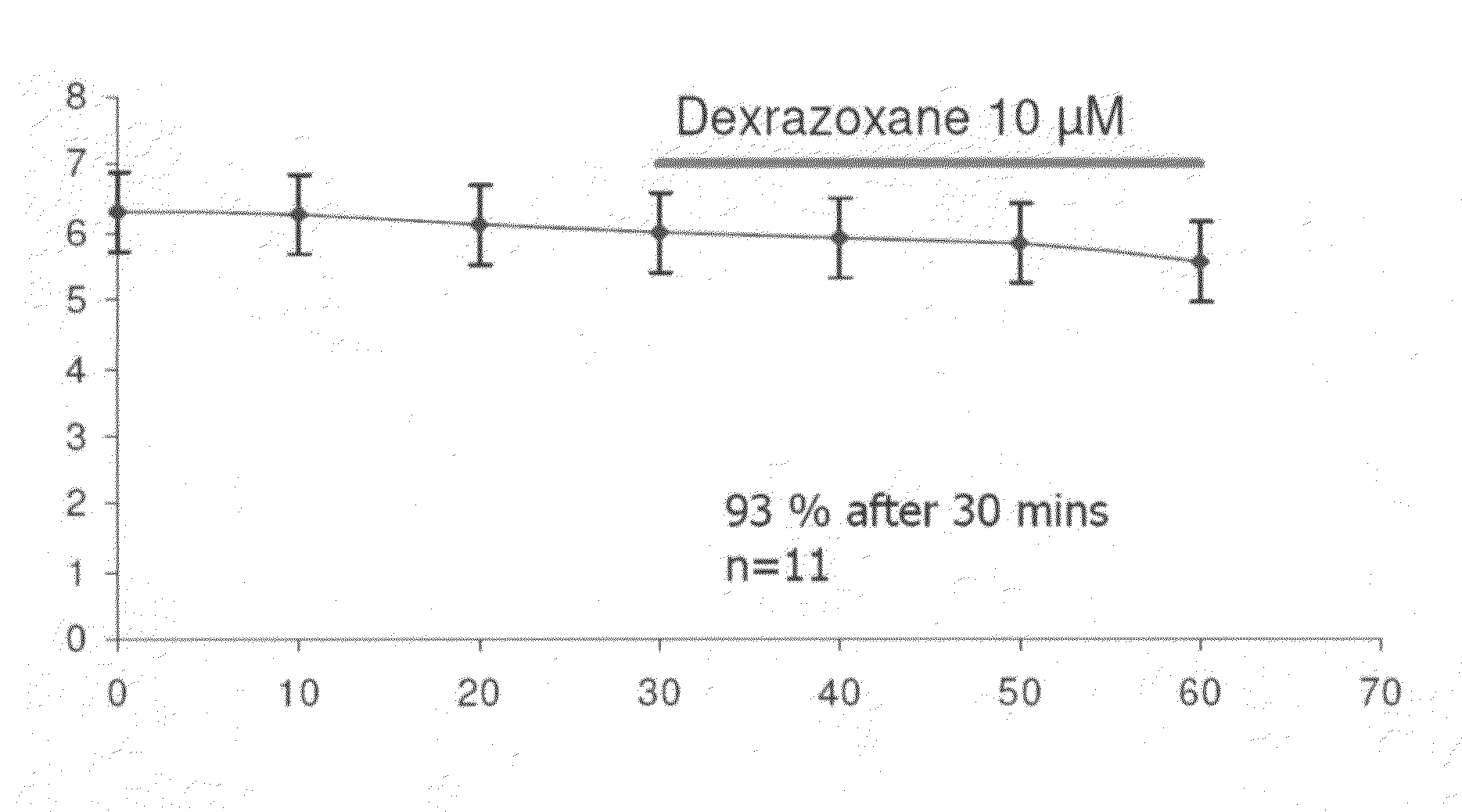
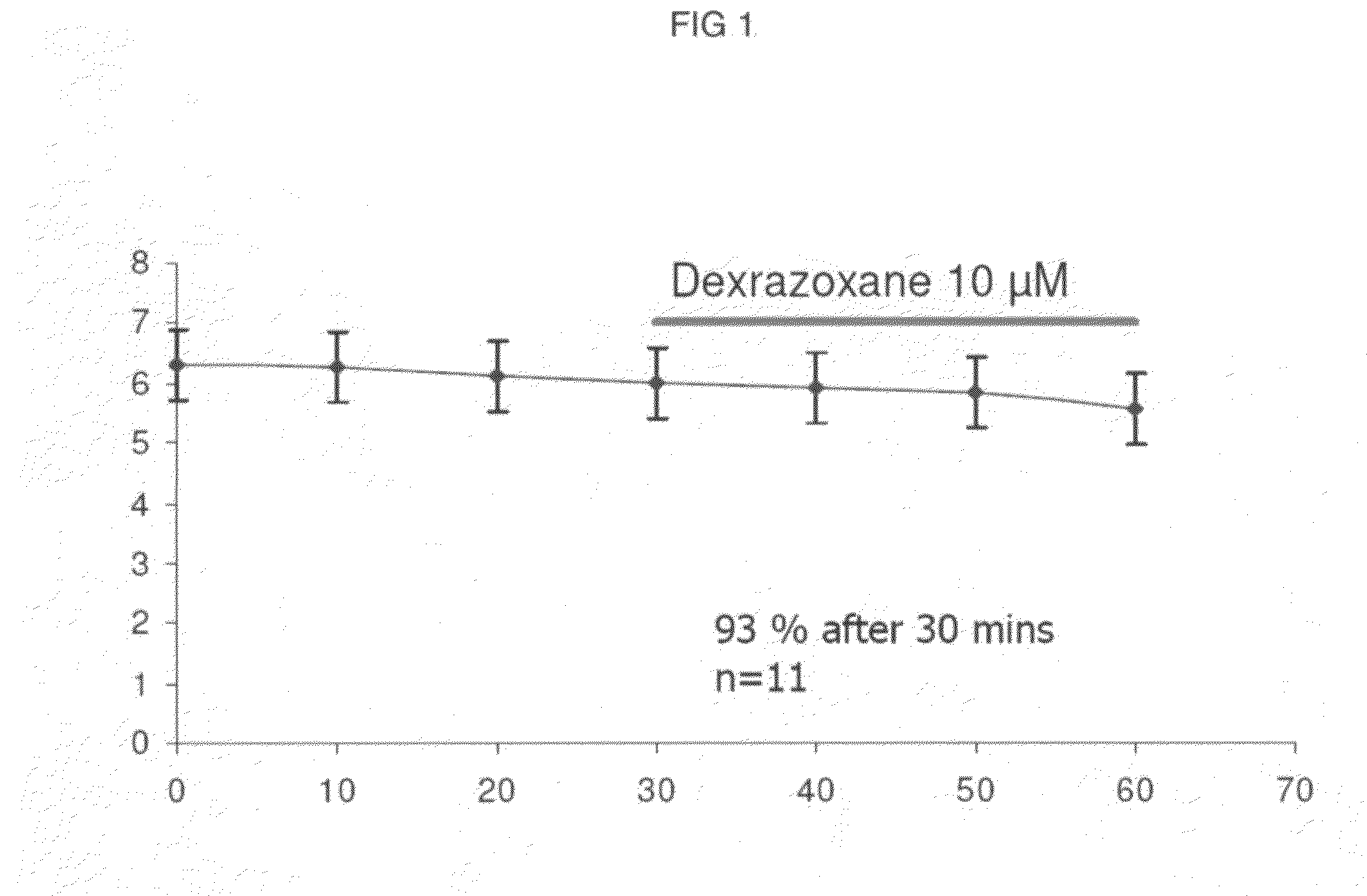
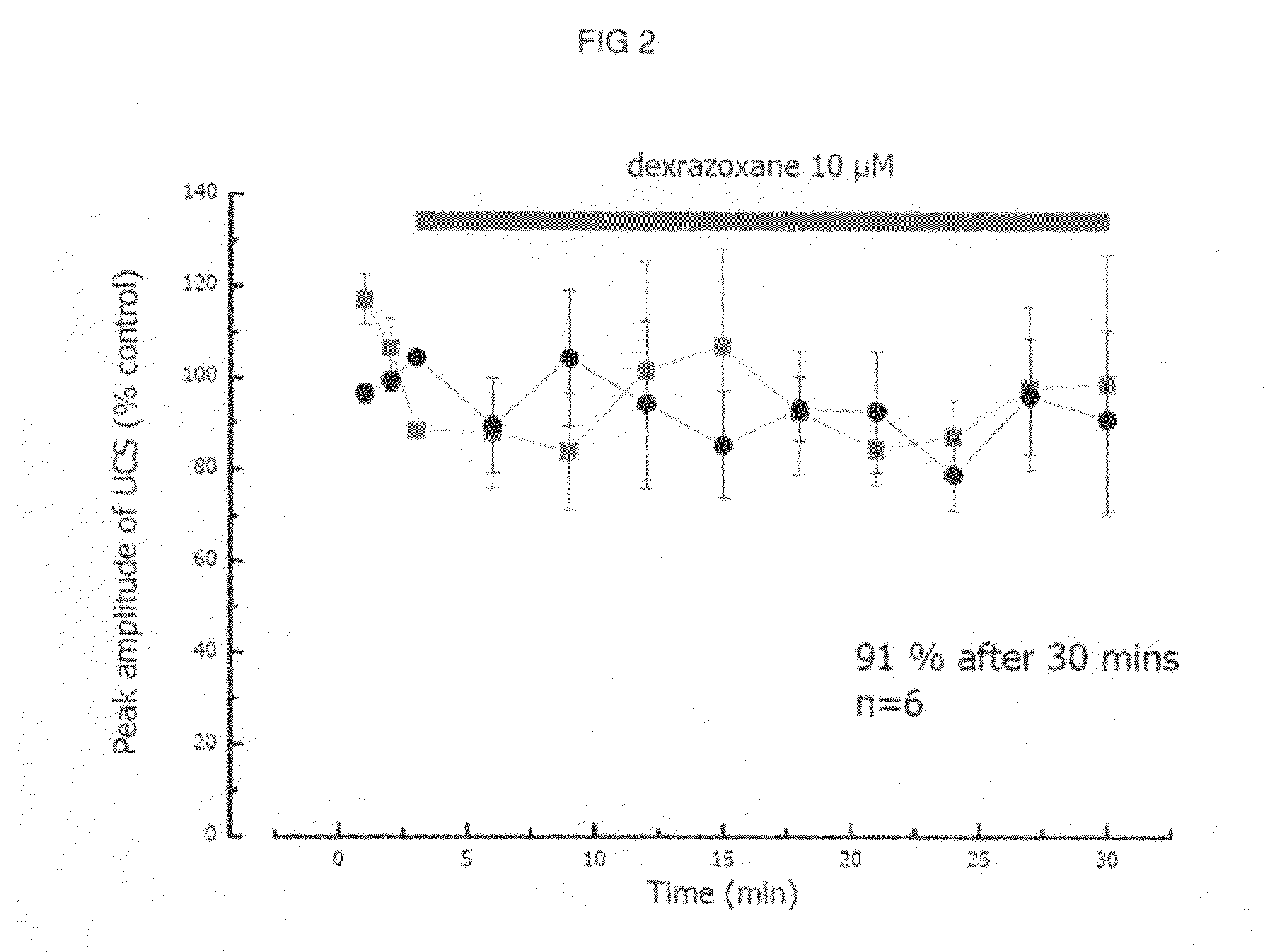
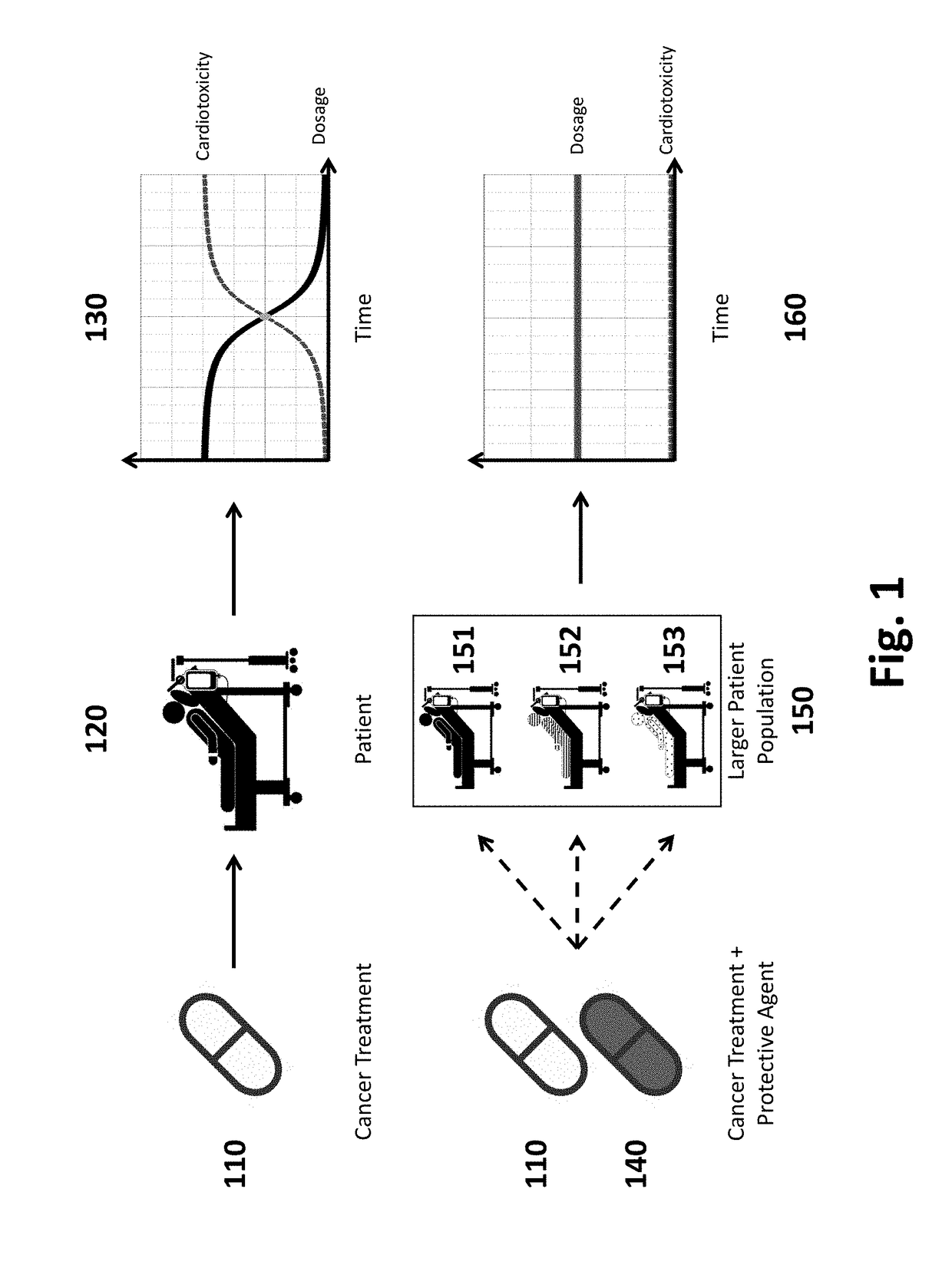
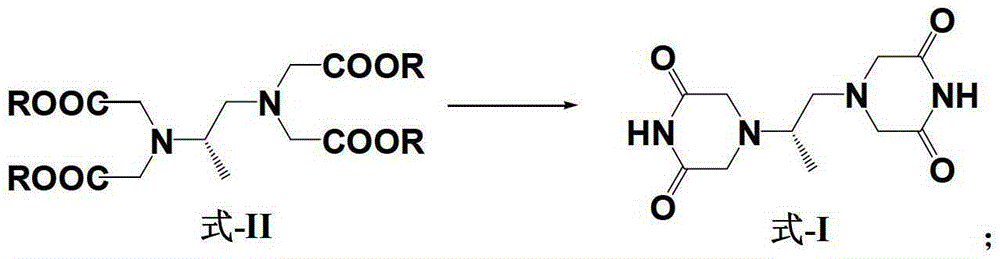
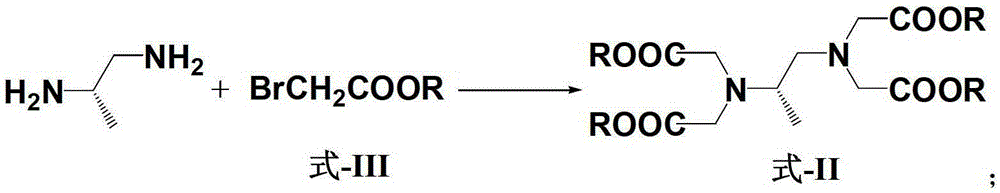
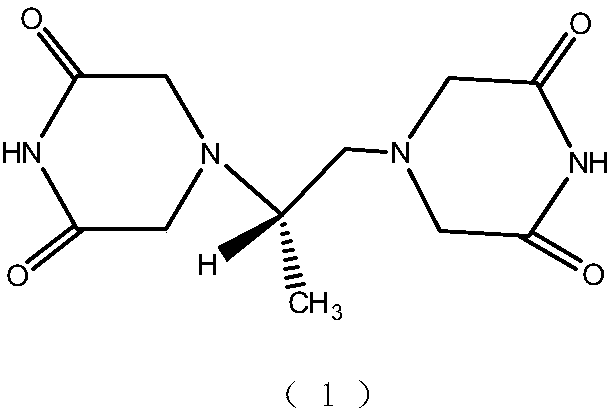
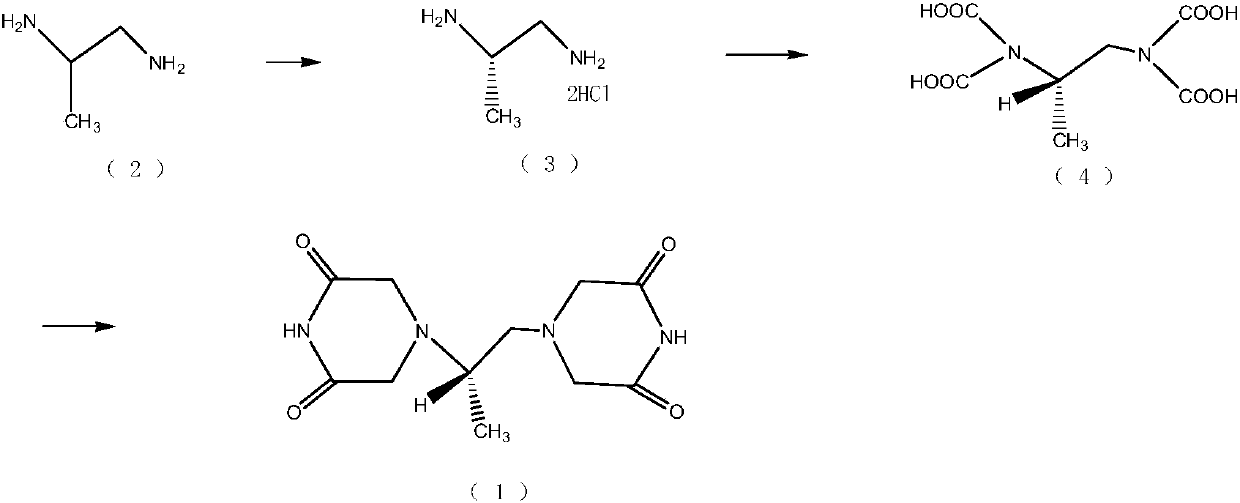
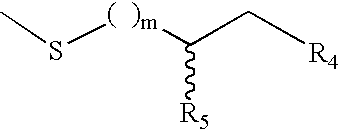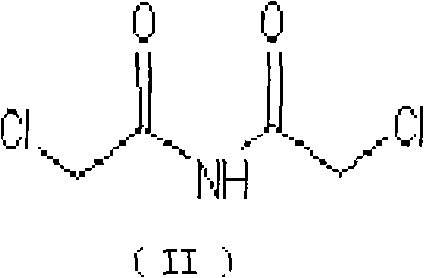Patents
Literature
36 results about "Dexrazoxane" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
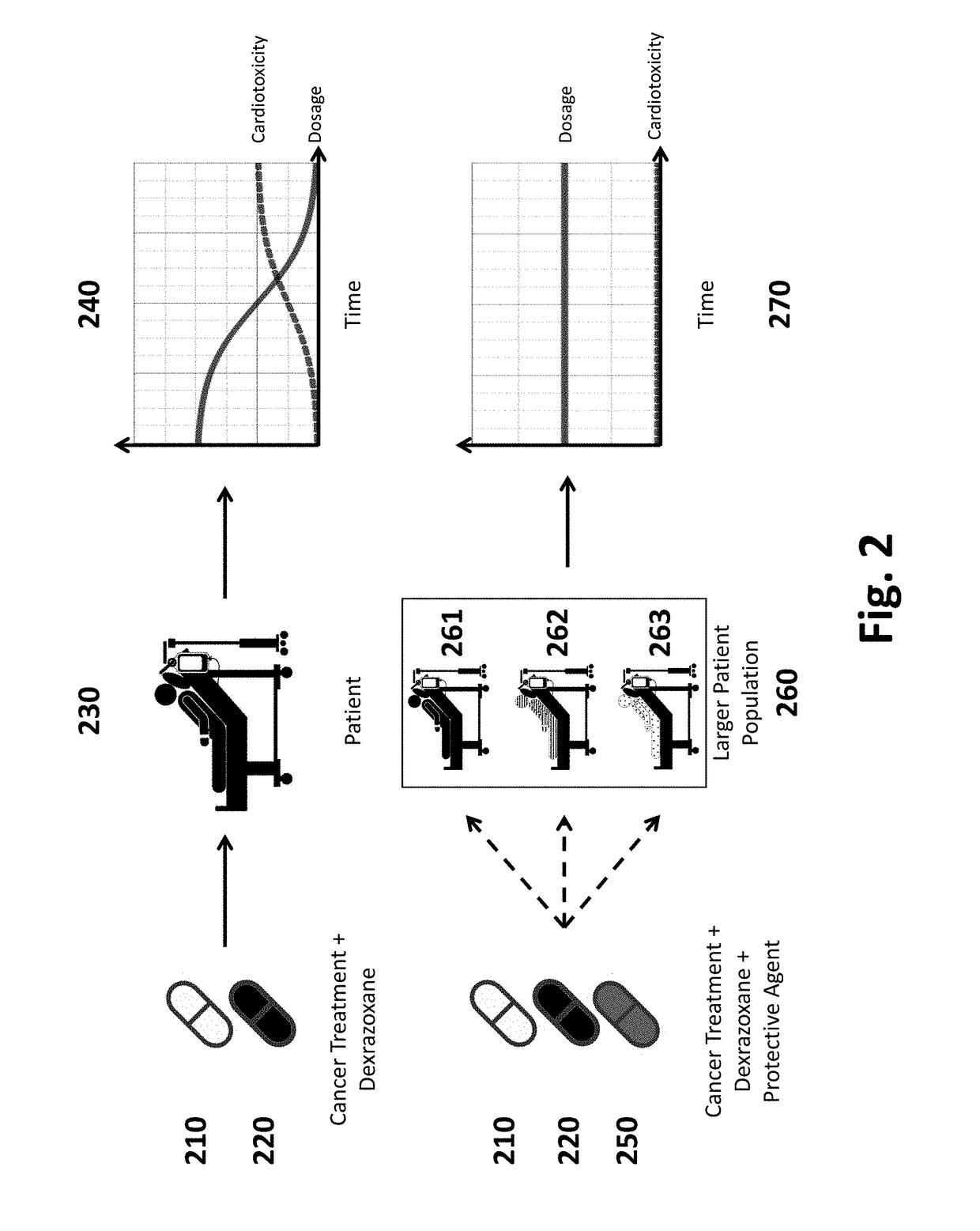
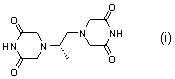
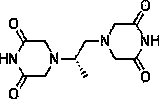
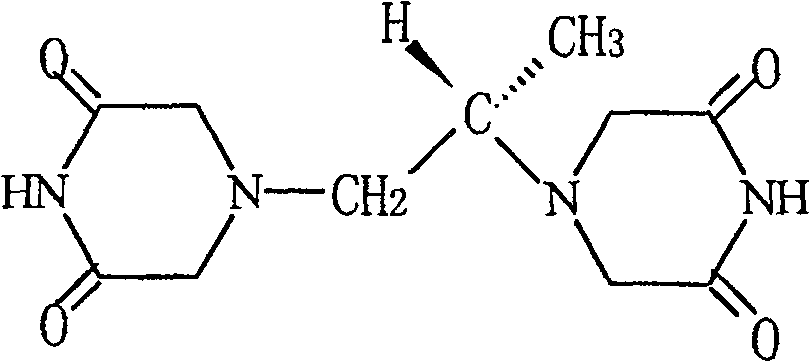
Dexrazoxane is used to reduce the risk and severity of heart damage caused by doxorubicin treatment and similar cancer chemotherapy medications.
Prevention and treatment of cardiac conditions
InactiveUS20080275036A1Efficient treatment methodMinimizing undesirable side effectOrganic active ingredientsBiocideHeart diseaseEndocrinology
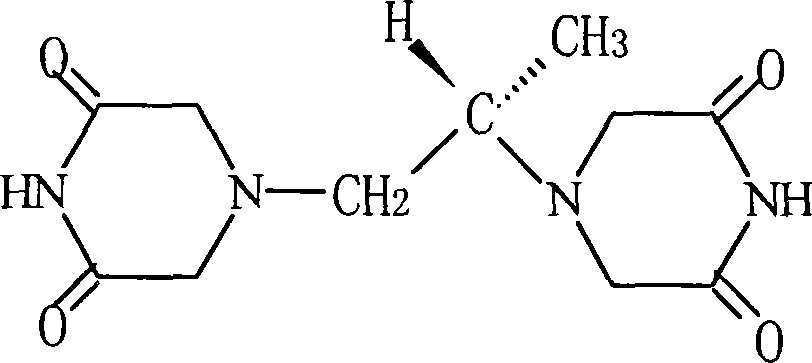
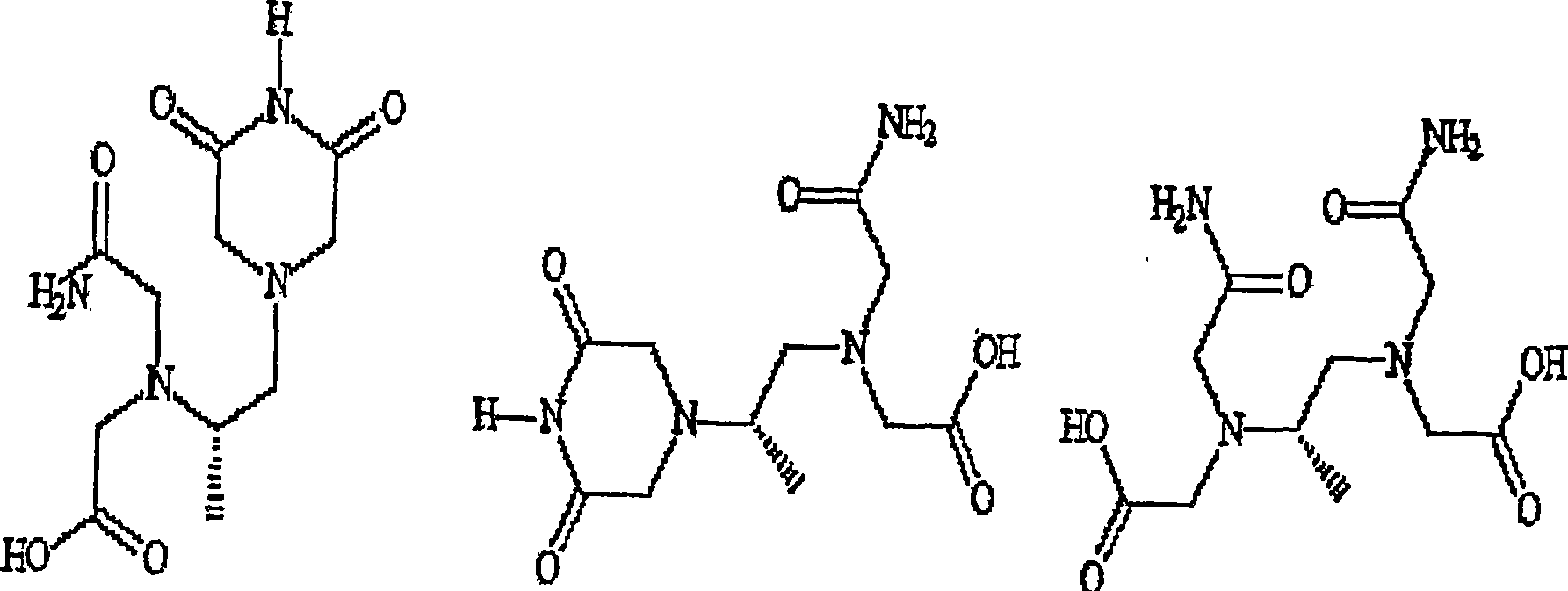
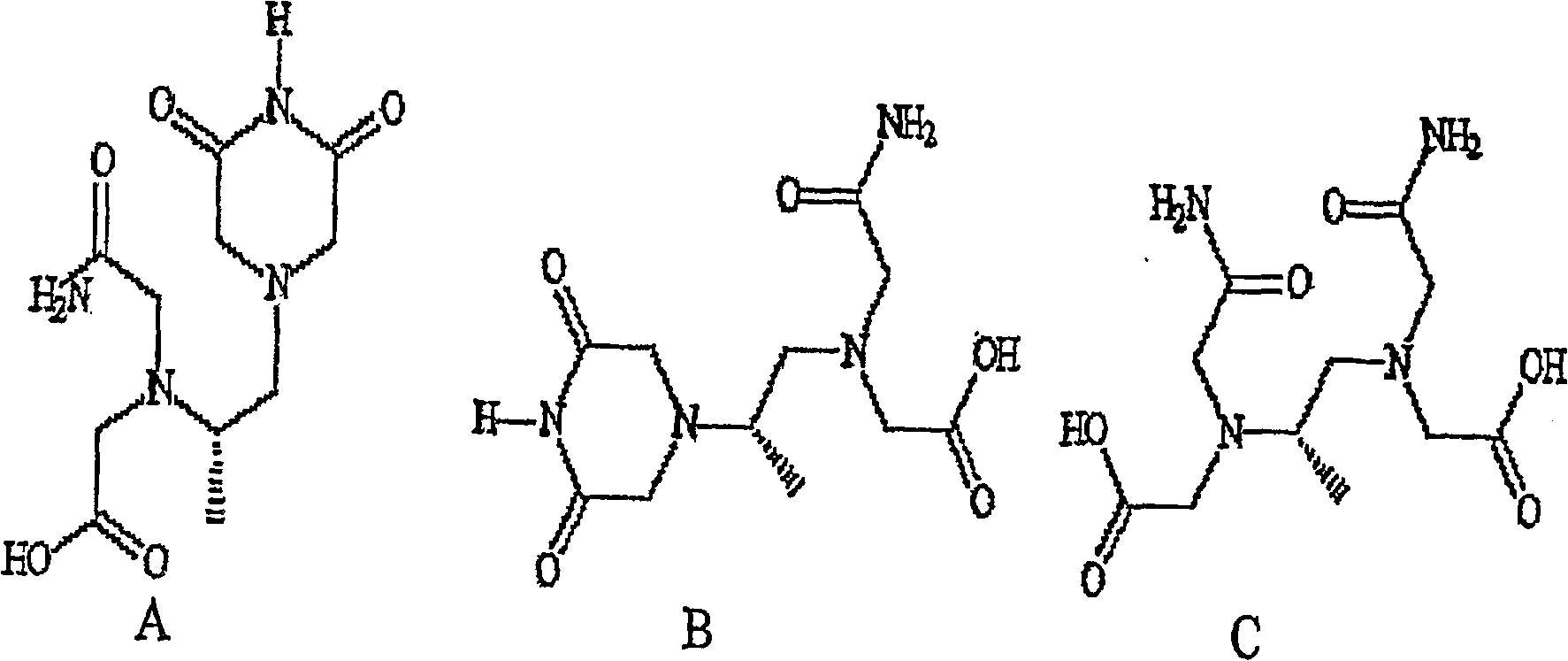
The present invention provides a method of treating conditions associated with iron and calcium overload comprising administering an effective amount of dexrazoxane or a non-dexrazoxane compound of formula (IA), (IB), or (IC) or a pharmaceutically acceptable salt, tautomer, or stereoisomer thereof.
Owner:APT PHARMA INC
Pharmaceutical compositions and methods for countering chemotherapy induced cardiotoxicity
InactiveUS20170224654A1Preventing heart failureImprove efficacyDipeptide ingredientsAntibody ingredientsDihydrouracilDihydrorobinetin
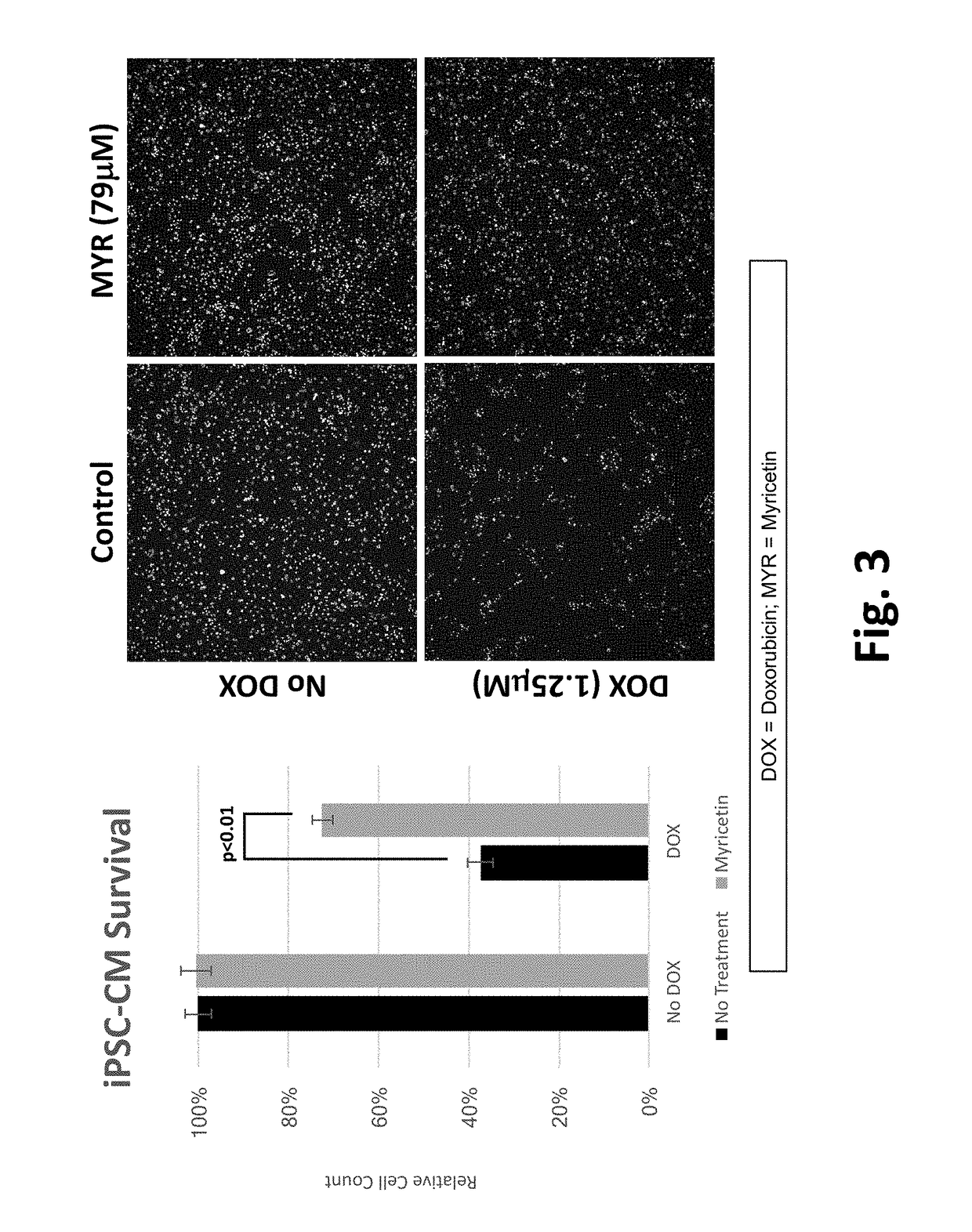
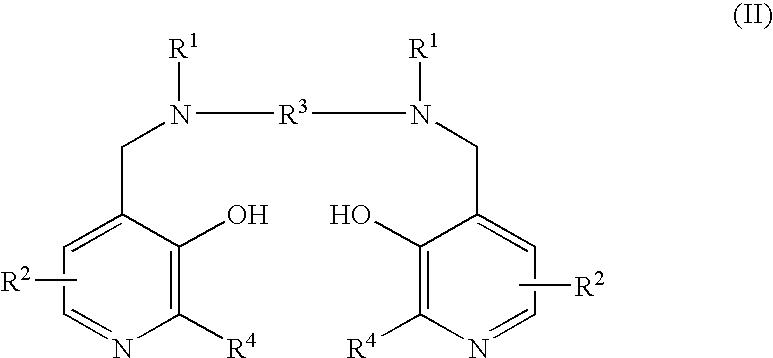
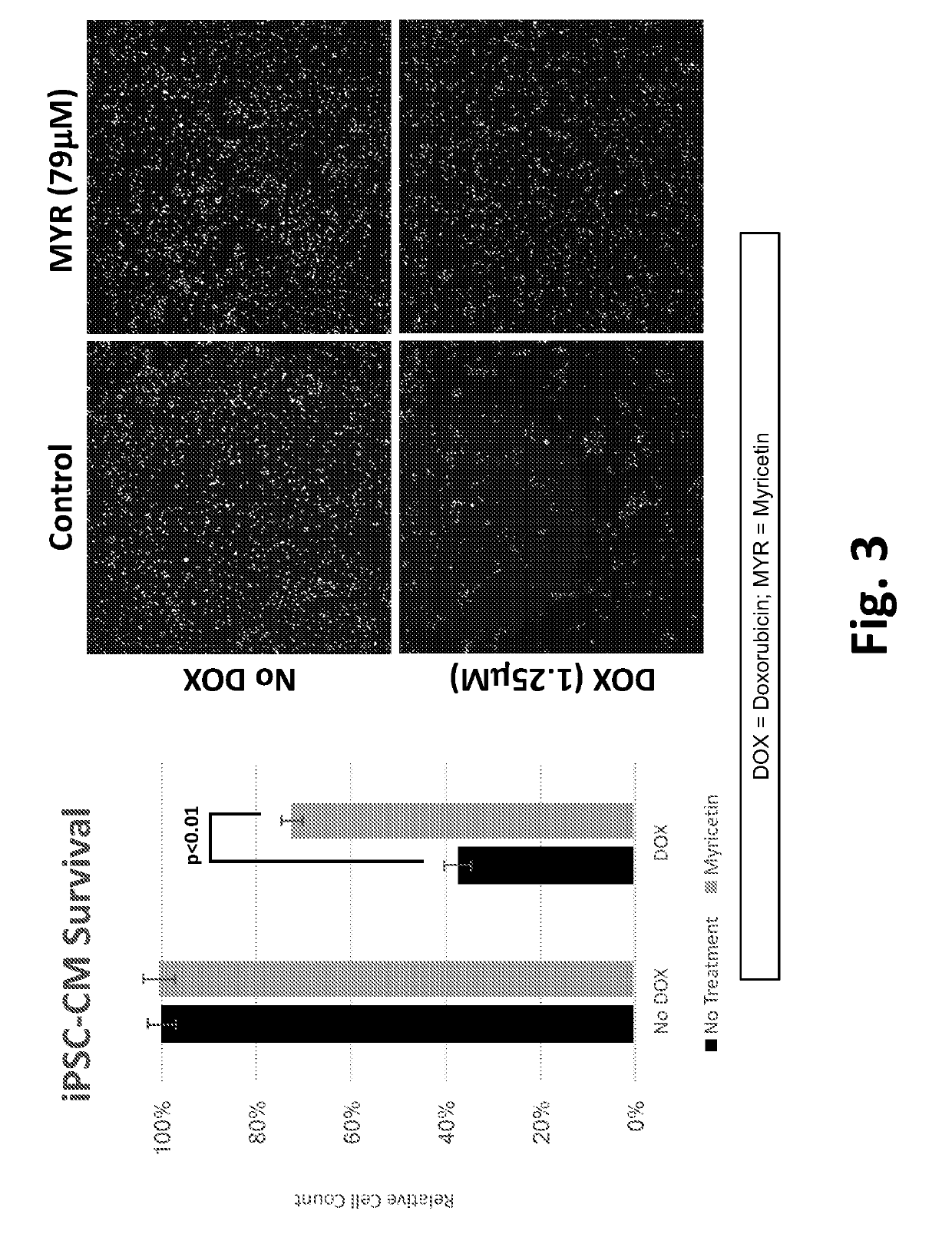
This disclosure provides methods and pharmaceutical compositions for reducing or eliminating cardiotoxicity, particularly cardiotoxicity induced by a cancer treatment or other therapy. In some cases, the methods and compositions prevent or reduce cardiotoxicity caused by anthracycline treatment. The methods provided herein often comprise administering a protective agent such as myricetin, tricetin, robinetin, ficetin, vitexin, quercetin, dihydrorobinetin, kaempferol, 7,3′,4′,5′-tetrahydroxyflavone, and myricitrin in conjunction with the administration of a cancer drug or other treatment. They may comprise administering a protective agent in combination with dexrazoxane. The compositions provided herein include co-formulations of a protective agent with a different protective agent or with a cancer treatment (e.g., anthracycline drug).
Owner:STEM CELL THERANOSTICS INC +1
Dexrazoxane analytical method
ActiveUS20200003737A1Effective and accurateIncrease the number ofOrganic chemistryComponent separationOrganic solventFluid phase
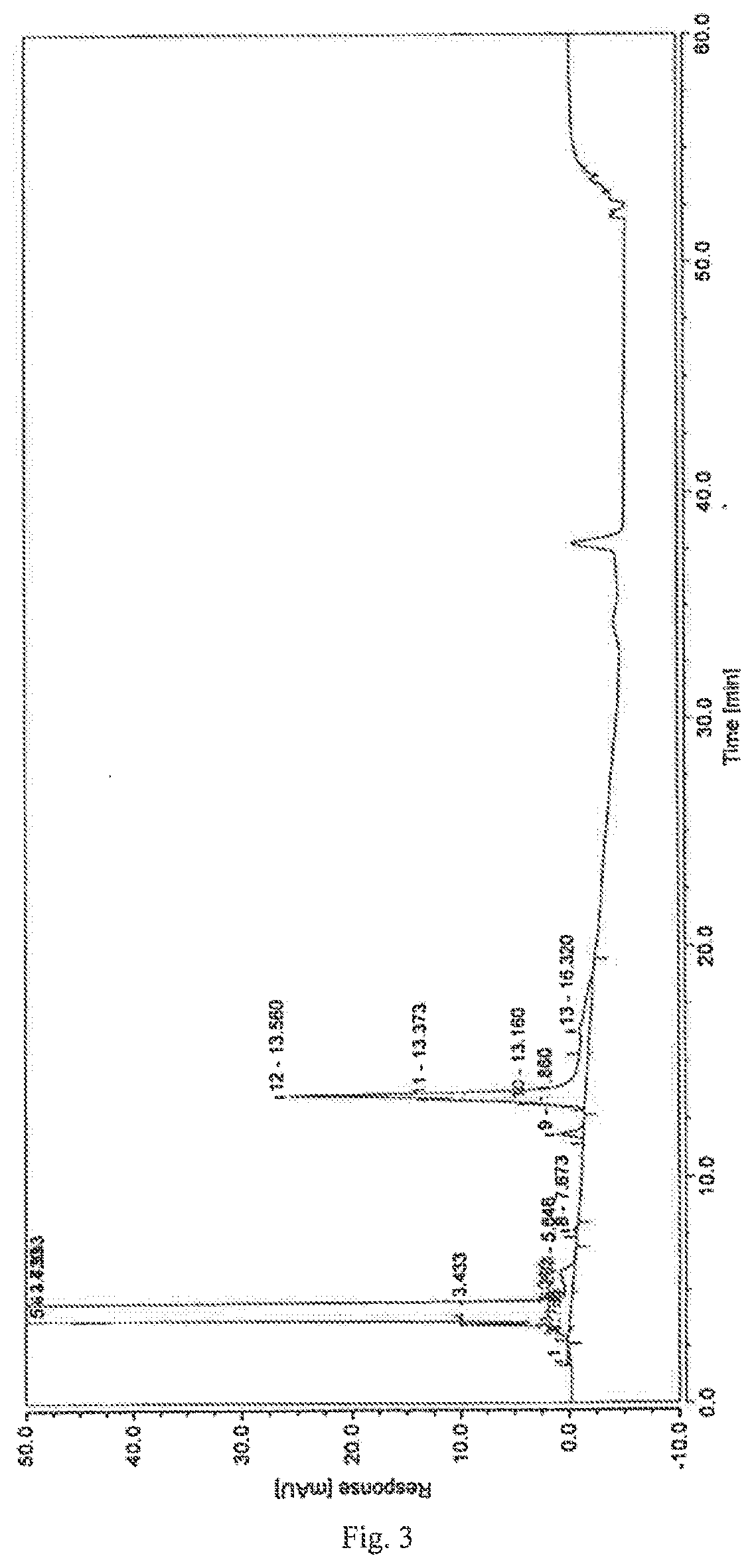
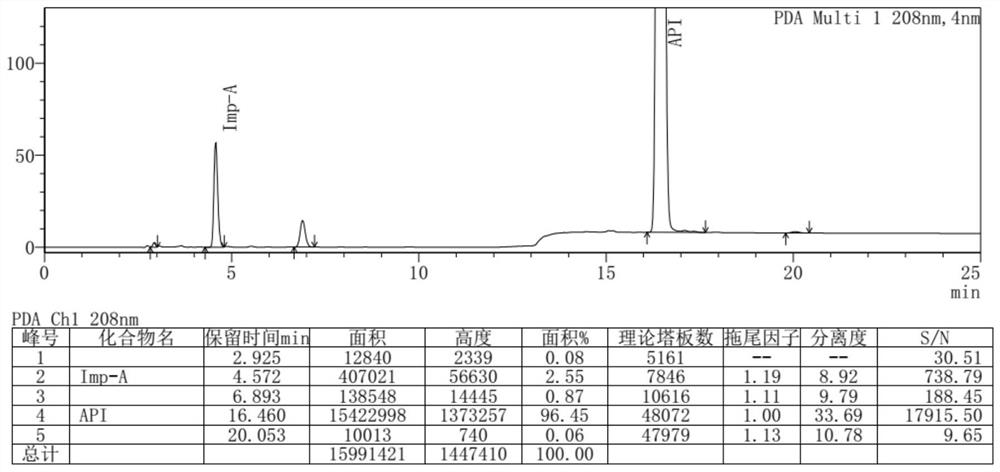
A high performance liquid chromatography method used for dexrazoxane-related substances is provided, and in the method, a low-density bonding reversed-phase C18 chromatographic column resistant to pure water is employed; a gradient elution is carried out with mobile phase A and mobile phase B as eluents, the mobile phase A being a buffer, and the mobile phase B being an organic solvent; the volume percent of mobile phase A in eluents in a first stage of the gradient elution is not lower than 90%, and the duration of the first stage of the gradient elution ranges from 15˜30 minutes. By means of the analytical method, dexrazoxane is effectively separated from main impurities, and the qualities of the active pharmaceutical ingredients of dexrazoxane and the preparations thereof could be better controlled.
Owner:JIANGSU AOSAIKANG PHARMA CO LTD
Dexrazoxane freezing-dried powder injection and preparation method thereof
InactiveCN101143134AFix stability issuesFreeze-dried wellOrganic active ingredientsPowder deliveryMicropore FilterFreeze-drying
The invention relates to a dexrazoxane freeze-dried powder injection and the preparation method. The preparation is used for resisting the cardiac toxicity which is induced by the cumulate quantity of adriamycin. The dexrazoxane freeze-dried powder injection contains the active components of the dexrazoxane and hydrochloric acid, the weight proportion of which is 1 to 0.05 to 0.5, and the preferential proportion is 1 to 0.2 to 0.5. The preparation method is that the hydrochloric acid is put into an aseptic vessel; the water for injecting is added till 80 percent of the preparation quantity, and the temperature is reduced and kept at 2 to 6 DEG C; the dexrazoxane is added to be mixed, and the hydrochloric acid of 1.0mol / L is dripped slowly into the solution to be solved while mixing the solution; the water for injecting is added till the full quantity; active carbon of 0.3 percent is added for absorbing for thirty minutes, and then the solution is decarbonized; after the medium body content is mensurated as being eligible, the solution is filtered by a 0.22 micron-micropore filtering filer; the filtrate is filled into a 25ml cillin bottle according to the filling quantity of 10ml each bottle, and the bottles are partially plugged by buna plugs and filled onto a plate to be sent into a freeze-drying box; a temperature probe is inserted, and the box door is closed; the filtrate is warmed, sublimed and dried be stages; nitrogen is puffed; the plugs are pressed; the filtrate is taken out from the box for rolling the openings, detecting the quality and packaging and the preparation can be obtained.
Owner:JIANGSU AOSAIKANG PHARMA CO LTD
Preparation method of dexrazoxane
ActiveCN102952088AImprove friendlinessShorten the production cycleOrganic chemistryPharmaceutical industryCombinatorial chemistry
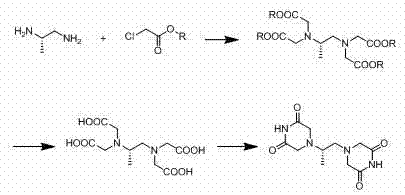
The invention relates to the field of medicament synthesis, in particular relates the synthetic field of anti-tumor medicaments, and more particularly relates to a preparation method of dexrazoxane. Aiming at the problems that the current synthetic route of dexrazoxane is complex, the cost is high, the post treatment steps are tedious and the product purity and product yield cannot be improved, the invention provides the preparation method of dexrazoxane. (S)-1,2-diaminopropane-tetraacetate can be obtained through only one step, thus not only is the production cycle shortened, but also the production cost is reduced. Besides, according to the technical scheme disclosed by the invention, reaction conditions such as high temperature, long cycle and high toxicity are avoided, so that the synthetic process is more environment-friendly, and is more suitable for being used in current pharmaceutical industry.
Owner:NANJING HAIRUN PHARM CO LTD
Dexrazoxane-containing composition and preparation method thereof, and dexrazoxane freeze-drying preparation and redissolving solvent thereof
InactiveCN103393609AFreeze-drying cycle is shortFast dissolutionOrganic active ingredientsPowder deliveryMedicinal chemistryPharmacology
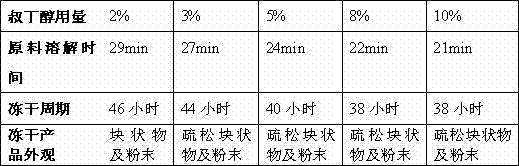
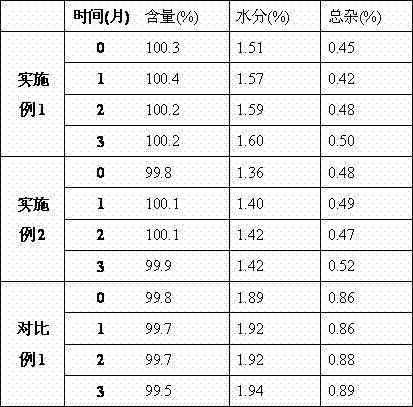
The invention relates to the field of medicine, in particular relates to the fields of preparation and application of dexrazoxane, and more particularly relates to a dexrazoxane-containing composition and a preparation method thereof, and a dexrazoxane freeze-drying preparation and a redissolving solvent thereof. The dexrazoxane-containing composition is a solution consisting of dexrazoxane, hydrochloric acid, water and tertiary butanol, wherein the dexrazoxane concentration of the composition is 20 to 30 mg / mL. The dexrazoxane freeze-drying preparation provided by the invention has excellent stability and the preparation method is easy to industrialize.
Owner:JIANGSU AOSAIKANG PHARMA CO LTD
Anti-cancer drugs slow release agent comprising anticancer antibiotics and booster thereof
Disclosed is an anticancer slow release agent which comprises slow release microspheres and dissolvent, wherein the slow release microballoons comprise anti-cancer active constituents and slow release auxiliary materials, the dissolvent being specific dissolvent containing suspension adjuvant. The anticancer antibiotics are selected from Idarubicin, Valtaxin, Pirarubicin and Mitoxantrone, The anti-metabolite drugs are selected from Pemetrexed, Carmustine, Tegafur, Zalcitabine, Emtritabine, Galocitabine, Ibacitabine, Ancitabine, Decitabine, Flurocitabine, Enocitabine, Imidazoletabine, Capecittabine, Gemcitabine, Fludrarbine, Raltitrexed, Dexrazoxane, Cladribine, Nolatrexed and folic acid, The slow release auxiliary materials are selected from EVAc, Polifeprosan, sebacylic acid copolymer, lactic acid, the viscosity of the suspension adjuvant is 100-3000cp (at 25-30 deg C), and is selected from sodium carboxymethylcellulose. The slow release microspheres can also be prepared into slow release implanting agent for injection or placement in or around tumor.
Owner:SHANDONG LANJIN PHARMA
Preparation method of high-purity dexrazoxane
ActiveCN102675227AIncrease the degree of steamingGood dispersionOrganic chemistryPressure reductionSodium salt
The invention relates to a preparation method of high-purity dexrazoxane, which comprises the following steps of: (1) cyclization reaction: carrying out cyclization reaction between (S)-1, 2-propane diamine-N, N, N', N'-tetracetic acid or disodium salt of (S)-1, 2-propane diamine-N, N, N', N'-tetracetic acid and formamide, wherein a high boiling point solvent is used; (2) preparation of salt-containing crude product: evaporating the mixture, which is obtained after reaction, for removing the formamide by pressure reduction and concentration, adding organic solvent into the mixture, and filtering to obtain solid; (3) preparation of crude product: adding dioxane into the salt-containing crude product, heating for backflow, filtering, concentrating the filtrate, adding organic solvent into the filtrate to obtain the crude product of dexrazoxane; and (4) refining: adding the crude product of dexrazoxane into N, N'-dimethyl formamide, heating for dissolving, dropwise adding the solvent, carrying out crystallization, filtering to obtain solid, washing the obtained solid with the solvent, drying to finally obtain the high-purity dexrazoxane. The method for synthesizing dexrazoxane is stable in yield and easy in condition control; the product purity is higher than 99.5% and residual organic solvent is little, and the synthesis cost can be reduced.
Owner:JIANGSU AOSAIKANG PHARMA CO LTD
Freezing-dried composite contg. dexrazoxane and its prepn. method
InactiveCN1537535ADecrease increaseOxidative stabilityOrganic active ingredientsPowder deliveryFreeze-dryingHigh heat
A freeze dried composition containing dexrazoxane is disclosed. Its preparing process is also disclosed, which features use of antioxidizing agent to make its product have high antioxidizing stability.
Owner:SHANGHAI INST OF PHARMA IND CO LTD +1
Method of treating multiple sclerosis
InactiveUS20040038904A1Toxic effect is reduced and minimizedConvenient for patientBiocideNervous disorderPharmaceutical drugProtective Agents
The present invention provides for use of an anthracycline, such as doxorubicin, alone or in combination with a protective agent, such as dexrazoxane, for treating multiple sclerosis.
Owner:PHARMACIA & UPJOHN CO
Pharmaceutical Compositions and Methods for Countering Chemotherapy Induced Cardiotoxicity
ActiveUS20190175544A1Avoid failureMinimizing risk of failureDipeptide ingredientsAntibody ingredientsCancer drugsVitexin
This disclosure provides methods and pharmaceutical compositions for reducing or eliminating cardiotoxicity, particularly cardiotoxicity induced by a cancer treatment or other therapy. In some cases, the methods and compositions prevent or reduce cardiotoxicity caused by anthracycline treatment. The methods provided herein often comprise administering a protective agent such as myricetin, tricetin, robinetin, ficetin, vitexin, quercetin, dihydrorobinetin, kaempferol, 7,3′,4′,5′-tetrahydroxyflavone, and myricitrin in conjunction with the administration of a cancer drug or other treatment. They may comprise administering a protective agent in combination with dexrazoxane. The compositions provided herein include co-formulations of a protective agent with a different protective agent or with a cancer treatment (e.g., anthracycline drug).
Owner:SCT II LLC +1
Preparation method of dexrazoxane and pharmaceutical salts thereof
ActiveCN101684100AEasy to routeLow costOrganic chemistryAntineoplastic agentsChemical synthesisAcetonitrile
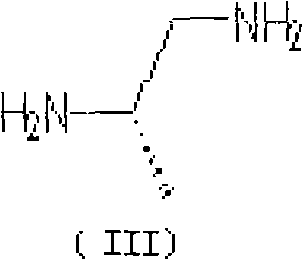
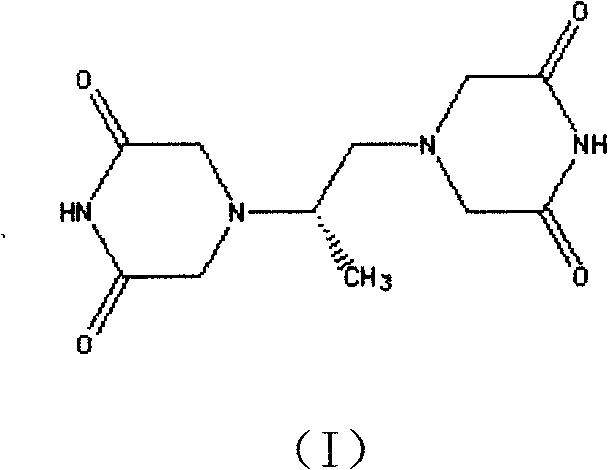
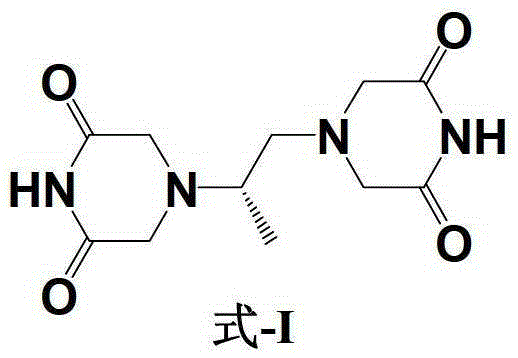
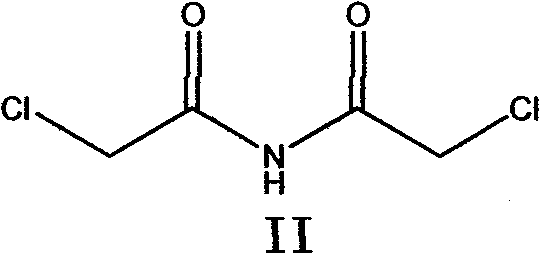
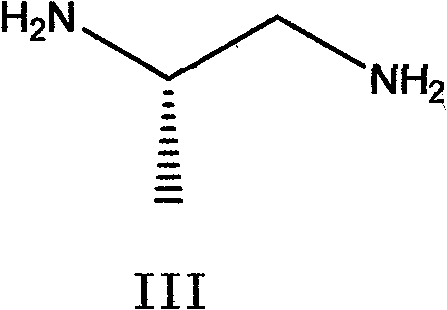
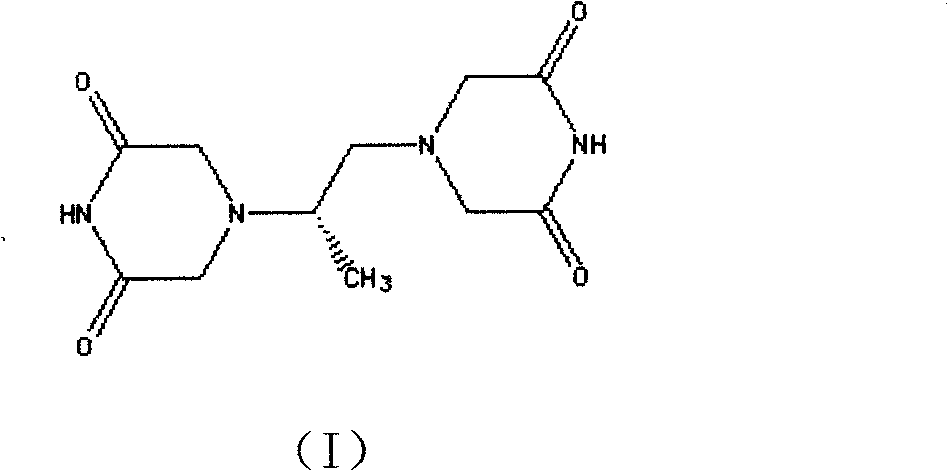
The invention belongs to chemical synthesis pharmacy technology field, the invention provides a preparation method of medicine dexrazoxane which has a function of antitumor, the process includes: N,N-dichloroacetyl imine (II) is prepared by chloroacetonitrile in the presence of acid, then the (+)- dexrazoxane (I) is obtained by condensation of the N,N-dichloroacetyl imine (II) and the (+)-1,2-propanediamine (III), the obtained dexrazoxane is made into pharmaceutically acceptable salt. The method according to the invention has advantages of simple route, convenient operation, easy industrialization and short period, and the obtained product has high purity. Route of the process is shown in the upper right.
Owner:CHONGQING HAITENG PHARMA
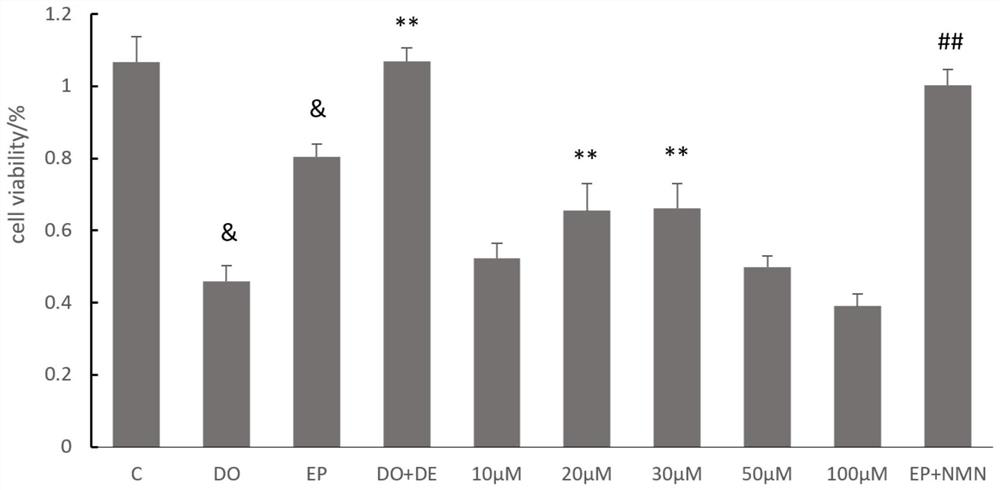
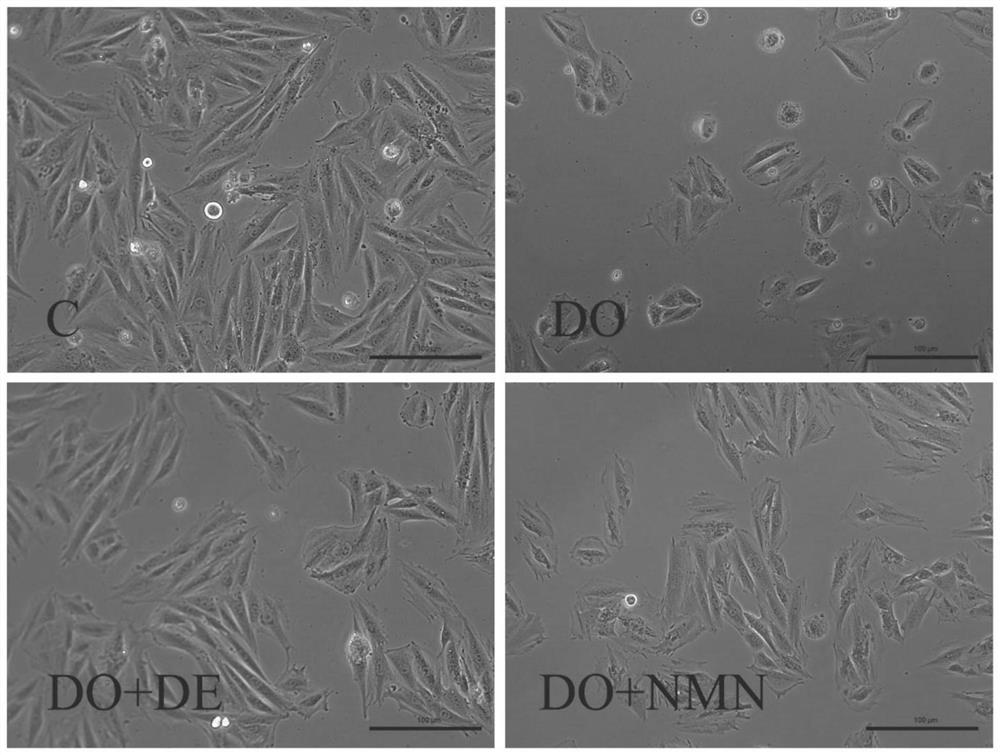
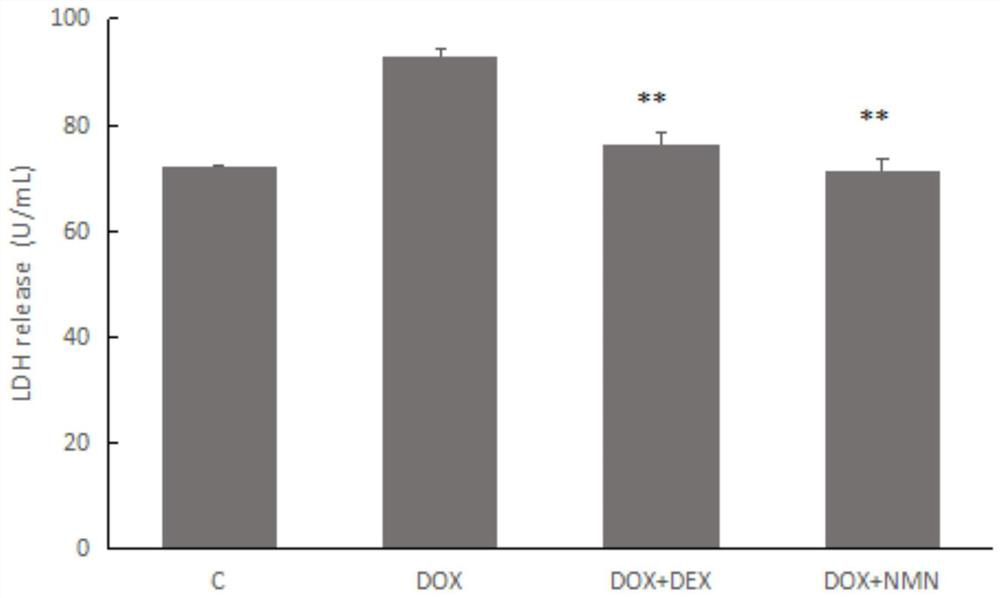
Nicotinamide mononucleotide and protective application thereof in myocardial injury of antitumor drug
PendingCN113143946ADiscover new value applicationsLow costOrganic active ingredientsAntineoplastic agentsSide effectHistone deacetylase
The invention belongs to the technical field of drugs for preventing and treating cardiac toxicity of chemotherapeutic drugs, and particularly relates to nicotinamide mononucleotide and protective application thereof in myocardial injury of an antitumor drug. The nicotinamide mononucleotide is converted into NAD <+> in a human body to play a physiological function, such as activating NAD <+> substrate dependent enzyme Sirtuins (histone deacetylase, also known as silencing regulatory protein), regulating cell survival and death, and maintaining an oxidation-reduction state. NMN is used as a substitute of Dex, and has a protective effect on myocardial injury caused by anthracycline chemotherapeutic drugs such as Dox, and the side effect of Dox in treating tumors is relieved.
Owner:TSINGHUA UNIV +2
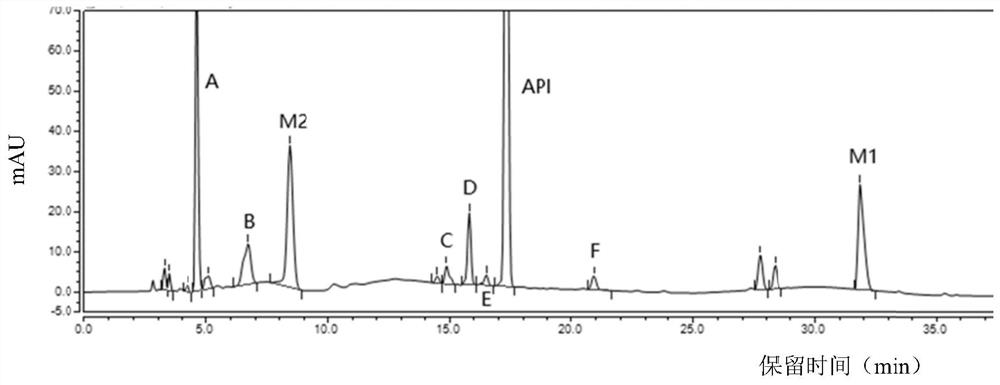
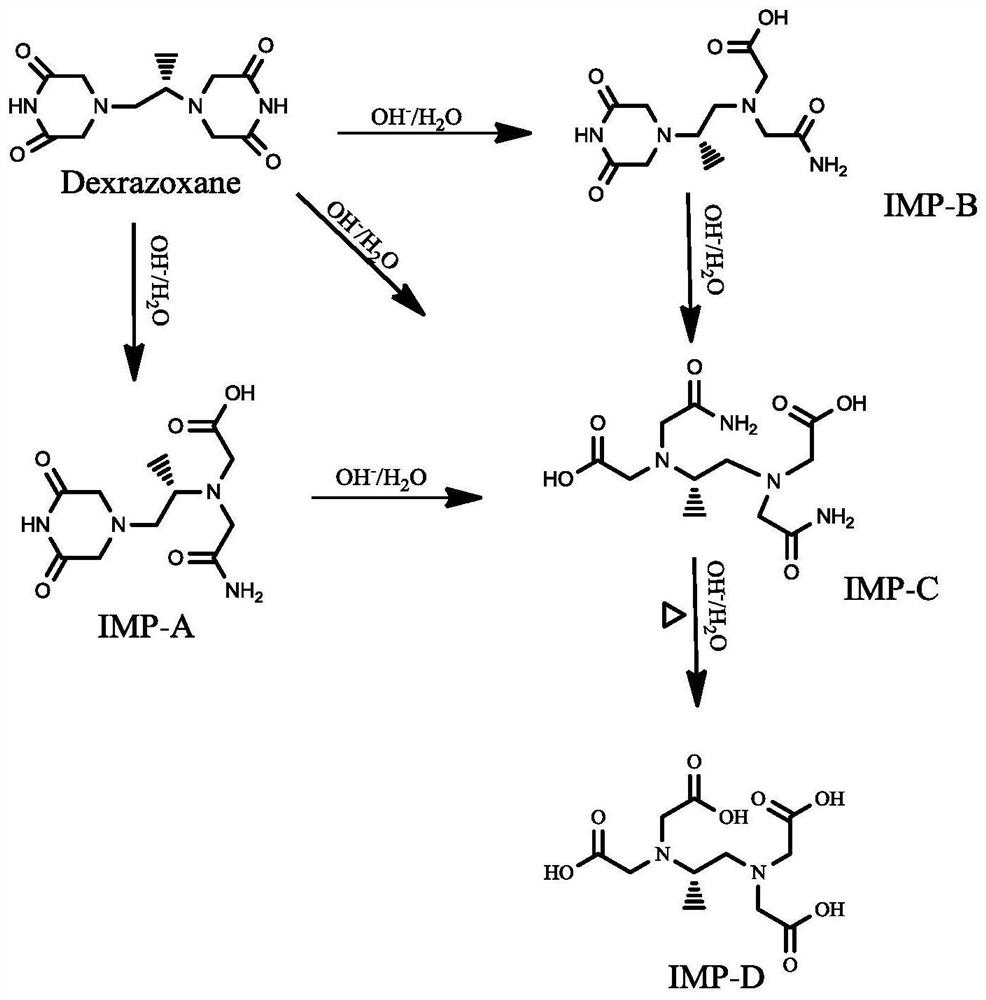
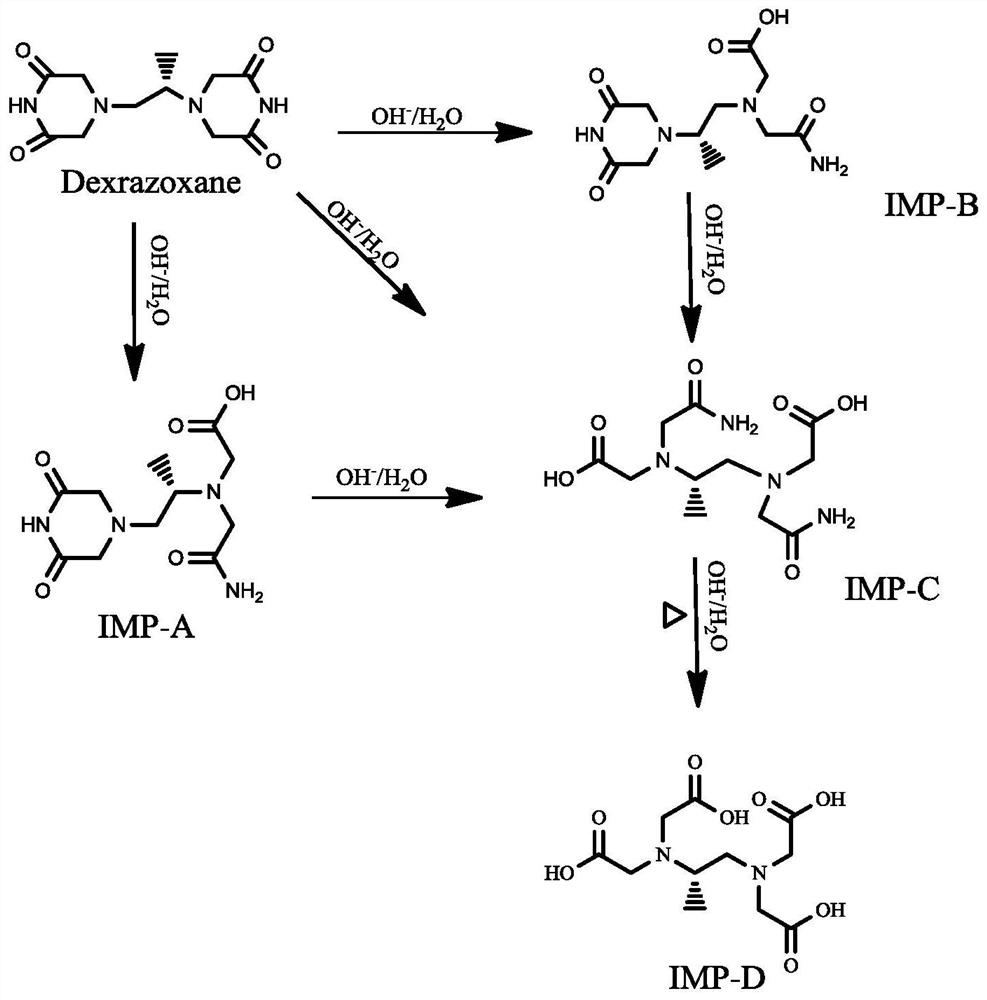
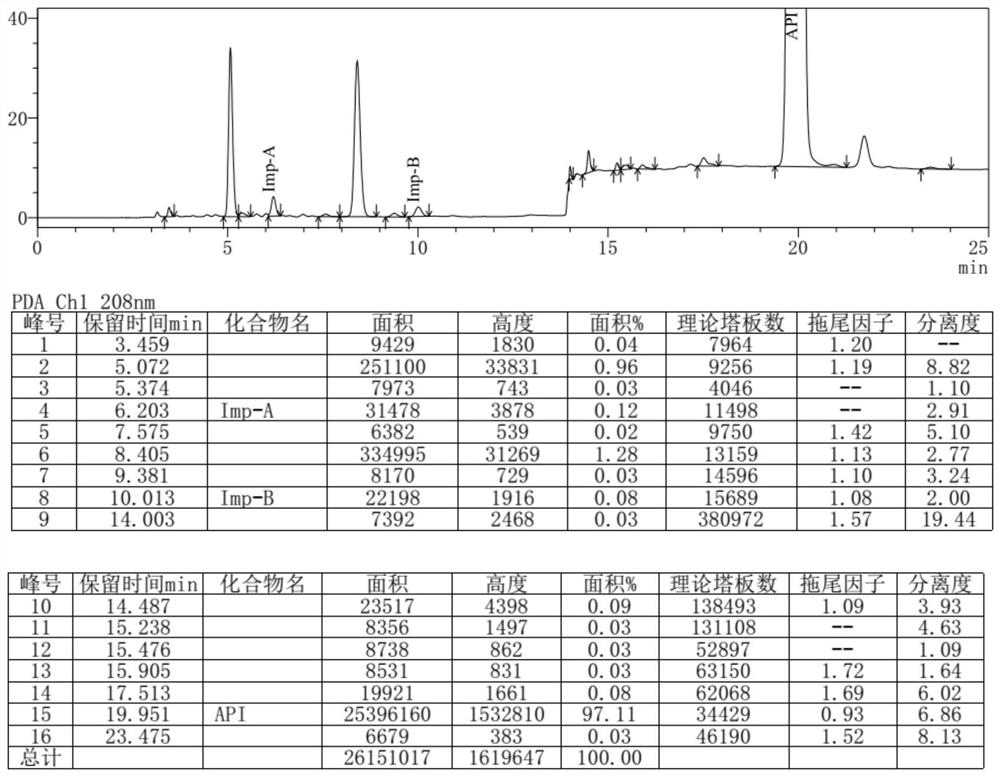
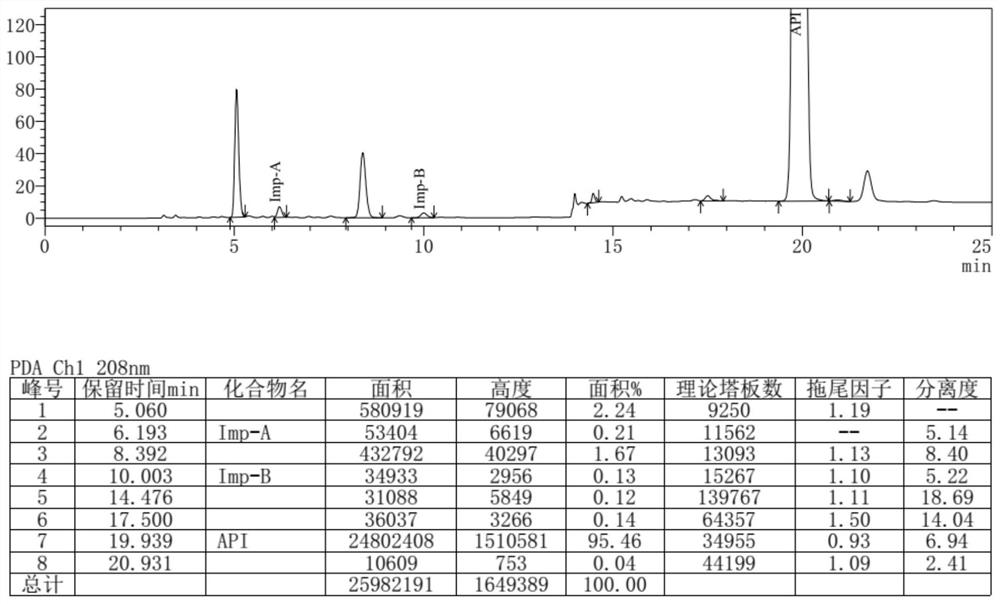
Separation and detection method of dexrazodone intermediate and impurities
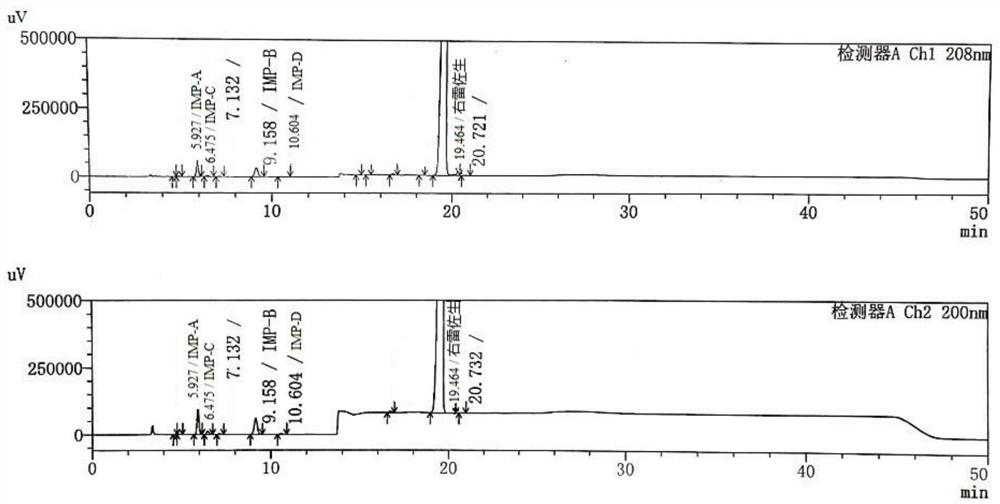
ActiveCN113390971ASimple separation and detectionFast separation and detectionComponent separationPhysical chemistryDiluent
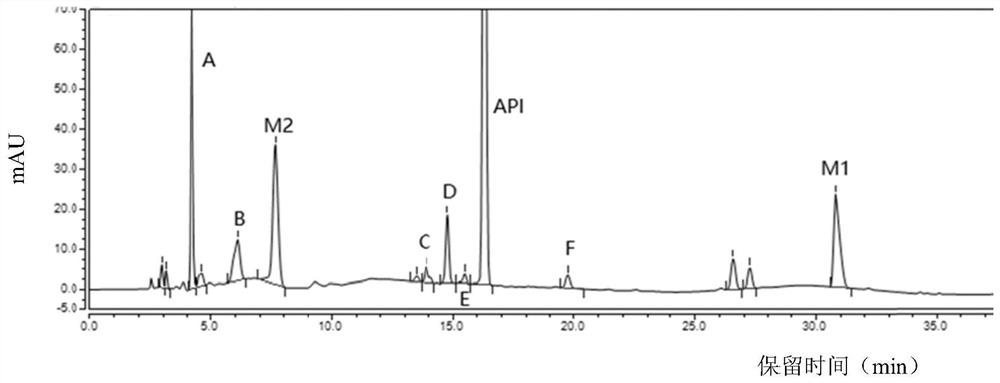
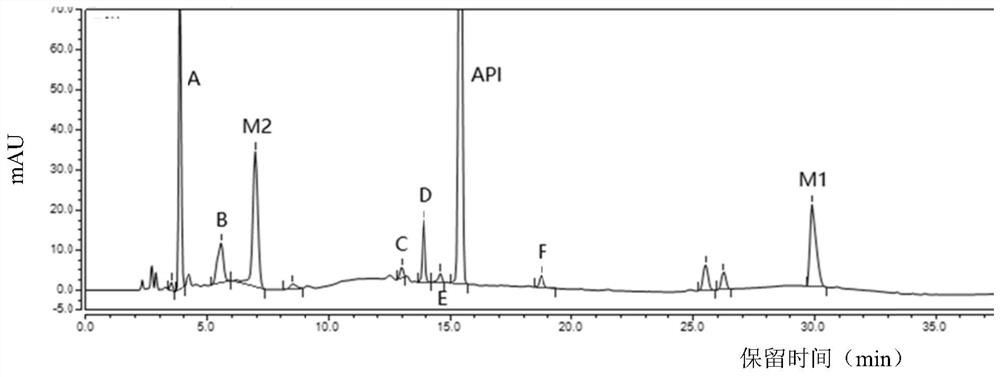
The invention provides a separation and detection method of a dexrazodone intermediate and impurities. The method comprises the following steps: (1) diluting dexrazodone containing the intermediates and the impurities by using a diluent to obtain a sample solution; and (2) detecting the sample solution by using a high performance liquid chromatograph, wherein a chromatographic column of the high performance liquid chromatograph is a CAPCELL PAK ADME chromatographic column. According to the separation and detection method disclosed by the invention, the dexrazodone intermediate M2, the intermediate M1, the impurity A, the impurity B, the impurity C, the impurity D, the impurity E and the impurity F can be effectively separated, good base line separation can be realized among peaks, when the flow velocity of a mobile phase or the column temperature of a chromatographic column is properly controlled, the peak separation degrees of most of the intermediates and the impurities are greater than 3.0, and an overall separation degree is high so that the quality of dexrazoxane and the intermediates thereof can be effectively controlled.
Owner:中润药业有限公司
Preparation method of high-purity dexrazoxane
ActiveCN102675227BIncrease the degree of steamingGood dispersionOrganic chemistryPressure reductionSodium salt
The invention relates to a preparation method of high-purity dexrazoxane, which comprises the following steps of: (1) cyclization reaction: carrying out cyclization reaction between (S)-1, 2-propane diamine-N, N, N', N'-tetracetic acid or disodium salt of (S)-1, 2-propane diamine-N, N, N', N'-tetracetic acid and formamide, wherein a high boiling point solvent is used; (2) preparation of salt-containing crude product: evaporating the mixture, which is obtained after reaction, for removing the formamide by pressure reduction and concentration, adding organic solvent into the mixture, and filtering to obtain solid; (3) preparation of crude product: adding dioxane into the salt-containing crude product, heating for backflow, filtering, concentrating the filtrate, adding organic solvent into the filtrate to obtain the crude product of dexrazoxane; and (4) refining: adding the crude product of dexrazoxane into N, N'-dimethyl formamide, heating for dissolving, dropwise adding the solvent, carrying out crystallization, filtering to obtain solid, washing the obtained solid with the solvent, drying to finally obtain the high-purity dexrazoxane. The method for synthesizing dexrazoxane is stable in yield and easy in condition control; the product purity is higher than 99.5% and residual organic solvent is little, and the synthesis cost can be reduced.
Owner:JIANGSU AOSAIKANG PHARMA CO LTD
Method for refining dexrazoxane
Owner:JIANGSU AOSAIKANG PHARMA CO LTD
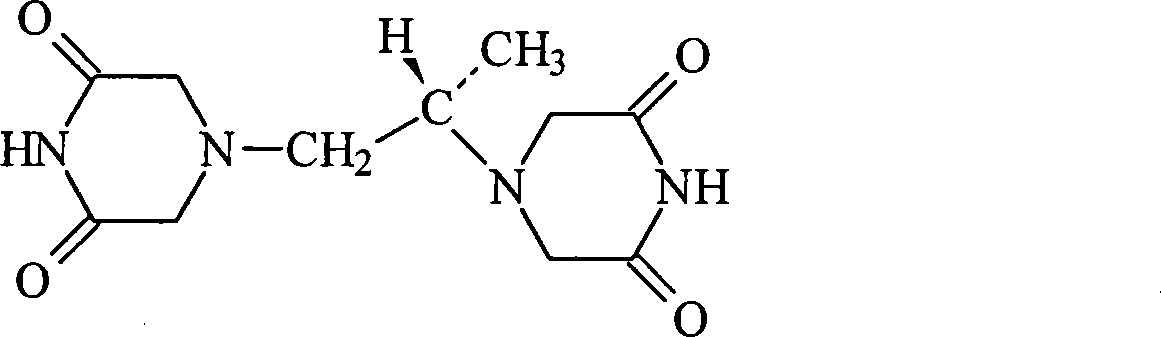
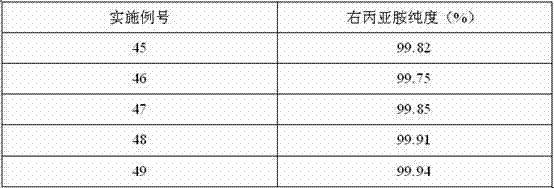
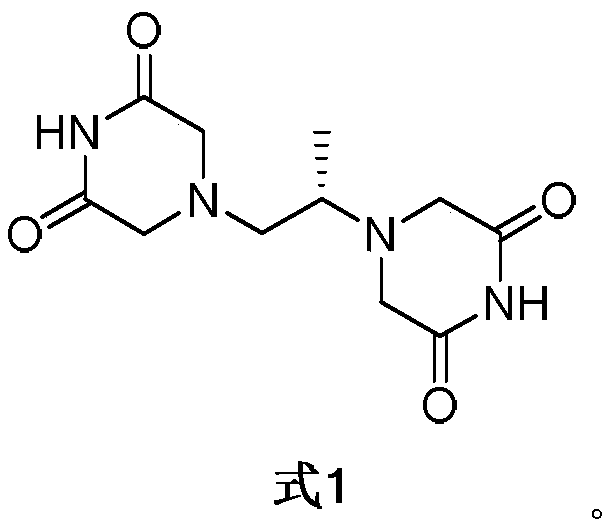
A kind of preparation method of dextropropanimine
The invention relates to a preparation method of dexrazoxane. In allusion to various problems of a present dexrazoxane synthetic route, such as complexity, high cost, high reaction temperature, low yield, tedious aftertreatment, long production cycle and the like, the invention provides a simple and efficient preparation method of dexrazoxane. Starting from (S)-1,2- diaminopropane hydrochloride or (S)-1,2-diaminopropane, only two steps are required to obtain a crude product dexrazoxane with the highest purity of 99.46%; and simple recrystallization is carried out to obtain a pure product with purity of 99.96%. The method has advantages of simple operation and proper temperature. By the method, the cycle of synthetic process is shortened greatly. In addition, yield and purity of the product are also raised greatly.
Owner:JIANGSU AOSAIKANG PHARMA CO LTD
Dexrazoxane preparation method
InactiveCN109836387AFacilitate the realization of industrial productionThe reaction steps are simpleOrganic chemistryPotassiumChloroacetic acids
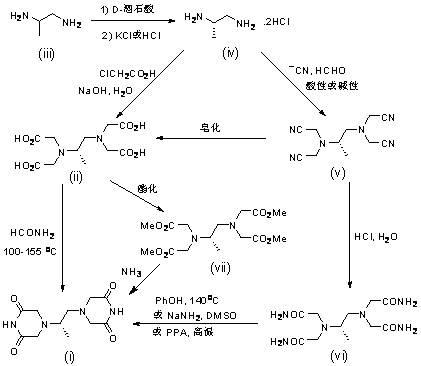
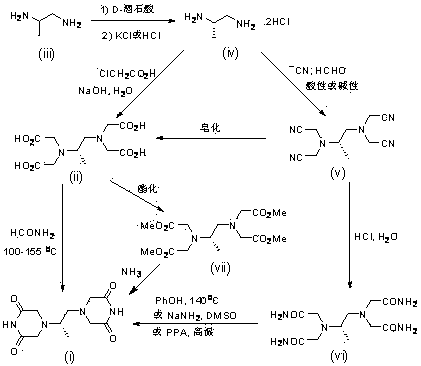
The invention belongs to the field of drug synthesis, and provides a completely-new dexrazoxane preparation method, which comprises: carrying out a reaction on (S)-1,2-propanediamine ditartrate splitby using inexpensive and easily-available (+ / -)-1,2-propanediamine as a starting raw material and using D-(-)-tartaric acid as a splitting agent and potassium chloride to obtain (S)-1,2-propanediaminedihydrochloride, carrying out condensation on the (S)-1,2-propanediamine dihydrochloride and chloroacetic acid to prepare (S)-N,N,N',N'-1,2-propanediaminetetraacetic acid, and finally carrying out cyclization to obtain dexrazoxane, wherein the total yield is 38.3%. According to the present invention, the route has advantages of simple reaction step, convenient post-treatment, no requirement of column chromatography separation, good product quality and the like.
Owner:辽宁博美医药科技有限公司
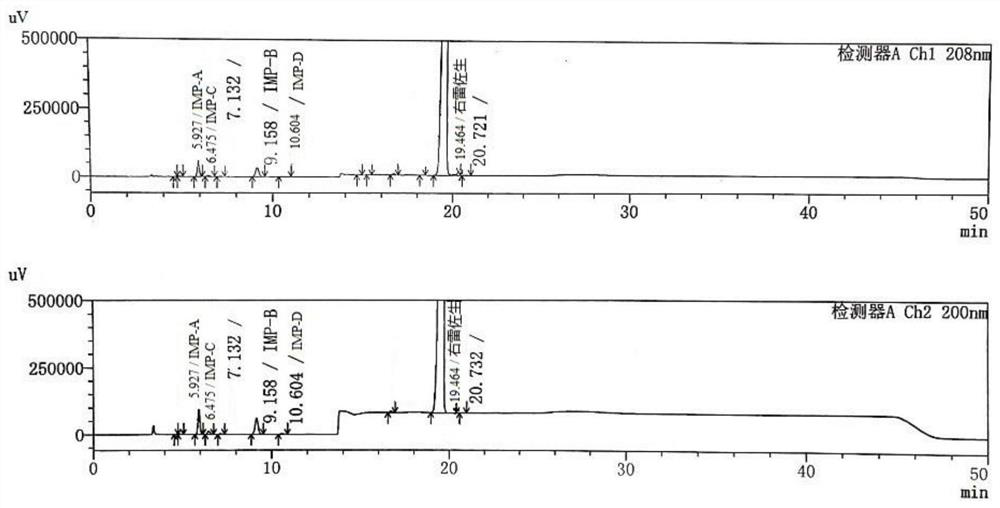
Method for detecting dexrazodone and related substances thereof
ActiveCN113466392AGuarantee quality and safetyEfficient separationComponent separationO-Phosphoric AcidFluid phase
The invention discloses a method for detecting dexrazodone and related substances thereof, dexrazodone and / or related substances in dexrazodone or a preparation thereof are qualitatively or / and quantitatively detected by adopting a high performance liquid chromatography method, and the detection conditions of liquid chromatography are as follows: a chromatographic column is C18 or an equivalent chromatographic column; and mobile phases comprise a mobile phase A and a mobile phase B, the mobile phase A is a phosphoric acid buffer solution, the mobile phase B is a mixed solution of the phosphoric acid buffer solution and acetonitrile in a volume ratio of (80-90): (10-20), and gradient elution is carried out. By adopting the method disclosed by the invention, the dexrazolone and nine related substances in the dexrazolone can be effectively detected at the same time, and verification experiments of system applicability, specificity, accuracy, precision, linear range, quantitation limit detection limit and durability show that the method can achieve the purpose of accurate quantitative analysis, and is beneficial to more strict quality control on the dexrazolone.
Owner:SICHUAN HUIYU PHARMA +1
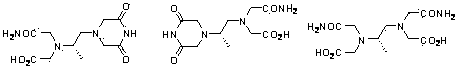
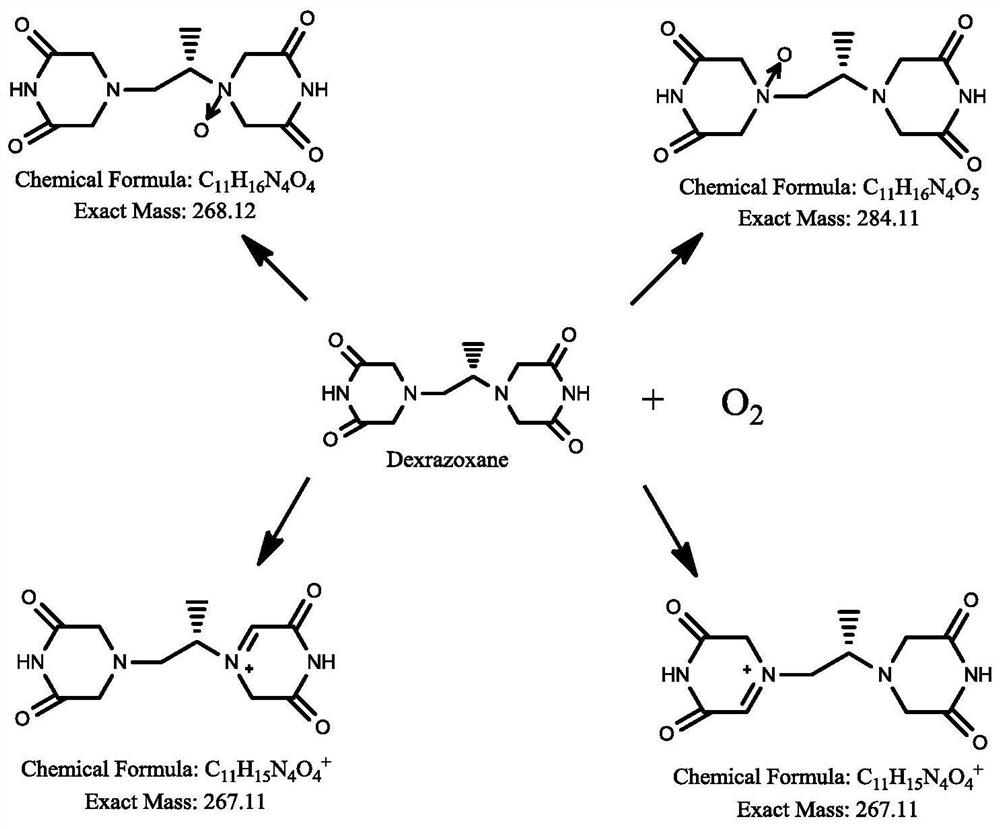
Dexrazoxane related substances and detection method thereof
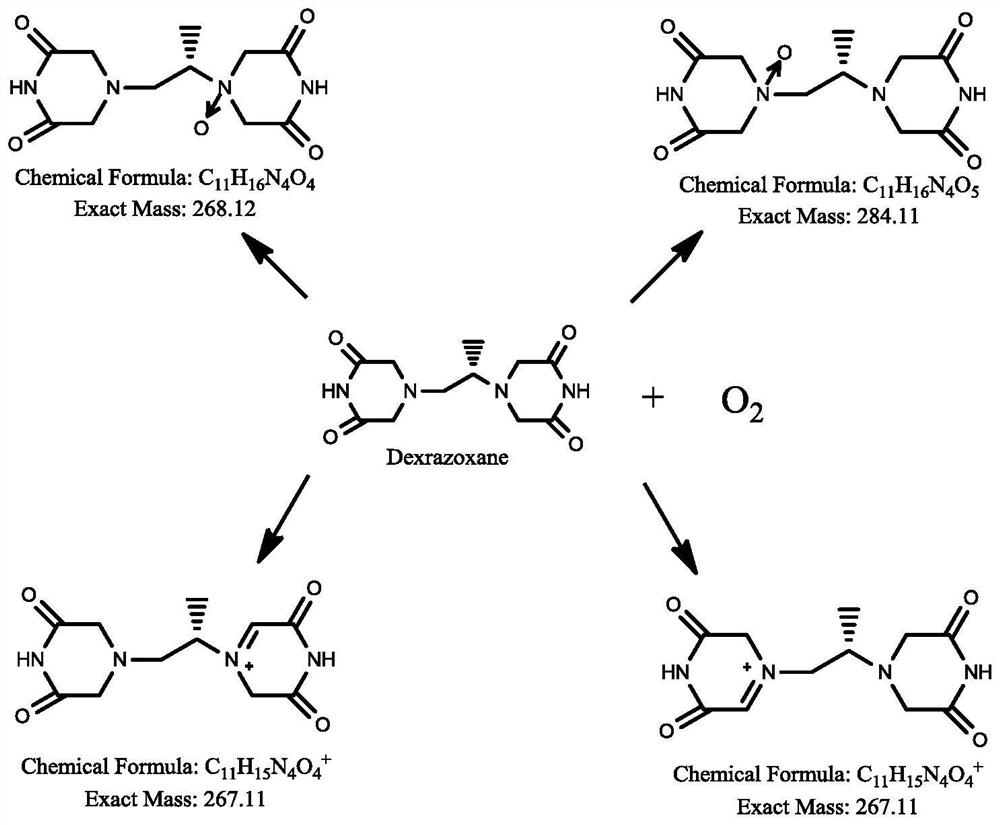
ActiveCN113640421AGuarantee quality and safetyEfficient separationComponent separationO-Phosphoric AcidFluid phase
The invention discloses dexrazoxane related substances and a detection method thereof, and provides four new oxidation impurities of dexrazoxane: an oxidation impurity 1, an oxidation impurity 2, a chromogenic impurity 1 and a chromogenic impurity 2; the related substances are qualitatively or / and quantitatively detected by adopting a high performance liquid chromatography method; the detection conditions of the liquid chromatography comprise that a chromatographic column is C18 or an equivalent chromatographic column; mobile phases comprise a mobile phase A and a mobile phase B, wherein the mobile phase A is a phosphoric acid buffer solution, the mobile phase B is a mixed solution of the phosphoric acid buffer solution and acetonitrile in a volume ratio of (80-90):(10-20), and gradient elution is carried out. By adopting the method disclosed by the invention, nine related substances of dexrazoxane can be effectively detected at the same time, and verification experiments of system applicability, specificity, accuracy, precision, linear range, quantitation limit detection limit and durability show that the method can achieve the purpose of accurate quantitative analysis, and more strict quality control of dexrazoxane is facilitated.
Owner:SICHUAN HUIYU PHARMA +1
Dexrazoxane preparation method
ActiveCN110804022AHigh purityLow purityOrganic chemistry methodsPhysical chemistryProcess engineering
The invention discloses a dexrazoxane preparation method. According to the preparation method, (S)-1,2-diaminopropane-tetraacetate and alkali metal salt thereof are used as raw materials, and ammoniumsalt is used as an ammonium source to prepare dexrazoxane. By the adoption of the preparation method, tedious technological operation is avoided, the technological operation is easier and more convenient, and therefore the preparation method is more adaptive to industrial amplifying production.
Owner:YANGTZE RIVER PHARM GRP CO LTD
Application of dexrazoxane in preparation of drug for treating neurodegenerative diseases
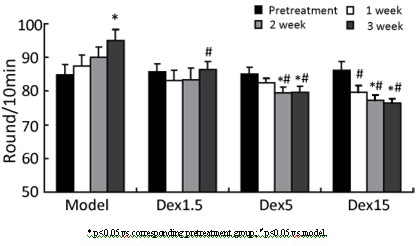
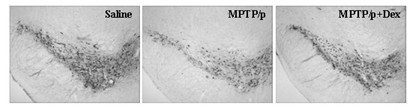
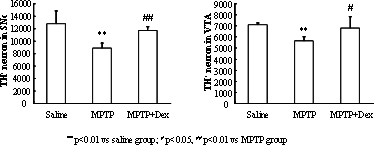
InactiveCN102349908AImprove behavioral disordersAvoid damageOrganic active ingredientsNervous disorderMPTPNeuro-degenerative disease
The invention provides application of dexrazoxane in preparation of drug for treating neurodegenerative diseases. Experiments prove that dexrazoxane can improve the behavior disorder of model rats of Parkinson's disease belonging to neurodegenerative diseases, inhibit the decrease of TH (helper T cell) nerve cells in mouse mesencephalic SNc (substantia nigra compact part) and ventral tegmental area (VTA), which is caused by MPTP / p (1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine / probenecid) modeling, improve the proliferation and activation of astrocyte and microglia, obviously inhibit the over-expression of alpha-synuclein in substantia nigra compact part and reticular part, and inhibit the reduction of survival rate of dopaminergic neuron cell strain SH-SY5Y cells, which is caused by H2O2 and MPP<+> (1-methyl-4-phenylpyridinium ion). The results show that dexrazoxane has the action of treating neurodegenerative diseases. Through the invention, dexrazoxane can be used as an active ingredient to prepare the drug for treating neurodegenerative diseases.
Owner:NANJING MEDICAL UNIV
Slow-released injection containing nolatrexed dihydrochloride and synergist thereof
The invention relates to a slow-release injection containing nolatrexed and a synergistic agent of the nolatrexed, which consists of a slow-release microballoon sphere and a solution medium, wherein, the slow-release microballoon sphere comprises active anti-cancer ingredients and slow-release accessories; and the solution medium is a special solution medium containing a suspending agent. The active anti-cancer ingredients are antimetabolites, such as pemetrexed, rumitrexed, raltitrexed, nolatrexed, carmofur, dexrazoxane, tegafur, zalcitabine, emtricitabine, ibatabine, ancitabine, decitabine, flurocitabine, enocitabine, imidazoletabine, capecitabine, gemcitabine, fludarabine or cladribine, and the like, and synergistic agents of the antimetabolites selected from topoisomerase inhibitors and / or tetrazine compounds; the slow-release accessories are selected from one of polifeprosan, di-fatty acid and decanedioic acid copolymer, polylactic acid copolymer and EVAC or the combination thereof; the viscosity of the suspending agent is 100cp to 3000cp (at the temperature of 20 DEG C to 30 DEG C) and the suspending agent is selected from carboxymethylcellulose sodium and the like. The slow-release microballoon sphere can also be prepared into a slow-release implant used for being injected or put in tumors or the surrounding of the tumors so as to enhance the effects of radiotherapy and chemotherapy.
Owner:SHANDONG LANJIN PHARMA +1
Preparation method of dexrazolone
ActiveCN114685383AAvoid harsh reaction conditionsEasy to operateOrganic chemistryWhite powderProcess engineering
The invention provides a preparation method of dexrazolone, which comprises the following step: carrying out ring closing reaction on (S)-1, 2-propylene diamine tetraacetic acid and urea to obtain the dexrazolone. The preparation method has the following beneficial effects: 1, urea is adopted as an amination nitrogen source, high-purity dexrazoxane can be obtained through conventional reaction operation, harsh reaction conditions are avoided, the process operability is high, and process production is facilitated; 2, the reaction is fast, the generation of partial process impurities is avoided, the purification pressure of the final product is reduced, the purity and yield of the prepared dexrazoxane are high, the production cost is greatly reduced, and the method is economical and environment-friendly; 3, post-treatment is simple, convenient and easy to operate, and stable production of a high-quality dexrazoxane raw material medicine is facilitated; and 4, the operation is simple, the process conditions are easy to control, the cost is lower, the yield of products among batches is stable, the refined dexrazoxane is white powder, the purity reaches 99.9% or above, and the maximum individual impurity is 0.05% or below.
Owner:SICHUAN HUIYU PHARMA +1
Anti-cancer drugs slow release agent comprising anticancer antibiotics and synergist thereof
Disclosed is an anticancer slow release agent which comprises slow release microspheres and dissolvent, wherein the slow release microballoons comprise anti-cancer active constituents and slow releaseauxiliary materials, the dissolvent being specific dissolvent containing suspension adjuvant. The anticancer antibiotics are selected from Idarubicin, Valtaxin, Pirarubicin and Mitoxantrone, The anti-metabolite drugs are selected from Pemetrexed, Carmustine, Tegafur, Zalcitabine, Emtritabine, Galocitabine, Ibacitabine, Ancitabine, Decitabine, Flurocitabine, Enocitabine, Imidazoletabine, Capecittabine, Gemcitabine, Fludrarbine, Raltitrexed, Dexrazoxane, Cladribine, Nolatrexed and folic acid, The slow release auxiliary materials are selected from EVAc, Polifeprosan, sebacylic acid copolymer, lactic acid, the viscosity of the suspension adjuvant is 100-3000cp (at 25-30 deg C), and is selected from sodium carboxymethylcellulose. The slow release microspheres can also be prepared into slow release implanting agent for injection or placement in or around tumor.
Owner:SHANDONG LANJIN PHARMA
Dexrazoxane freeze-dried powder injection and preparation method thereof
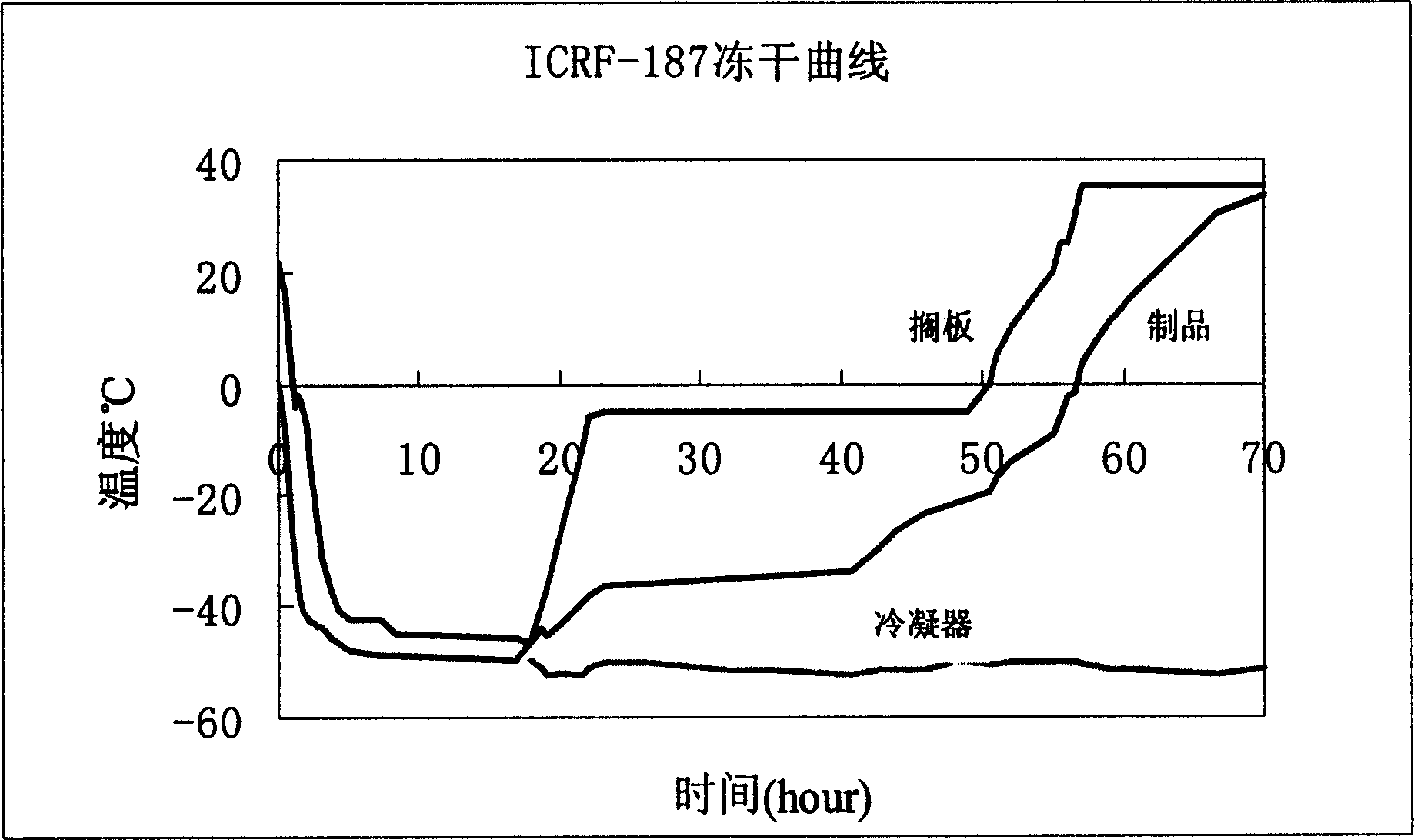
The invention relates to a dexrazoxane freeze-dried powder injection and a preparation method thereof. The dexrazoxane freeze-dried powder injection consists of a dexrazoxane salt, sodium acetate and mannitol, the above compositions are used to prepare a solution, the solution is processed according to the following conditions: pre-freezing at -40 DEG C to -45 DEG C for 4 h, vacuumizing, keeping the vacuum degree at 20 pa, slowly heating to -20 DEG C to -25 DEG C, and keeping the temperature for 10 h, slowing heating to 25 DEG C to 30 DEG C and keeping the temperature for 5 h, so that the powder injection is obtained. The powder injection is diluted with common injection water for intravenous injection without being diluted by using a specific diluent, and application is convenient.
Owner:BEIJING SUNHO PHARMA
Cell transport preserving fluid and application thereof
The invention discloses a cell transport preserving fluid and application thereof. The cell transport preserving fluid is prepared from the following components in mass concentration: 0.01 mg / ml to 2.5 mg / ml of glucose, 0.05 mg / ml to 10 mg / ml of sodium chloride, 0.05 mg / ml to 3 mg / ml of potassium chloride, 0.05 mg / ml to 1 mg / ml of calcium chloride, 0.05 mg / ml to 20 mg / ml of sodium lactate, 0.05 mg / ml to 2.5 mg / ml of amifostine, 0.05 mg / ml to 3.0 mg / ml of dexrazolone and the balance of water. The cell transport preserving fluid provided by the invention can maintain the activity of various cells for about 7 days, and has a certain advantage in the aspect of maintenance time compared with other preserving fluids; the cell transportation preserving fluid is liquid used in the transportation process of human cells, the components are simple and easy, all components can be normally input into a human body, no human-derived or animal-derived components exist, and the cell transportation preserving fluid plays a great role in development of cells for clinical treatment towards the drug development direction.
Owner:北京益华生物科技有限公司
Method for refining dexrazoxane
Owner:JIANGSU AOSAIKANG PHARMA CO LTD
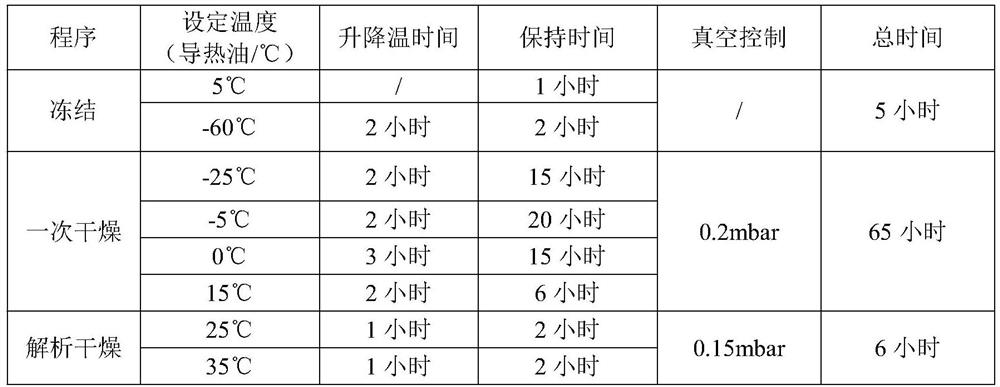
Freeze-dried preparation containing dexrazolone and preparation method of freeze-dried preparation
ActiveCN114306251AReduce moistureImprove stabilityOrganic active ingredientsPowder deliveryDrugs preparationsVacuum pump
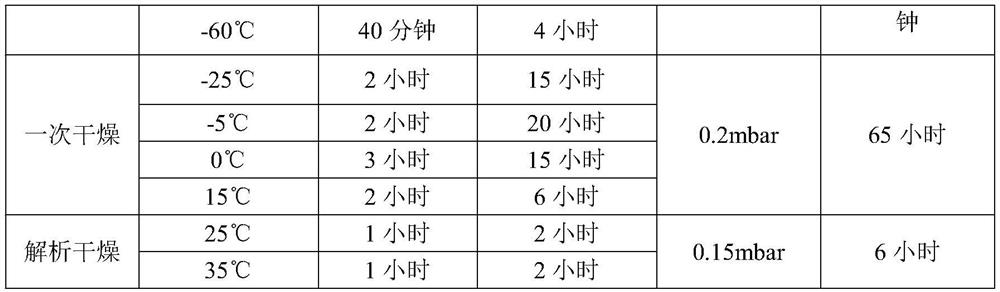
The invention discloses a lyophilized preparation containing dexrazodone and a preparation method thereof, and belongs to the technical field of pharmaceutical preparations. A solution for preparing the dexrazoxane freeze-dried preparation is composed of dexrazoxane, hydrochloric acid and water according to a certain proportion, the concentration of dexrazoxane is 30-40 mg / mL, and the pH value of the solution is 1.6-2.0. The invention further provides a method for preparing the dexrazoxane freeze-dried preparation, the dexrazoxane freeze-dried preparation is kept at the temperature of-55 DEG C to-60 DEG C for 4-5 hours, then a vacuum pump is started, the vacuum degree is reduced to be below 0.2 mbar, primary drying is carried out at the temperature of-25 DEG C to 15 DEG C, the primary drying time is 60-70 hours, finally, desorption drying is continued at the temperature of 0.15 mbar and the temperature of 25-35 DEG C, and the drying time is 5-10 hours. The prepared freeze-dried product has water content of less than 1%, has good stability and can be stored at room temperature.
Owner:LEPU PHARMACEUTICAL CO LTD
Preparation method of dexrazoxane and pharmaceutical salts thereof
ActiveCN101684100BEasy to routeLow costOrganic chemistryAntineoplastic agentsChemical synthesisPharmacy technology
The invention belongs to chemical synthesis pharmacy technology field, the invention provides a preparation method of medicine dexrazoxane which has a function of antitumor, the process includes: N,N-dichloroacetyl imine (II) is prepared by chloroacetonitrile in the presence of acid, then the (+)- dexrazoxane (I) is obtained by condensation of the N,N-dichloroacetyl imine (II) and the (+)-1,2-propanediamine (III), the obtained dexrazoxane is made into pharmaceutically acceptable salt. The method according to the invention has advantages of simple route, convenient operation, easy industrialization and short period, and the obtained product has high purity. Route of the process is shown as follow.
Owner:CHONGQING HAITENG PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com