vegetables preservationFreeze-dryingNatural state
The invention discloses a processing method of slightly lyophilized Chinese wolfberries. The method comprises the following steps of soaking, cleaning, freeze-drying protection and freeze-drying. Themethod mainly relates to the following steps in detail: adding a freeze-drying protective agent containing 1-10% w / w of trehalose and 0.5-1% w / w of calcium chloride to washed Chinese wolfberries; uniformly spraying the protective agent on the surface of the Chinese wolfberries by adopting atomization spray heads; putting the Chinese wolfberries into a freeze-drying machine, and starting vacuumizing under the condition of no heating and no freezing; keeping a negative pressure for 10 to 30 minutes as the pressure drops to 60 Pa; and maintaining a vacuum degree for 2-5h. according to the lyophilized fresh Chinese wolfberry fruits produced by adopting a vacuum freeze-drying technology, the method maintains the original natural state of fresh Chinese wolfberry fruits, and the color, the appearance shape and the appearance and mouth feel after rehydration of the lyophilized Chinese wolfberry fruits are basically the same as that of the picked fresh Chinese wolfberry fruits. The method maintains the integrity and stiffness of dried Chinese wolfberry cells, and reduces the production cost of products, and the products are easier to preserve in marketing sale process.
Owner:早康枸杞股份有限公司
Fructus momordicae freeze-dried micropowder and preparation method thereof
InactiveCN106174198AMaintain colorKeep the scentFood freezingFood dryingAdditive ingredientFreeze-drying
The invention discloses fructus momordicae freeze-dried micropowder. The fructus momordicae freeze-dried micropowder is prepared through the steps of fruit sorting, cleaning, slicing, fixation, natural air drying, prefreezing, secondary freezing, deep freezing, superfine grinding and the like. Compared with the prior art, according to the method, the technology is simple and easy to control, the freeze-drying time is short, energy consumption is low, and the production efficiency is high; a hot-water fixation technology is adopted, activity of enzymes of polyphenol oxidase and the like in peel of fructus momordicae can be passivated, fructus momordicae can be sterilized, and it is guaranteed that eating is safe and hygienic; by means of the three-time freezing technology, moisture in fructus momordicae is subjected to orderly sublimation drying, and peel and pulp separation caused by moisture drying is avoided; meanwhile, the color, fragrance, taste, nutritional ingredients and the like of fructus momordicae are well maintained; after the fine powder is obtained, the use rate of fructus momordicae is greatly increased, absorption is enhanced, the taste is improved, and application is wider.
Owner:陈素云
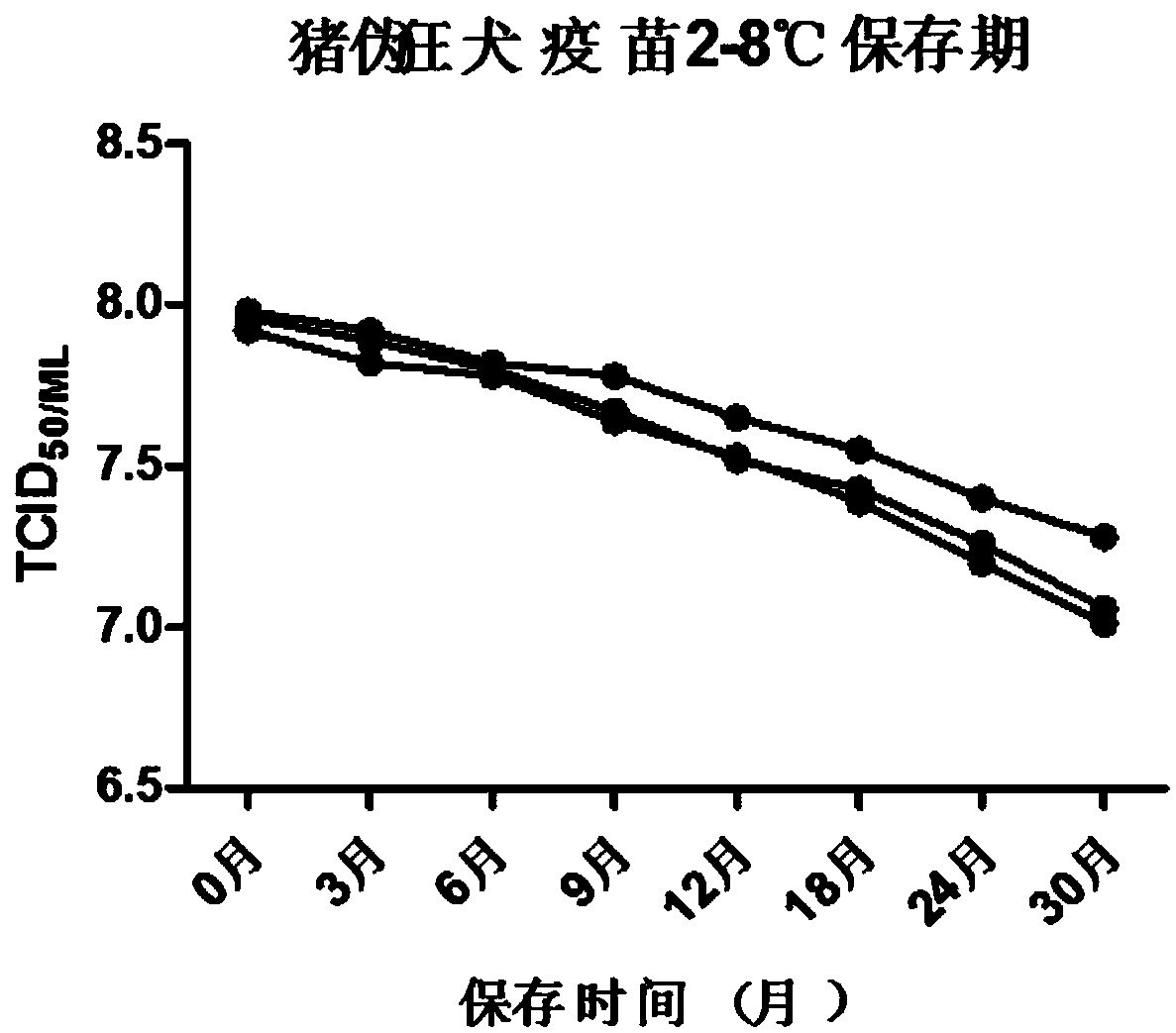
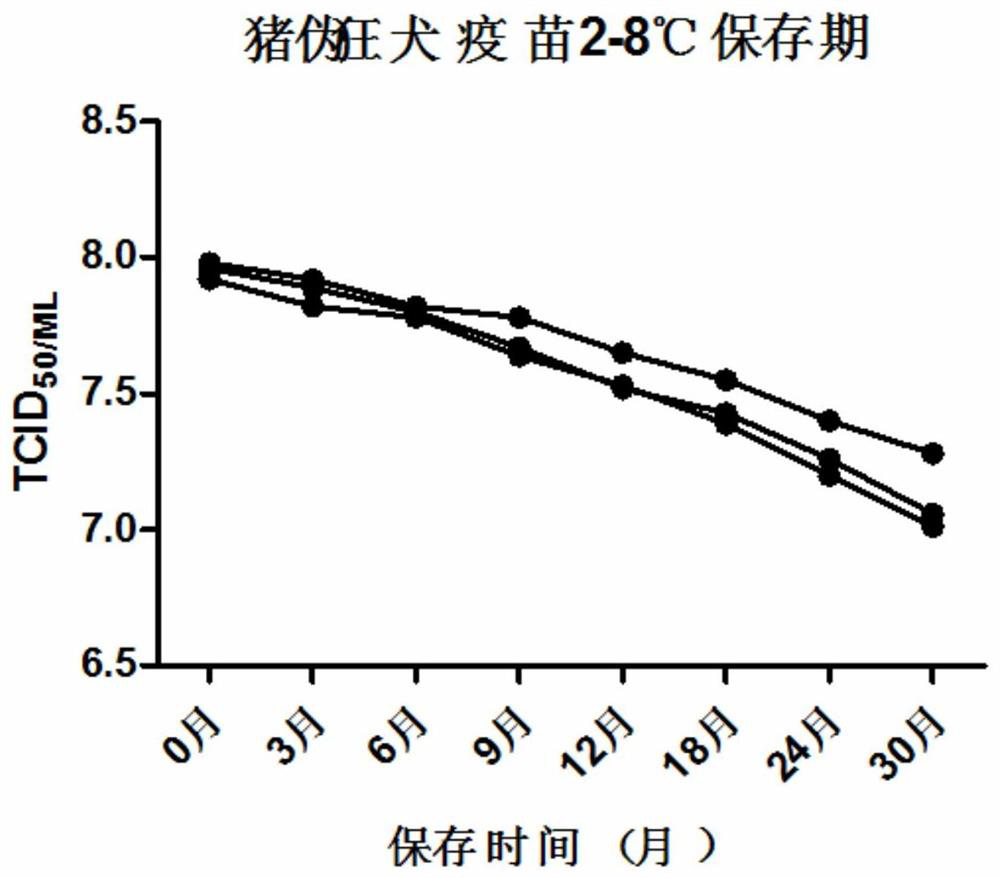
Porcine pseudorabies live vaccine freeze-drying heat-resisting protective agent, preparation method and application thereof
ActiveCN108938574AFreeze-drying time is shortLow costPowder deliveryViral antigen ingredientsFreeze-dryingPseudorabies
The invention provides a porcine pseudorabies live vaccine freeze-drying heat-resisting protective agent, a preparation method and an application thereof. The protecting agent is characterized by being free of gelatin and proteins. The protecting agent contains the following components in percentage by weight: 2-8% of saccharose, 3-6% of isomaltulose, 1-5% of D-sorbitol, 1-5% of glycerinum, 0.1-5%of hydroxypropyl methylcellulose, 0.1-3.5% of methionine, 0.01-0.05% of poly-D-lysine, Hanks (containing phenol red) and the balance of double pure water, totaling 100%. The porcine pseudorabies livevaccine prepared by the freeze-drying heat-resisting protective agent is relatively good in heat resistance, the freeze-drying loss is 0.5-0.65Lg, the heat resistant loss at 37 DEG C for 10 days is 0.2-0.25Lg, and the storage life loss at 2-8 DEG C for 30 days is 0.7-0.9Lg. The low-temperature cold chain problem in storage and transportation of the vaccine is solved, and in particular the rural low-temperature cold chain problem in a remote region is solved.
Owner:JIANGSU ACAD OF AGRI SCI
Fludarabine phosphate freeze-dried powder injection and preparation method thereof
ActiveCN102091046BFull and uniform appearanceGood resolubilityPowder deliveryOrganic active ingredientsFreeze-dryingCLARITY
The invention belongs to the technical field of medicines, and relates to a fludarabine phosphate freeze-dried powder injection and a preparation method thereof. The fludarabine phosphate freeze-dried powder injection consists of fludarabine phosphate and mannitol in a mass ratio of 1:(0.6-1.2). The method for preparing the fludarabine phosphate freeze-dried powder injection comprises the following steps of: adding water for injection into a liquid preparing tank, adding the fludarabine phosphate in a prescription amount, adding alkali, stirring until the mixture is dissolved completely; and adding the mannitol, regulating the pH value, adding active carbon for decolorizing, filtering for decolorizing, performing fine filtering by using a filter membrane, subpackaging, and performing freeze drying. The fludarabine phosphate freeze-dried powder injection has excellent formability, clarified appearance of solution to be frozen, high redissolution performance of freeze-dried products, high clarity after redissolution, low impurity content and moisture content, high stability and controllable quality.
Owner:HAINAN JINRUI PHARMA CO LTD
A kind of multivitamin parenteral nutrition nanosphere freeze-dried injection and preparation method thereof
ActiveCN109010292BSolve the co-solution problemInfinite dilutionPowder deliveryHydroxy compound active ingredientsBiotechnologyParenteral nutrition
The invention discloses a multi-vitamin parenteral nutrition nanosphere freeze-dried injection, which is composed of 13 kinds of vitamins, medium-chain triglycerides, soybean lecithin, 15-hydroxystearic acid polyethylene glycol ester, glycerin , mannitol and water, wherein the mass ratio of soybean lecithin to polyethylene glycol 15-hydroxystearate is 1: (1.4-4); medium-chain triglycerides, soybean lecithin and 15-hydroxystearate The mass ratio of the total weight of polyethylene glycol stearate is 1: (1-4). In the present invention, fat-soluble vitamins are made into a lipid nanosphere solution, which can be co-dissolved with water-soluble vitamin solutions, and the problem of co-dissolution of fat-soluble vitamins and water-soluble vitamins is solved in an ingenious way. The multivitamin parenteral nutrition nanosphere freeze-dried finished product of the present invention is a clear and transparent liquid after reconstitution with water, and can be diluted with infinite aqueous solution. The product of the present invention has high safety, high quality, good stability, and easy operation of the production process. , low production cost.
Owner:CHINESE MEDICINES GUANGZHOU
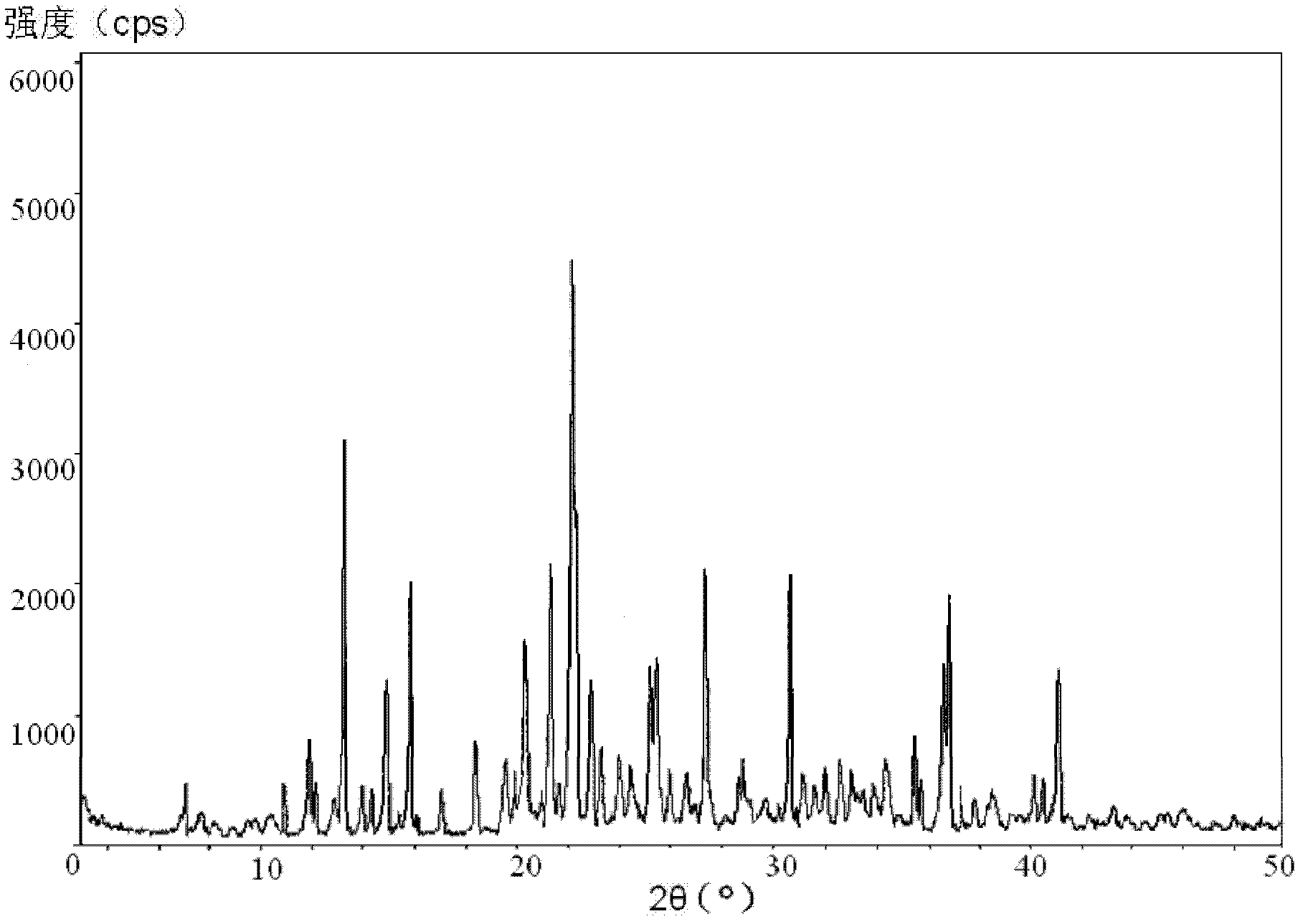
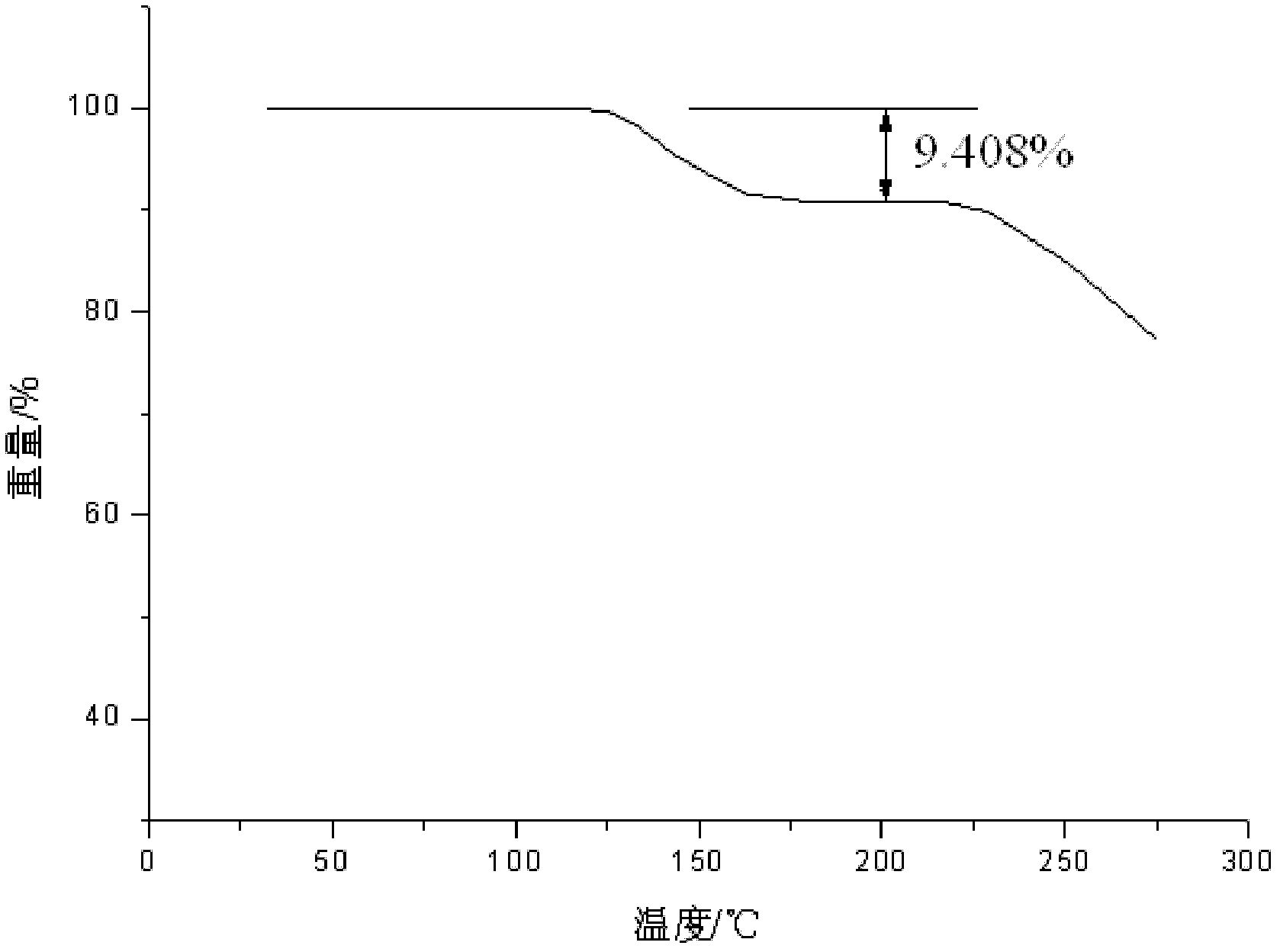
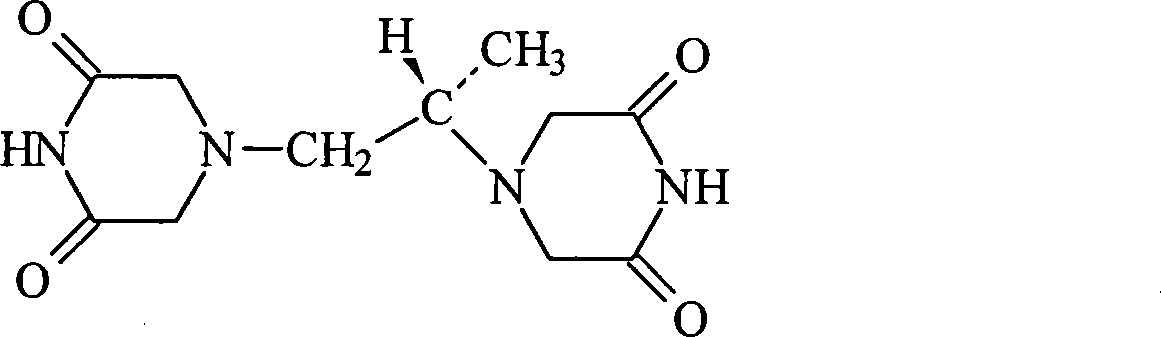
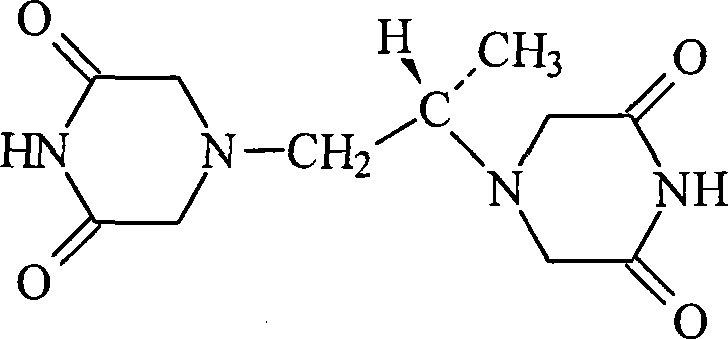
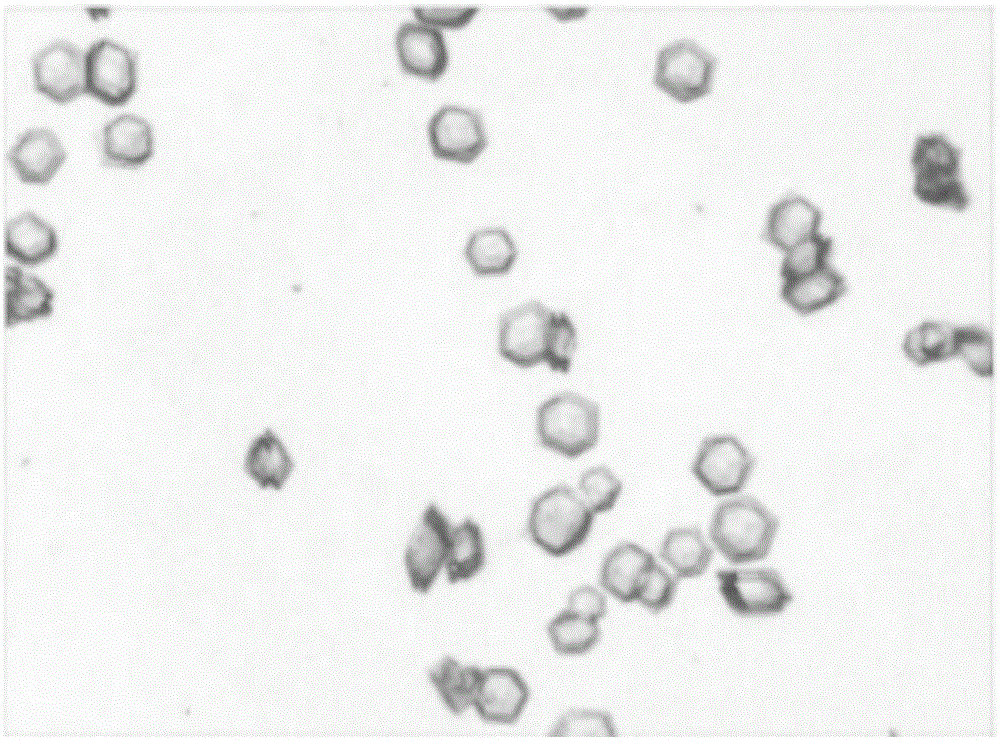
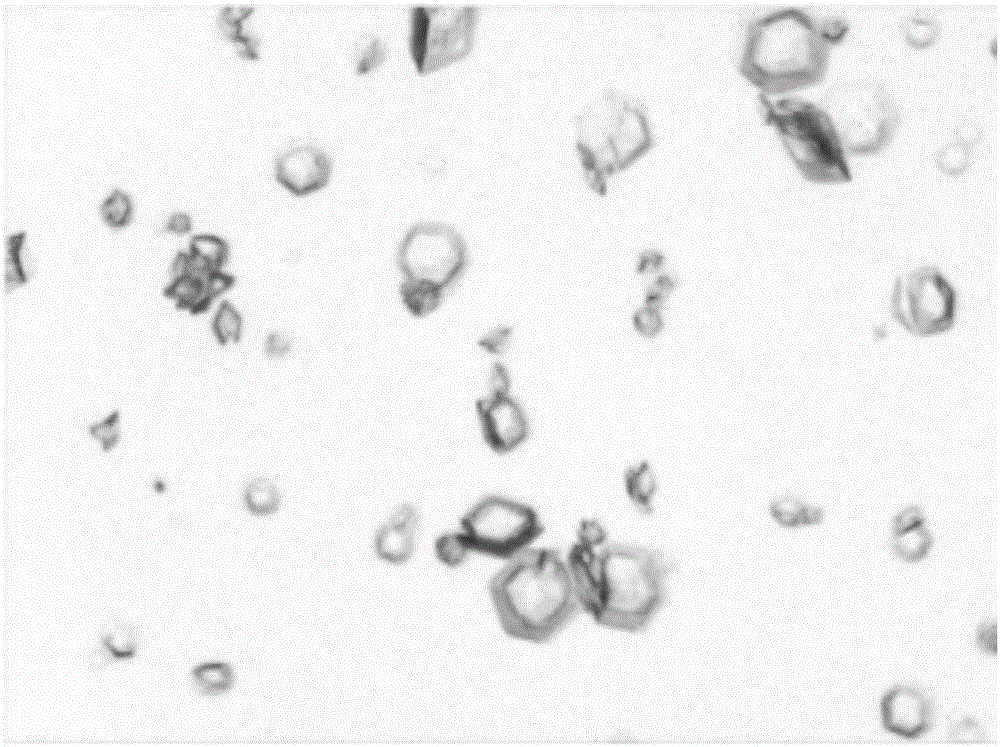
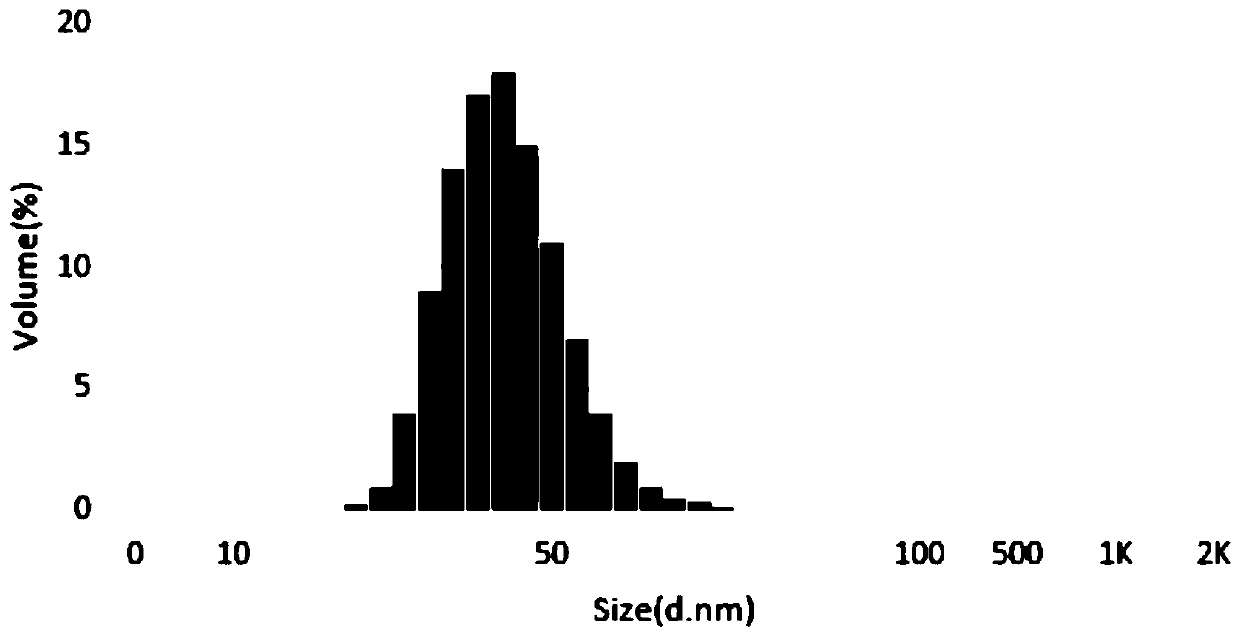
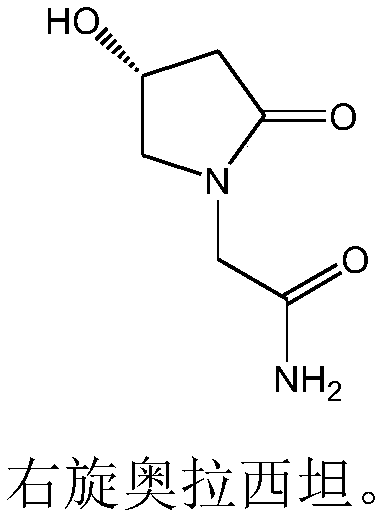
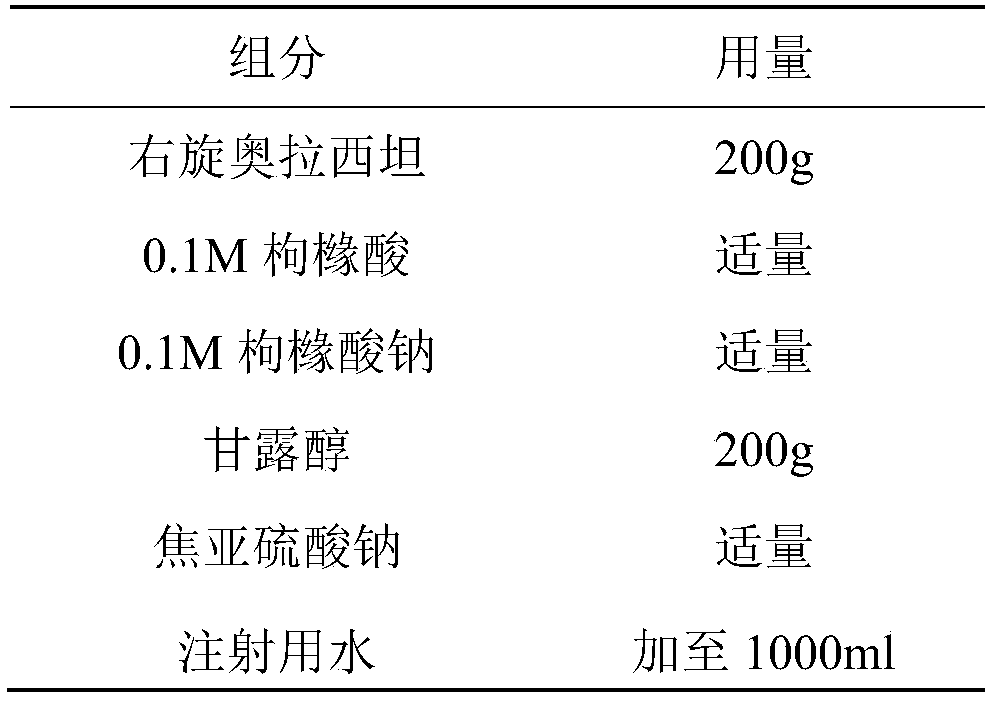
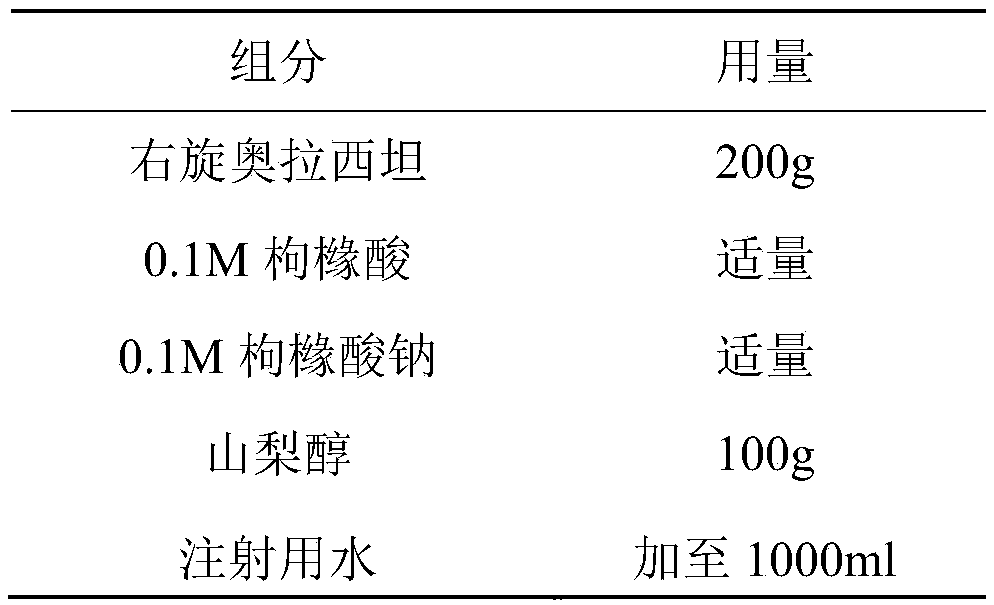
Freeze-dried dextro-oxiracetam preparation for injection and preparation method thereof
InactiveCN110314143AImprove stabilityHigh purityPowder deliveryOrganic active ingredientsSocial benefitsFreeze-drying
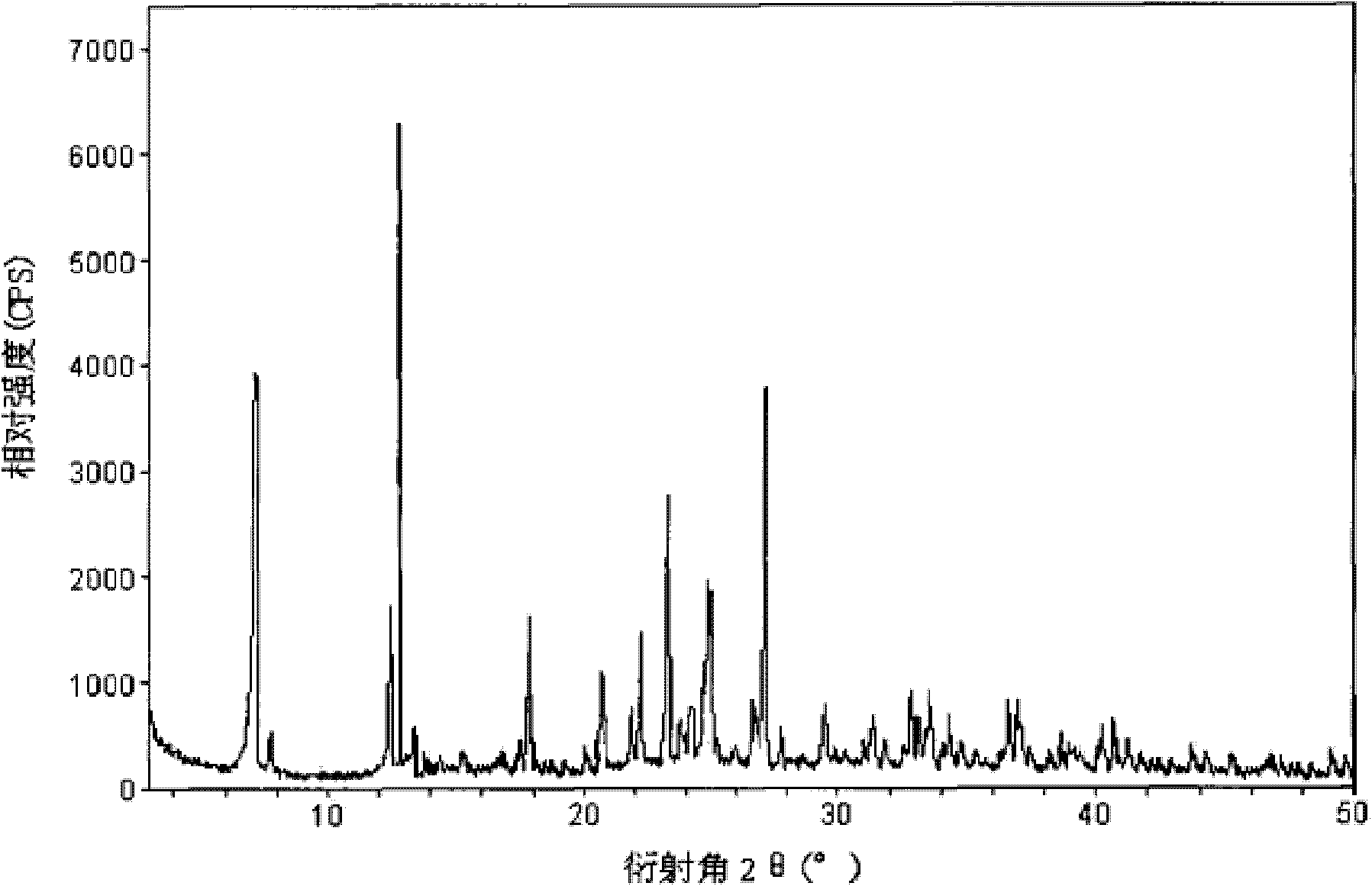
The invention provides a freeze-dried dextro-oxiracetam preparation. freeze-dried dextro-se oxiracetam crystal compounds with diffraction peaks at the diffraction angles 2theta of 17.76+ / -0.2 degrees,20.16+ / -0.2 degrees, 21.20+ / -0.2 degrees, 24.17+ / -0.2 degrees and 25.88+ / -0.2 degrees, which are subjected to X powder diffraction through Cu Ka radiation, are adopted as active ingredients to be subjected to freeze drying with a freeze-drying excipient by means of a specific freeze-drying technology to obtain the free-dried dextro-se oxiracetam preparation which has high purity, low impurity content and high stability and can be stored for a long time without deterioration. The preparation has the advantages that a preparation technology is simple, convenient, feasible and high in repeatability, industrial large-scale production is easily realized, and considerable economic and social benefits can be generated.
Owner:CHONGQING RUNZE PHARM CO LTD
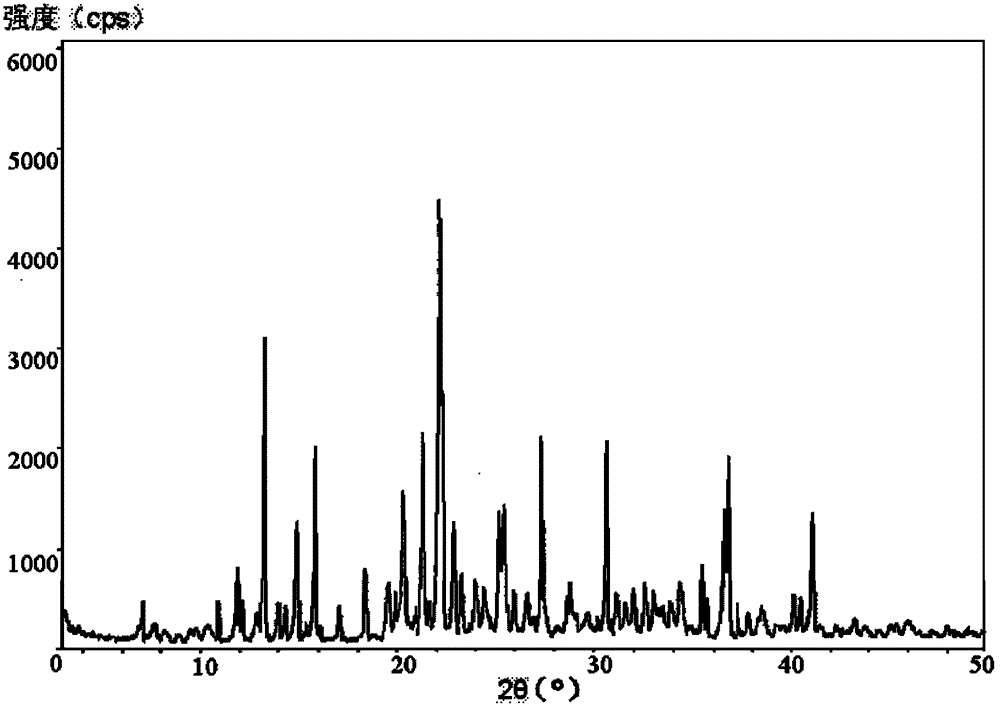
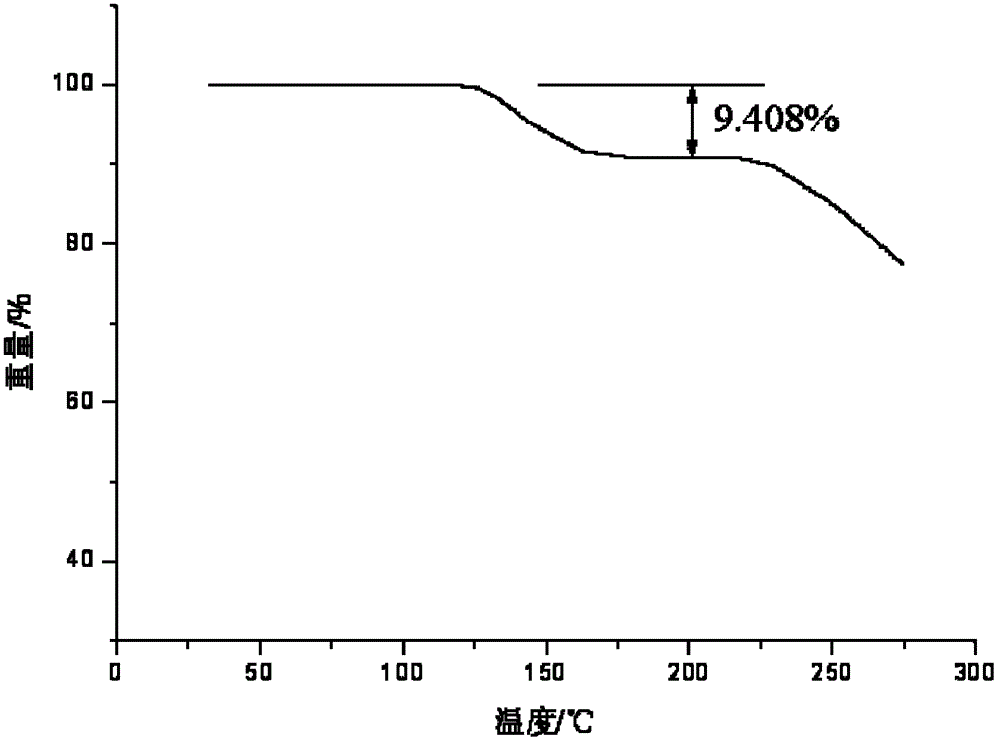
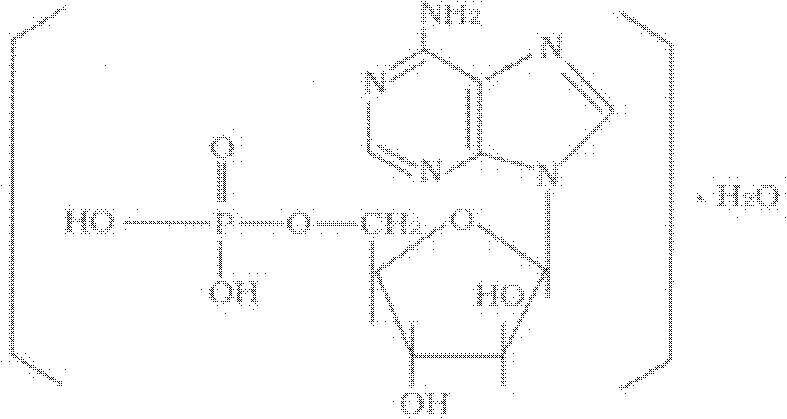
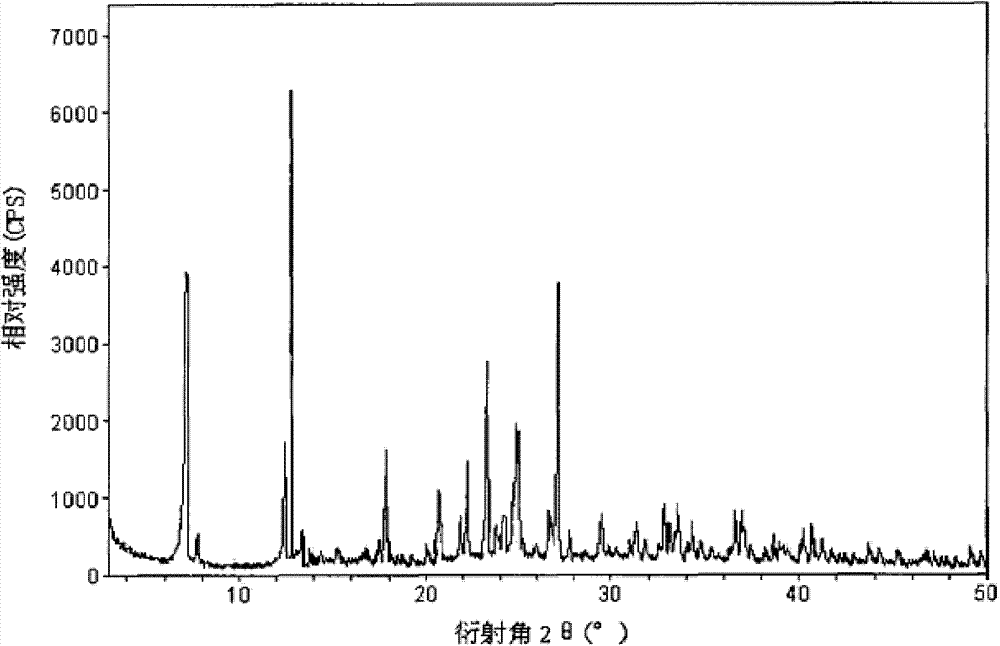
Vidarabine monophosphate freeze-dried powder injection and preparation method thereof
ActiveCN102379853BGood formabilityHigh clarityOrganic active ingredientsPowder deliveryFreeze-dryingPhosphoric acid
The invention relates to a vidarabine monophosphate freeze-dried powder injection and a preparation method thereof, and the vidarabine monophosphate freeze-dried powder injection is prepared by freeze-drying vidarabine monophosphate, sodium hydroxide solution and water for injection, wherein the sodium hydroxide solution is used as a pH regulator, and the sodium hydroxide solution is used until the pH value of liquid medicine is 7.0-7.5. The vidarabine monophosphate freeze-dried powder injection provided by the invention is few in types of auxiliary materials, low in using amount, good in forming property of a freeze-dried product, good in re-dissolving property, clear in appearance of the solution before freezing, good in clarity after being freeze-dried, low in content of impurities, good in stability and controllable in quality, and can reduce the potential safety hazard of the vidarabine monophosphate freeze-dried powder injection, and improve the efficacy of the vidarabine monophosphate freeze-dried powder injection.
Owner:HAINAN JINRUI PHARMA CO LTD
Momordica grosvenori drying process and momordica grosvenori dried product prepared through same
InactiveCN106136129AThe process is simple and easy to controlFreeze drying time is shortFruits/vegetable preservation by irradiation/electric treatmentFood dryingPunchingFreeze-drying
The invention belongs to the technical field of food processing, and particularly discloses a momordica grosvenori drying process and a momordica grosvenori dried product prepared through the momordica grosvenori drying process. The drying process comprises the steps of fruit selection, cleaning, fixation, punching, disinfection, freezing and the like. Compared with the prior art, the process is simple and easy to control, the freeze drying time is short, energy consumption is low, and production efficiency is high. Activity of polyphenol oxidase and the like in momordica grosvenori is restrained through microwave fixation, then, ultraviolet light is adopted for sterilization and disinfection, the storage period of momordica grosvenori freeze-dried superfine powder is effectively prolonged, and using safety and health of the momordica grosvenori freeze-dried superfine powder are ensured; by means of a vacuum freezing sublimation drying process, the problem that pulp and peel are separated due to water loss is avoided, the shape, color, luster, fragrance, taste, nutritive ingredients and the like of momordica grosvenori are well kept, and the momordica grosvenori drying method is worthy of being applied and popularized.
Owner:陈素云
Freeze-dried siraitia grosvenorii slices and preparation method thereof
InactiveCN106136128AMaintain colorKeep the scentFruits/vegetable preservation by heatingFood dryingFreeze-dryingDrying time
The invention discloses freeze-dried siraitia grosvenorii slices and a preparation method thereof and belongs to the technical field of food processing. The freeze-dried fructus siraitiae slices are prepared through processes of selecting, slicing, carrying out fixation, naturally drying by air, pre-freezing, carrying out secondary freezing, deeply freezing and the like. Compared with the prior art, the method has a simple and easily-controlled process, short freezing and drying time, low energy consumption and high production efficiency; a hot water fixation technology is adopted so that the activity of enzymes including polyphenol oxidase and the like in siraitia grosvenorii peels is deactivated; three times of freezing processes are adopted so that moisture in siraitia grosvenorii is volatilized in sequence and the separation of peels and pulp, caused by moisture drying, is avoided; meanwhile, the color and luster, aroma, taste, nutrient components and the like of the siraitia grosvenorii are kept very well.
Owner:陈素云
Mini fast vacuum freeze-drier and its drying method
InactiveCN1202401CFreeze-dried fastMaintain structureDrying solid materials without heatFreeze-dryingRoom temperature
The invention discloses a miniature rapid vacuum freeze dryer and a freeze drying method. It belongs to vacuum freeze drying equipment and process technology. The vacuum desiccator is mainly composed of a freeze-drying chamber, a refrigerant contained in the freeze-drying chamber, a vacuum device connected with the freeze-drying chamber, a condensation receiving chamber and a sample drying chamber. The method for freeze-drying samples by using the above-mentioned vacuum freeze-dryer is to place the sample drying chamber containing the sample in the refrigerant in the freeze-drying chamber to pre-freeze the sample, and then quickly place the pre-frozen sample drying chamber and the freeze-drying chamber Connected with each other, using an air bath at room temperature or an external heating source as the heating medium, and freeze-drying under vacuum conditions. The main advantages of the present invention are that the structure of the freeze dryer is simple, portable and flexible, the production cost is very low, the operation process is simple, the equipment runs smoothly, the energy consumption is low, the pre-freezing speed is fast, and the freeze-drying time is short.
Owner:TIANJIN UNIV
Whole fruit drying process of grosvenor momordica fruit
InactiveCN106212643AAvoid separationConsistent colorFruit and vegetables preservationPunchingMomordica
The invention belongs to the technical field of food processing, and in particular discloses a whole fruit drying process of grosvenor momordica fruit. The drying process including fruit selection, washing, enzyme deactivation, punching and freezing. The process has the advantages of simple process, easiness to control, short freeze drying time, low energy consumption, high production efficiency and low cost, and is applicable to large-scale industrial production. A water steam enzyme deactivation technology is used to passivate the enzyme activity of polyphenoloxidase in the peel of grosvenor momordica fruit, to sterilize the grosvenor momordica fruit, and ensure food safety and health; and three freezing processes effectively avoid the separation of peel and pulp due to dryness, so that the freeze-dried grosvenor momordica fruit product is integrated and beautiful, and has color and taste consistent with fresh fruits.
Owner:陈素云
Dantrolene sodium freeze-dried powder injection for injection and preparation method thereof
ActiveCN102302461BGood formabilityHigh clarityOrganic active ingredientsPowder deliveryForeign matterCLARITY
The invention belongs to the technical field of medicines, and particularly relates to a dantrolene sodium freeze-dried powder injection for injection and a preparation method thereof. The freeze-dried powder injection is prepared by freeze drying dantrolene sodium, lactobiose, a pH regulator and water for injection, wherein a mass ratio of the dantrolene sodium to the lactobiose is (1:0.5) to (1:2.5). The preparation method comprises the following steps of: adding the dantrolene sodium to the lactobiose in a prescription amount into the water for the injection, stirring, regulating the pH value to 9.0 to 10.5 by using the pH regulator, adding active carbon for injection, removing a heat source, decolorizing, filtering for decarburizing, performing fine filtering by using a filter membrane, packaging in separate bags, freezing and drying. The dantrolene sodium freeze-dried powder injection is studied on the basis of a freeze-dried process, namely the dantrolene sodium freeze-dried powder injection is cooled, heated by a small margin and is cooled again, so that the moisture of the freeze-dried product is reduced, and the freeze-dried product is high in redissolution and visible foreign matters and does not have insoluble granules. The dantrolene sodium freeze-dried powder injection for the injection is high in resolubility, clarity and stability, and low impurity content.
Owner:HAINAN JINRUI PHARMA CO LTD
Method for preparing freeze-dried superfine powder of siraitia grosvenorii
InactiveCN105995736AThe process is simple and easy to controlFreeze drying time is shortFood freezingFruits/vegetable preservation by irradiation/electric treatmentFreeze-dryingAdditive ingredient
The invention discloses a method for preparing freeze-dried superfine powder of siraitia grosvenorii. The method comprises the following steps: (1) selecting the siraitia grosvenorii; (2) performing dicing; (3) performing microwave fixation; (4) performing ultraviolet disinfection; (5) performing pre-refrigerating; (6) performing refrigerating for the second time; (7) performing deep-refrigerating; and (8) performing superfine comminution. Compared with the prior art, the method disclosed by the invention has the advantages that the activity of enzymes such as polyphenol oxidase in the siraitia grosvenorii is restrained by microwave fixation, and then sterilization and disinfection are performed with ultraviolet rays, so that the storage life of the freeze-dried superfine powder of the siraitia grosvenorii is effectively prolonged, and safe and hygienic use of the freeze-dried superfine powder is guaranteed; a vacuum freezing sublimation drying technology is adopted, so that separation of peel and flesh, caused by dryness of moisture is avoided, and besides, the color, the fragrance, the mouth feel, the nutrient components and the like of the siraitia grosvenorii are well maintained. After the superfine powder is prepared, the utilization rate of the siraitia grosvenorii is greatly increased, absorption is strengthened, the mouth feeling is improved, and the using range is broad.
Owner:陈素云
Lafutidine lyophilized powder injection and preparing method thereof
InactiveCN101199527BAvoid degradationPrevent precipitationOrganic active ingredientsPowder deliveryMANNITOL/SORBITOLCITRATE ESTER
Owner:JIANGSU AOSAIKANG PHARMA CO LTD
Freeze-drying process of recombinant protease K
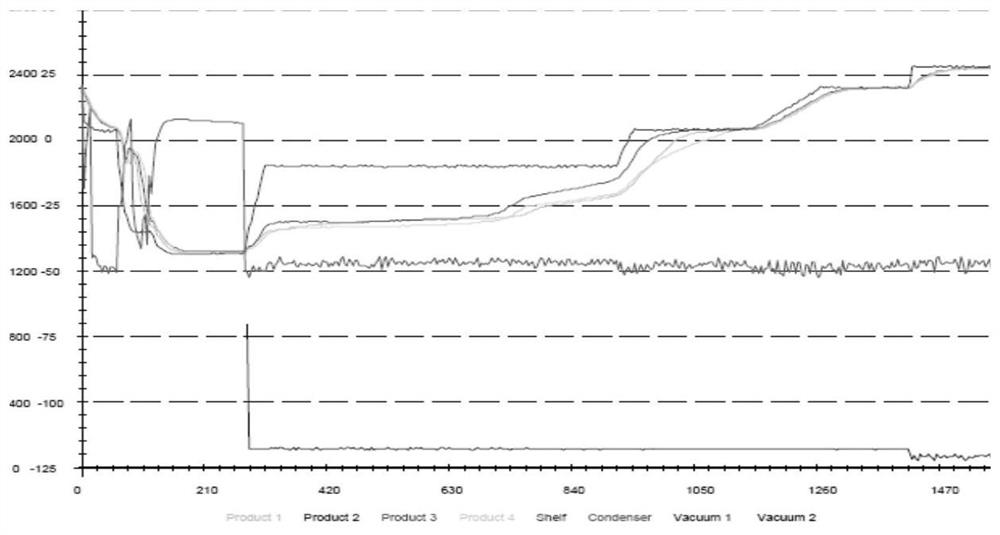
ActiveCN114277019AEasy to operateFreeze-drying time is shortHydrolasesEnzyme stabilisationBiochemical engineeringBiology
The invention provides a freeze-drying process of recombinant protease K. The freeze-drying process comprises the following steps: S1, purifying a protease k solution, and concentrating the protease k solution; s2, adding a protease freeze-drying preservation solution in equal proportion; s3, performing a freeze-drying procedure: firstly adding a protease solution for pre-freezing, and then performing freeze-drying in a vacuum state, wherein a freeze-drying temperature curve is as follows: keeping for 0.5-1.5 hours at-25 DEG C to-15 DEG C, keeping for 5-7 hours at-15 DEG C to-5 DEG C, keeping for 3-5 hours at-5 DEG C to 5 DEG C, keeping for 1-3 hours at 0-10 DEG C, keeping for 1-3 hours at 5-15 DEG C, and keeping for 7-9 hours at 10-20 DEG C; and then freeze-drying is finished. The freeze-drying process of the recombinant protease K provided by the invention is simple to operate, low in cost and short in freeze-drying time, and the product is easy to dissolve and long in shelf life.
Owner:合肥巅峰生物科技有限公司
Freeze-dried heat-resistant protective agent for porcine pseudorabies live vaccine, its preparation method and use
ActiveCN108938574BFreeze-drying time is shortLow costPowder deliveryViral antigen ingredientsCelluloseCold chain
The invention provides a porcine pseudorabies live vaccine freeze-drying heat-resisting protective agent, a preparation method and an application thereof. The protecting agent is characterized by being free of gelatin and proteins. The protecting agent contains the following components in percentage by weight: 2-8% of saccharose, 3-6% of isomaltulose, 1-5% of D-sorbitol, 1-5% of glycerinum, 0.1-5%of hydroxypropyl methylcellulose, 0.1-3.5% of methionine, 0.01-0.05% of poly-D-lysine, Hanks (containing phenol red) and the balance of double pure water, totaling 100%. The porcine pseudorabies livevaccine prepared by the freeze-drying heat-resisting protective agent is relatively good in heat resistance, the freeze-drying loss is 0.5-0.65Lg, the heat resistant loss at 37 DEG C for 10 days is 0.2-0.25Lg, and the storage life loss at 2-8 DEG C for 30 days is 0.7-0.9Lg. The low-temperature cold chain problem in storage and transportation of the vaccine is solved, and in particular the rural low-temperature cold chain problem in a remote region is solved.
Owner:JIANGSU ACAD OF AGRI SCI
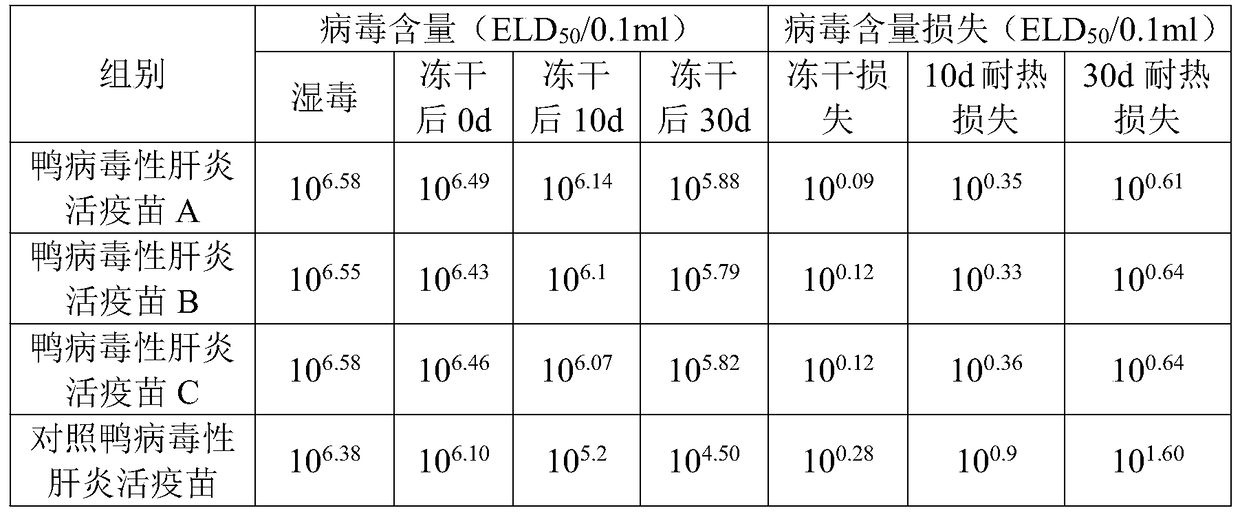
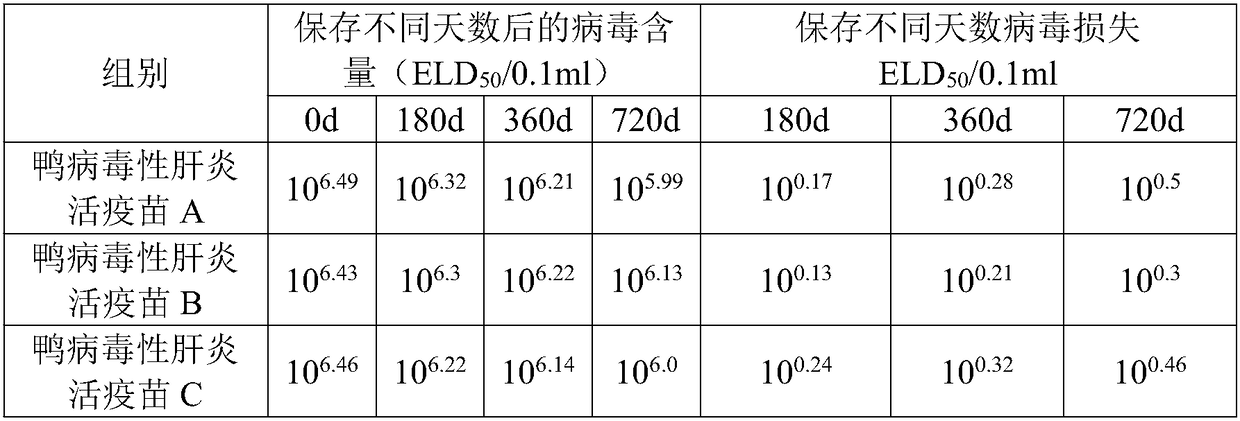
Heat-resistant freeze-drying protectant for duck viral hepatitis live vaccine and its preparation method and application
ActiveCN105233296BSolve the low temperature cold chain problemLow costPowder deliveryViral antigen ingredientsHigh temperature storageCold chain
The invention provides a heat-resisting freeze-drying protecting agent for duck viral hepatitis (DVH) live vaccine and a preparation method and application thereof, and belongs to the field of veterinary use biological product manufacturing. The heat-resisting freeze-drying protecting agent includes 70-100 g / L of saccharose, 1-10 g / L of Pullulan, 6-10 g / L of gelatin, 10-20 g / L of reduced glutathione, 10-20 g / L of dipotassium phosphate, 5-10 g / L of monopotassium phosphate, 10-30 g / L of bovine serum albumin (BSA) and 10-20 g / L of methionine. The invention further discloses the preparation method for protection of the heat-resisting freeze-drying protecting agent and the live vaccine including the heat-resisting freeze-drying protecting agent. The protecting agent can effectively protect antigenic activity in the freeze-drying and high-temperature storage processes and is safe to ducks, the low-temperature cold-chain problem of the vaccine in the storage and transportation processes is effectively solved, and the low-temperature cold-chain problem in remote area villages is solved particularly; meanwhile, the storage and transportation cost of the vaccine is lowered.
Owner:JIANGSU ACAD OF AGRI SCI
Chymotrypsin composition freeze-dried powder and preparation method thereof
ActiveCN102860988BFull and uniform appearanceGood resolubilityPowder deliverySenses disorderSolubilityPorosity
The invention relates to a chymotrypsin preparation, particularly relates to chymotrypsin composition freeze-dried powder and a preparation method thereof. The hymotrypsin composition freeze-dried powder comprises chymotrypsin, dextran 20 and sorbitol, wherein the ratio of chymotrypsin to dextran 20 to sorbitol is 1 million units: 1-1.8g: 0.05-0.6g. The average porosity of the hymotrypsin composition freeze-dried powder is 85-98%. The invention further relates to a preparation method of the hymotrypsin composition freeze-dried powder. The hymotrypsin composition freeze-dried powder disclosed herein has the advantages of full and uniform appearance, high porosity, good re-solubility, high stability, simple prescription, low proportion of pharmaceutic adjuvants, low side effect to human body, safety and reliability. According to the invention, the preparation technology of the hymotrypsin composition freeze-dried powder is further improved, the temperature, liquid volume, and freeze-drying process are improved, and sterilization is conducted twice.
Owner:HAINAN HERUI PHARMA
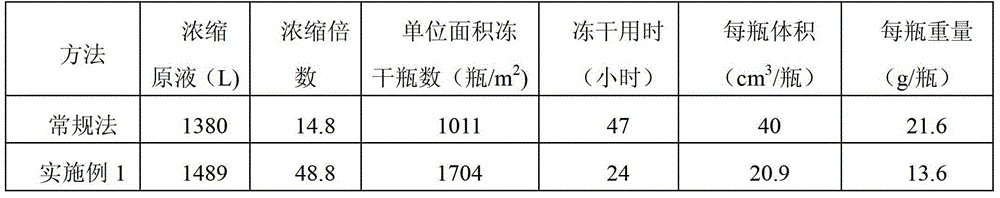
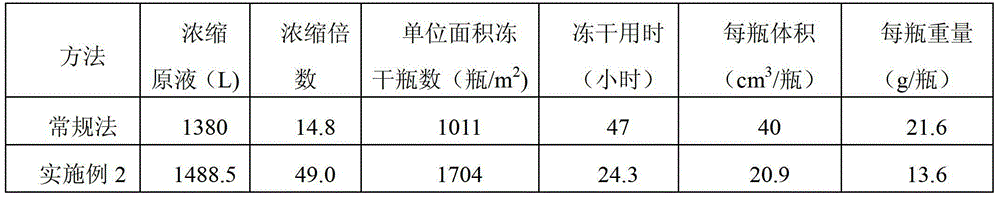
Method for preparing gemcitabine hydrochloride lyophilized powder
ActiveCN102793677BGood appearance structureQuality improvementOrganic active ingredientsPowder deliveryGemcitabine HydrochlorideLow vacuum
The invention relates to the field of pharmaceutic preparations and discloses a method for preparing gemcitabine hydrochloride lyophilized powder. According to the method for preparing the gemcitabine hydrochloride lyophilized powder, a primary pre-freezing process and a stage drying process are used for lyophilization; appropriate vacuum degrees and temperature are matched at all drying stages to avoid the problem that hydrochloric acid is removed from gemcitabine hydrochloride under high vacuum degree, and drying efficiency is low due to low vacuum degree; meanwhile, temperature is slowly raised at different speed at different drying stages, and the problem that the gemcitabine hydrochloride is deacidified due to too high heating speed can be solved. Compared with the prior art, the method for preparing the gemcitabine hydrochloride lyophilized powder has the advantages that the method is easy to operate, lyophilization time is short, and drying temperature is low; the prepared gemcitabine hydrochloride lyophilized powder product is high in outer structural quality and redissolution performance; a redissolution solution is clear and transparent, and opalescence is avoided; relative substances are low in content, and the prepared gemcitabine hydrochloride lyophilized powder is safe and reliable in quality; and the method is suitable for preparing the gemcitabine hydrochloridelyophilized powder and can be widely applied to large-scale production of the gemcitabine hydrochloride lyophilized powder.
Owner:CHONGQING LUMMY PHARMA
Preparation method of freeze-dried egg yolk antibody
ActiveCN103275218BImprove ultrafiltration concentration efficiencySmall footprintEgg immunoglobulinsImmunoglobulins against virusesYolkHollow fibre
The invention provides a preparation method of a freeze-dried egg yolk antibody. A refined egg yolk antibody solution used as a raw material is concentrated and freeze-dried to obtain the freeze-dried egg yolk antibody. The preparation method is characterized in that the technical process of the concentration comprises the following steps: (1) taking the refined egg yolk antibody solution, adding flocculant, standing, filtering, and sterilizing to obtain a primary filtrate; and (2) performing ultrafiltration and concentration on the primary filtrate obtained in the step (1) through a hollow fiber ultrafilter, thus obtaining a final concentrated solution. Compared with the prior art, the preparation method provided by the invention is high in concentration multiple, low in preparation cost, short in freeze-drying time, simple in process and suitable for large-scale antibody concentration.
Owner:重庆永健生物技术有限责任公司
Freezing-dried clofarabine powder injection and its preparation method
InactiveCN100591330CLow content of related substancesEasy to storeOrganic active ingredientsPowder deliveryMANNITOL/SORBITOLMedicine
The present invention relates to a Clofarabine freeze drying powder and the preparation method, which comprises Clofarabine and at least one biological acceptable excipient. Wight ratio of the Clofarabine and the excipient is from one to 10 till one to 50. The excipient is selected from one kind of mannitol and lactose. The optional choice is the mannitol. The preparation method is as following. The Clofarabine is melted with the injection water and the excipient is added. Followed by well mixing , filtration, packaging, adding stopper and loading in the plate. The freeze dryer is opened in advance. The platelayer is cooled with the heat conduction oil until temperature of the platelayer increases till minus 40 DEG Cto minus 50 DEG C. The Clofarabine solution packaged in the sterile silinbottle can be sent to the freeze dryer quickly.The box door is shut and the plate temperature is kept at minus 40 DEG Cto minus 50 DEG C and the box temperature is decreased. When the sample temperature reaches minus 40 DEG C, The plate cooling is stopped and the condenser is opened. At the same time the product temperature is maintaineed by cooling for 3 hours. When the temperature of the condenser reaches minus 40 DEG, the vacuum system is opened. After temperature holding, four steps of heating, sublimation, drying, plugging, box pressing and opening sealing can be started.
Owner:JIANGSU AOSAIKANG PHARMA CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com