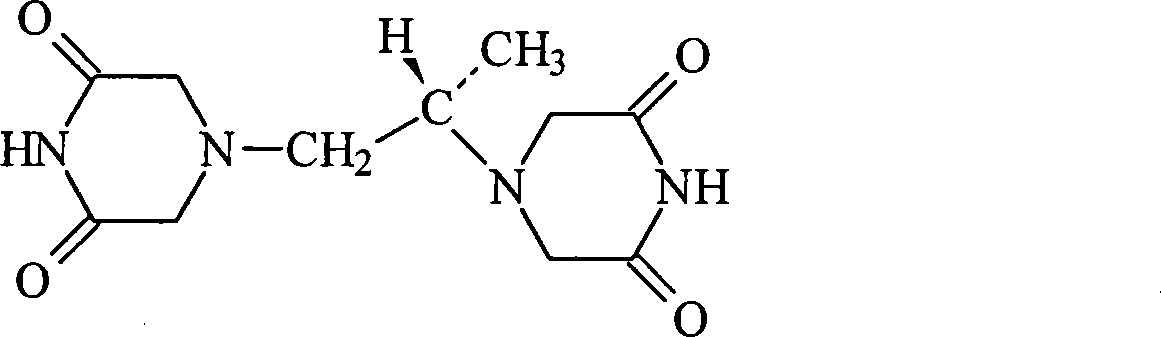
Dexrazoxane freezing-dried powder injection and preparation method thereof
A technique for freeze-dried powder injection and dextropropyl imine, which is applied in the field of dextropropyl imine freeze-dried powder injection and its preparation, achieving the effect of less incompatibility, excellent stability, and stable transportation and storage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] prescription
[0021] Dextropropyl imine 250g
[0022] Concentrated hydrochloric acid 37ml
[0023] Add water for injection to 10000ml
[0024]
[0025] A total of 1000 bottles were made
[0026] Take concentrated hydrochloric acid and put it in a sterile container, add water for injection to 80,000ml, cool down and keep warm at 4°C, add dextranimine, stir well, slowly add 1.0mol / L hydrochloric acid to dissolve under stirring, add water for injection to 10,000ml , add 30g activated carbon to adsorb for 30 minutes, decarbonize, after measuring the content of intermediates to pass, filter with 0.22μm microporous membrane, fill the filtrate in 25ml vials according to the filling capacity of 10ml per bottle, and partially plug the butadiene rubber stopper, Put it into a plate, put it into the freeze-drying box, insert the temperature probe, close the door, open the freeze-dryer in advance, use heat transfer oil to cool the plate layer, un...
Embodiment 2
[0028] prescription
[0029] Dextropropyl imine 250g
[0030] Concentrated hydrochloric acid 120ml
[0031] Add water for injection to 10000ml
[0032]
[0033] A total of 1000 bottles were made
[0034]Take concentrated hydrochloric acid and put it in a sterile container, add water for injection to 80,000ml, cool down and keep warm at 2°C, add dextropropyl imine, stir well, slowly add 1.0mol / L hydrochloric acid to dissolve under stirring, add water for injection to 10,000ml , add 30g activated carbon to adsorb for 30 minutes, decarbonize, after measuring the content of intermediates to pass, filter with 0.22μm microporous membrane, fill the filtrate in 25ml vials according to the filling capacity of 10ml per bottle, and partially plug the butadiene rubber stopper, Put it into a plate, put it into the freeze-drying box, insert the temperature probe, close the door, open the freeze-dryer in advance, use heat transfer oil to cool the plate lay...
Embodiment 3
[0036] prescription
[0037] Dextropropyl imine 250g
[0038] Concentrated hydrochloric acid 310ml
[0039] Add water for injection to 10000ml
[0040]
[0041] A total of 1000 bottles were made
[0042] Take concentrated hydrochloric acid and put it in a sterile container, add water for injection to 80,000ml, cool down and keep warm at 6°C, add dextropropylimine, stir well, slowly add 1.0mol / L hydrochloric acid until dissolved under stirring, add water for injection To 10000ml, add 30g activated carbon to absorb for 30 minutes, decarbonize, after the determination of intermediate content is qualified, filter with 0.22μm microporous membrane, fill the filtrate into 25ml vials with 10ml per bottle, and partially plug with butyl rubber Plug, put on a plate, send it into the freeze-drying box, insert the temperature probe, close the box door, open the freeze-dryer in advance, use heat transfer oil to cool the plate until the temperature of the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com