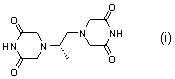
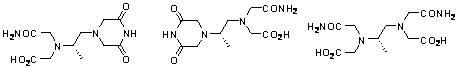
Preparation method of high-purity dexrazoxane
A high-purity technology of dextropropylimine, which is applied in the field of pharmaceutical and chemical engineering, can solve the problems of low refining yield, toxic and side effects of patients, and long-term crystallization, so as to achieve easy control of process conditions, stable product yield, and time-saving short effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
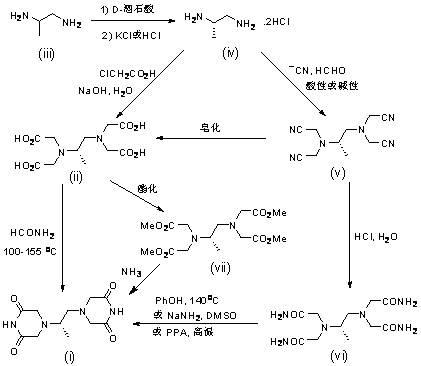
[0042] 80kg of water, 116kg of chloroacetic acid, 24kg of 50% sodium hydroxide solution, 30kg of (S)-1,2-propanediamine hydrochloride and 0.5kg of potassium iodide in 70kg of water were added to the 500L reactor in turn. Keep temperature 15-25 o C for 4 hours, then heated to 60 o C was reacted for 20 hours. About 30kg of concentrated hydrochloric acid was added to adjust the pH value to about 5.3. keep 60 o C was concentrated under reduced pressure, and about 220kg of water was distilled off. Filtrate while hot, transfer the filtrate to a 1000L crystallization kettle, and add 600kg of methanol in batches. Crystallize for about 5 hours and centrifuge. Filter cake 70 o C was dried for 8 hours to obtain 62.5kg of tetraacetic acid with a content of 78.3% and a moisture content of 5.2%.
Embodiment 2
[0044] Put tetraacetic acid (100g), formamide (150mL) and polyethylene glycol 300 (10g) into the reaction flask in turn, and heat to 100g under reduced pressure. o C reaction for 1 hour, the obtained clear solution was heated to 150-155 o C, continue vacuum distillation reaction for 5 hours. Keep vacuum distillation to internal temperature 120-130 o C No distillate. cool down to 20 o C, ethanol (100 mL) was added to give a yellow solid. The solid was put into a reaction flask containing dioxane (200mL), heated to reflux for about 20 minutes, filtered, the filtrate was concentrated under reduced pressure to obtain most of the dioxane, added 80mL of methanol, stood still for about 30 minutes, filtered, and the filter cake was vacuum 50 o C was dried for 3 hours to give 34.1 g of crude dextropropimine (pale yellow solid).
Embodiment 3
[0046] Put tetraacetic acid (100g), formamide (150mL) and polyethylene glycol 600 (40g) into the reaction flask in turn, and heat to 100g under reduced pressure. o C reaction for 1 hour, the obtained clear solution was heated to 150-155 o C, continue vacuum distillation reaction for 5 hours. Keep vacuum distillation to internal temperature 120-130 o C No distillate. cool down to 50 o C, a mixed solution (100 mL) of tert-butanol and tetrahydrofuran was added to obtain a yellow solid. The solid was put into a reaction flask containing dioxane (200mL), heated to reflux for about 20 minutes, filtered, the filtrate was concentrated under reduced pressure to obtain most of the dioxane, added 80mL of tert-butanol, stood still for about 30 minutes, filtered, and the filter cake Vacuum 50 o C was dried for 3 hours to obtain 32.8 g of crude dextranimine.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com