Freezing-dried composite contg. dexrazoxane and its prepn. method
A technology of dexrazoxane and composition, which is applied in the field of freeze-dried composition containing dexrazoxane and its preparation, and can solve the problems of instability to light, affecting solubility, non-degradation, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Formula: dexrazoxane 20mg / ml, sodium metabisulfite 1mg / ml, hydrochloric acid 0.1mol / ml.
[0027] Preparation method: first prepare 1000ml of 0.1mol / l hydrochloric acid, inject nitrogen gas into the above-mentioned hydrochloric acid solution, add the prescribed amount of antioxidant, add the main drug dexrazoxane, and divide into 30ml / 15ml freeze-dried bottles, each bottle 25ml / 12.5ml, the liquid level of the lyophilized liquid is about 25mm, and it is cooled to -45°C in a lyophilizer to lyophilize.
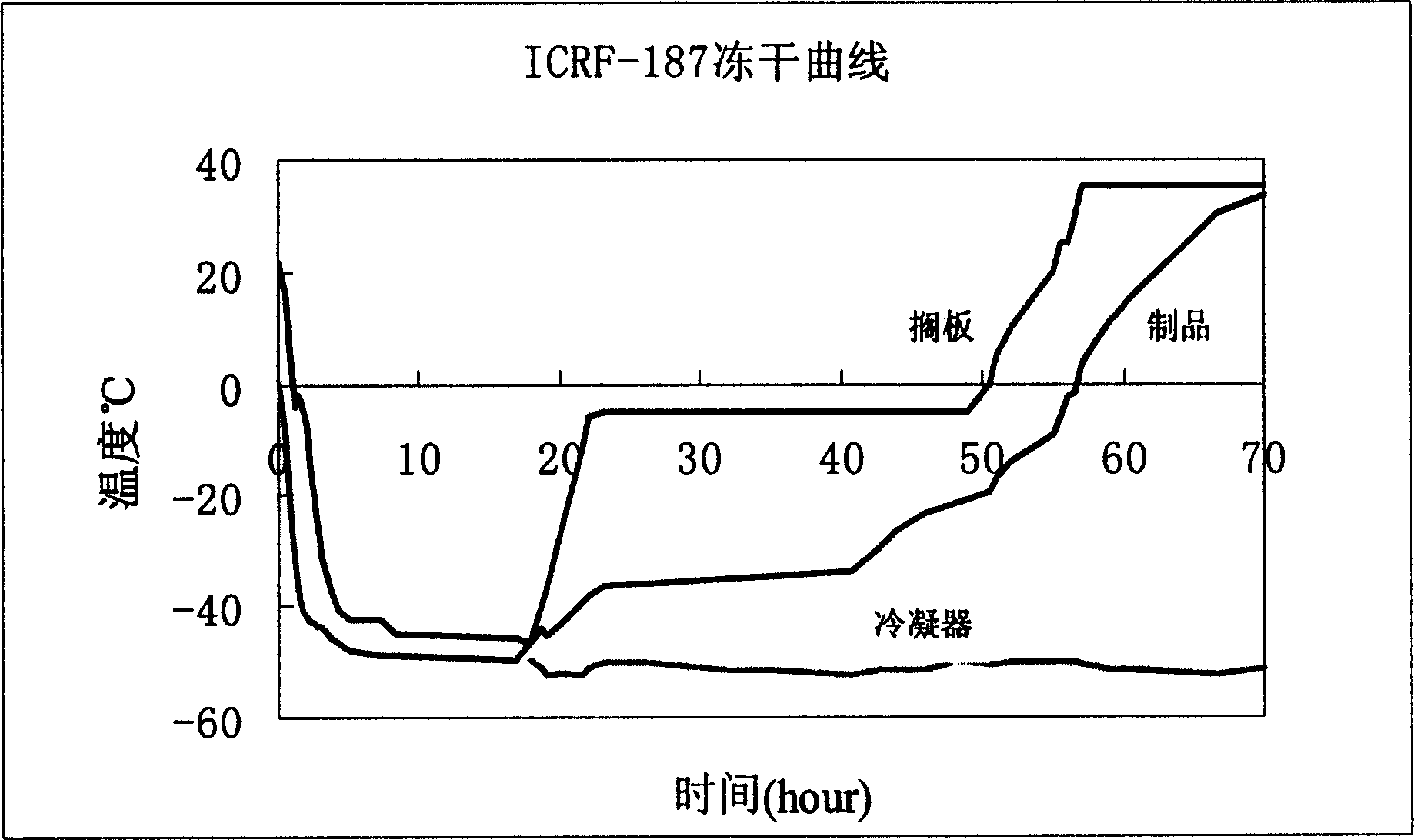
[0028] Raise the temperature to -25°C at a heating rate of 15°C / hr, the water will sublimate, and the sublimation time is 18 hours; then, under the condition of 10°C, continue to sublimate the water to dry the remaining water, and the sublimation drying time is 20 hours, freeze-drying curve such as figure 1 , to obtain dexrazoxane for injection described in this patent. The moisture content of the freeze-dried product is 1.89%, and the color is white.
Embodiment 2
[0030] Formula: dexrazoxane 30mg / ml, sodium bisulfite 10mg / ml, hydrochloric acid 0.15mol / ml.
[0031] Preparation method: first prepare 1000ml of 0.5mol / l hydrochloric acid, inject nitrogen gas into the above hydrochloric acid solution, add the prescribed amount of antioxidant, add the main drug dexrazoxane, divide into 30ml freeze-dried bottles, 25ml per bottle, and freeze-dry The height of the liquid level is about 25mm, and it is cooled to -54°C in a lyophilizer for lyophilization.
[0032] Raise the temperature to -15°C at a heating rate of 2°C / hr, the water will sublimate, and the sublimation time is 25 hours; then, under the condition of 15°C, the water will continue to sublimate to dry the remaining water, and the sublimation drying stage time For 20 hours, the dexrazoxane for injection described in this patent can be obtained. The moisture content of the freeze-dried product is 2.8%, and the color is white.
Embodiment 3
[0034]Using the same method and formula as in Example 1, substituting L-ascorbic acid for sodium metabisulfite, the dexrazoxane for injection described in this patent was obtained. The moisture content of the freeze-dried product is 2.1%, and the color is white.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com