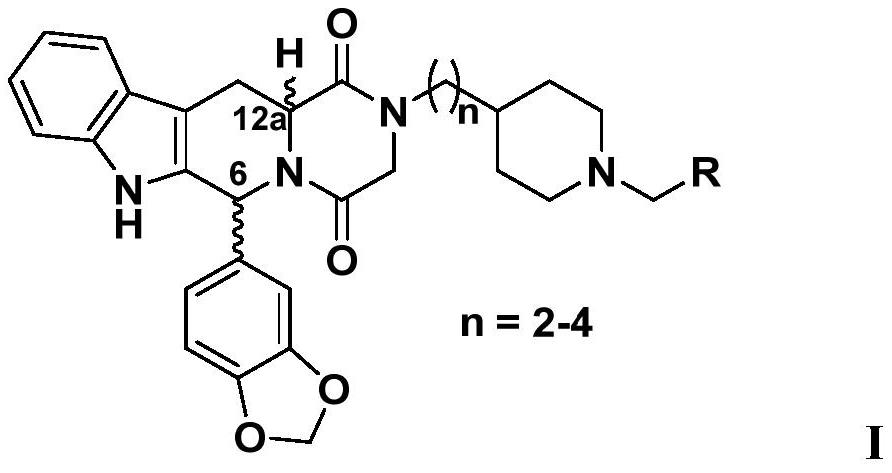
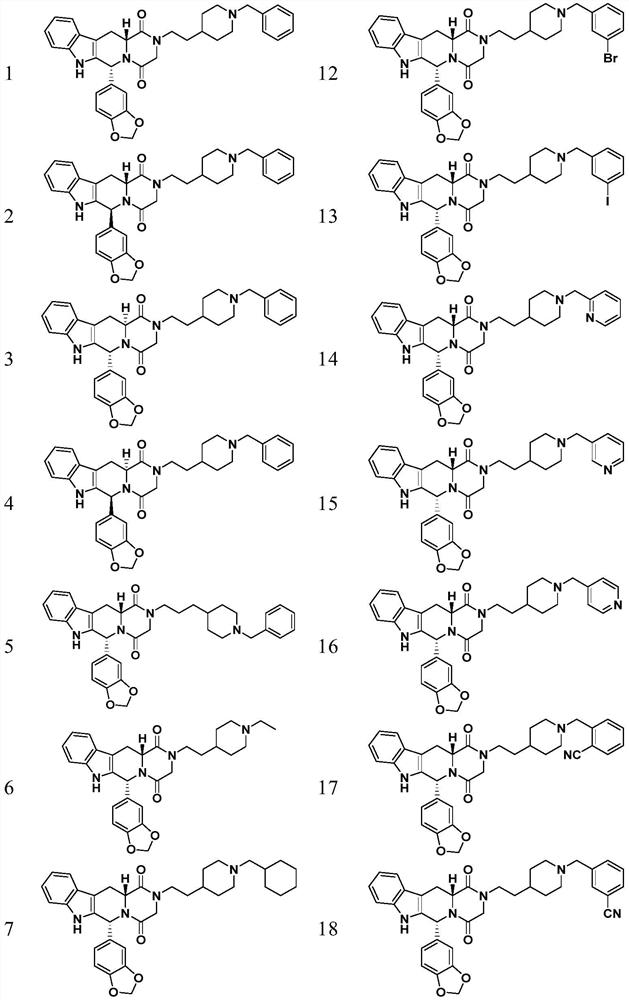
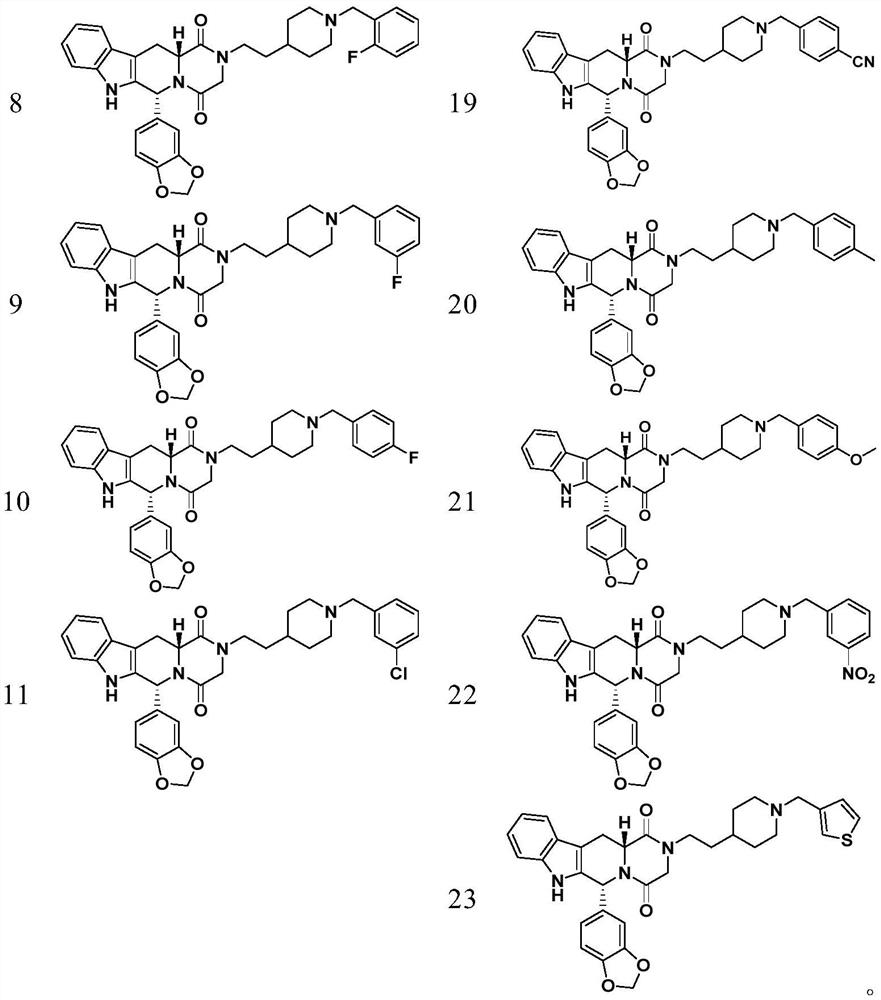
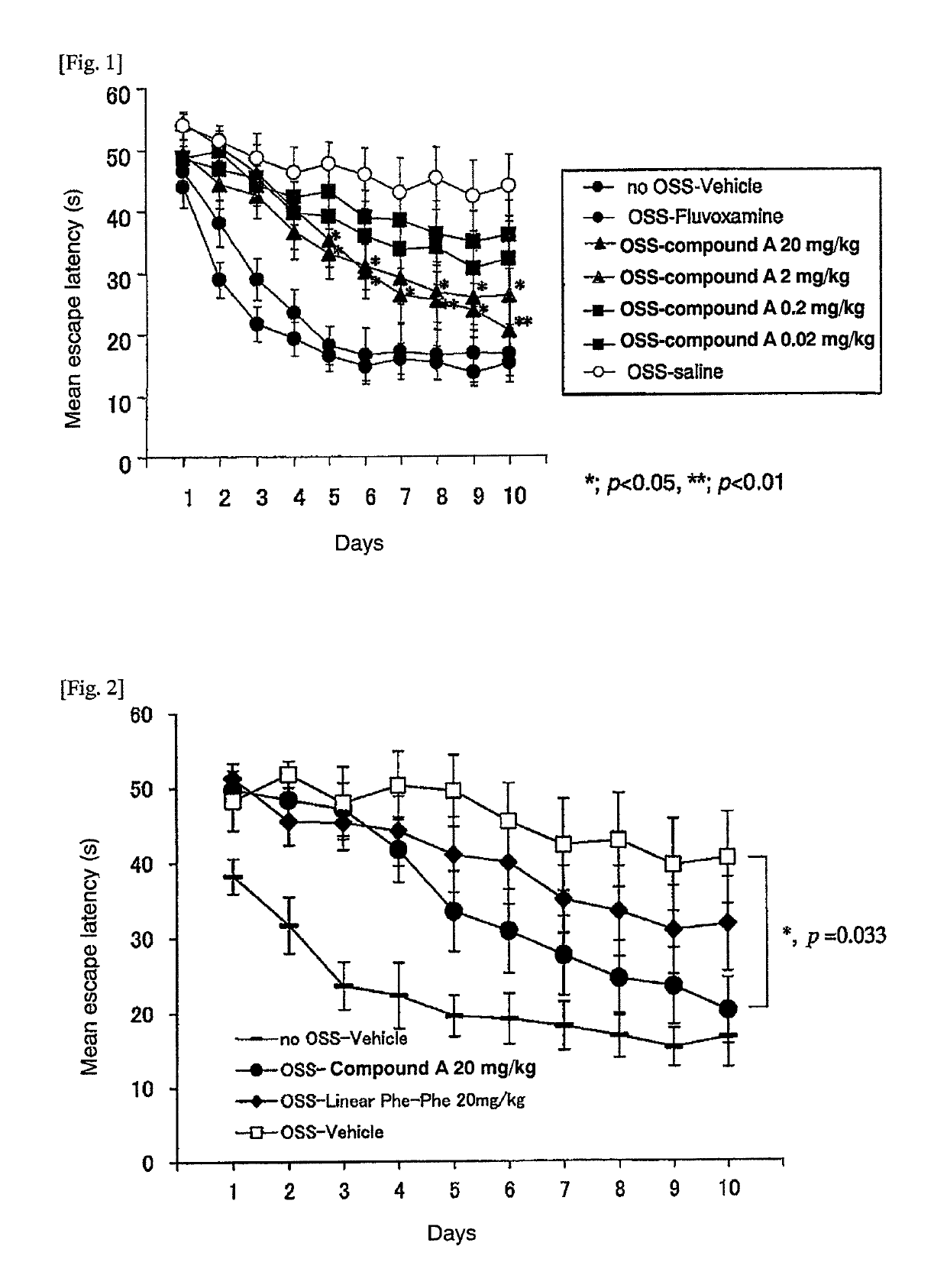
Patents
Literature
107 results about "Piperazinedione" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A crystalline antibiotic, fermentation product of actinomycetes with alkylating activity. Piperazinedione alkylates DNA molecules, thereby prevents completion of DNA replication leading to cell cycle arrest. Check for http://www.cancer.gov/Search/ClinicalTrialsLink.aspx?id=39556&idtype=1 active clinical trials or http://www.cancer.gov/Search/ClinicalTrialsLink.aspx?id=39556&idtype=1&closed=1 closed clinical trials using this agent. (http://nciterms.nci.nih.gov:80/NCIBrowser/ConceptReport.jsp?dictionary=NCI_Thesaurus&code=C1401 NCI Thesaurus)
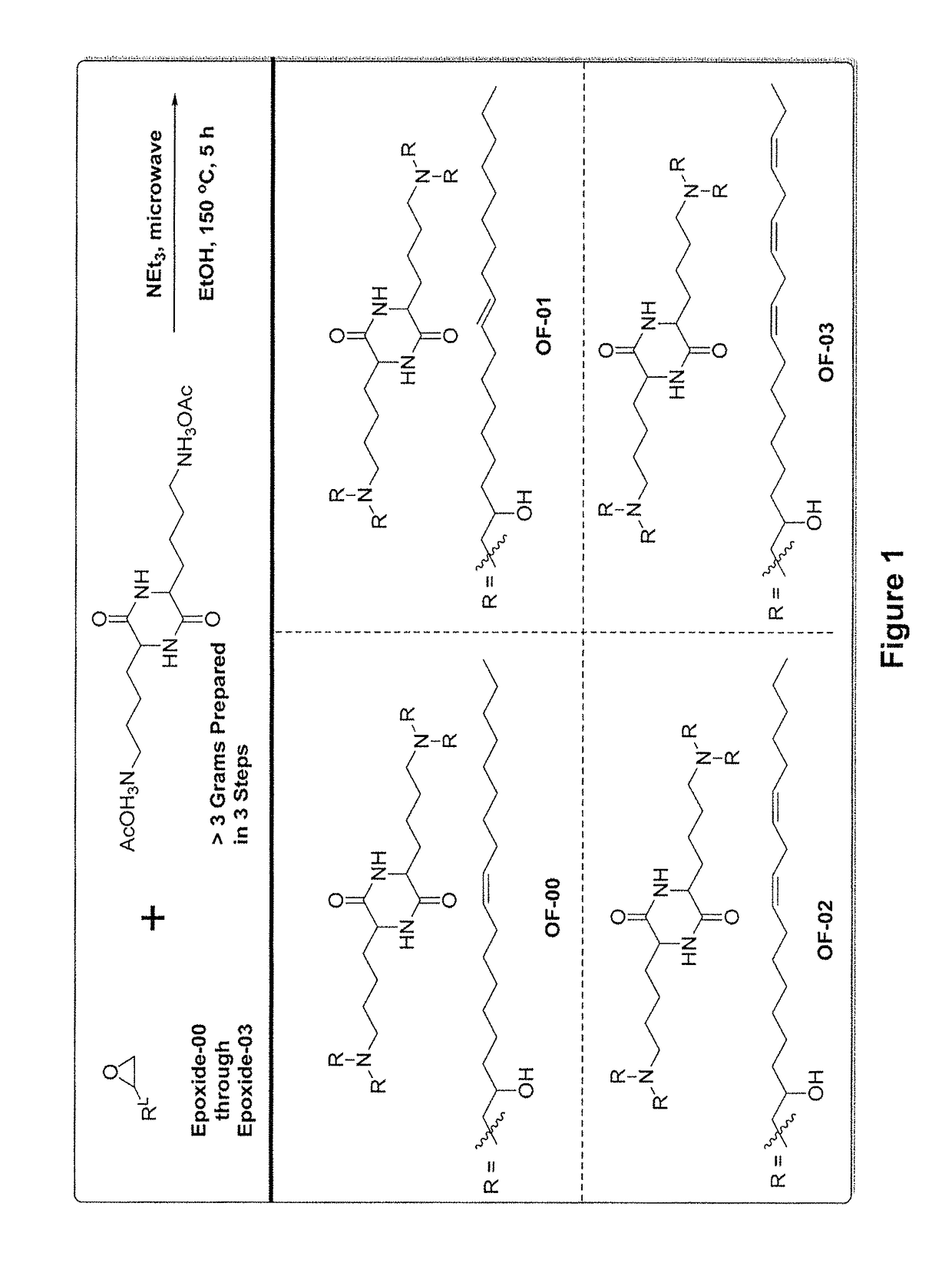
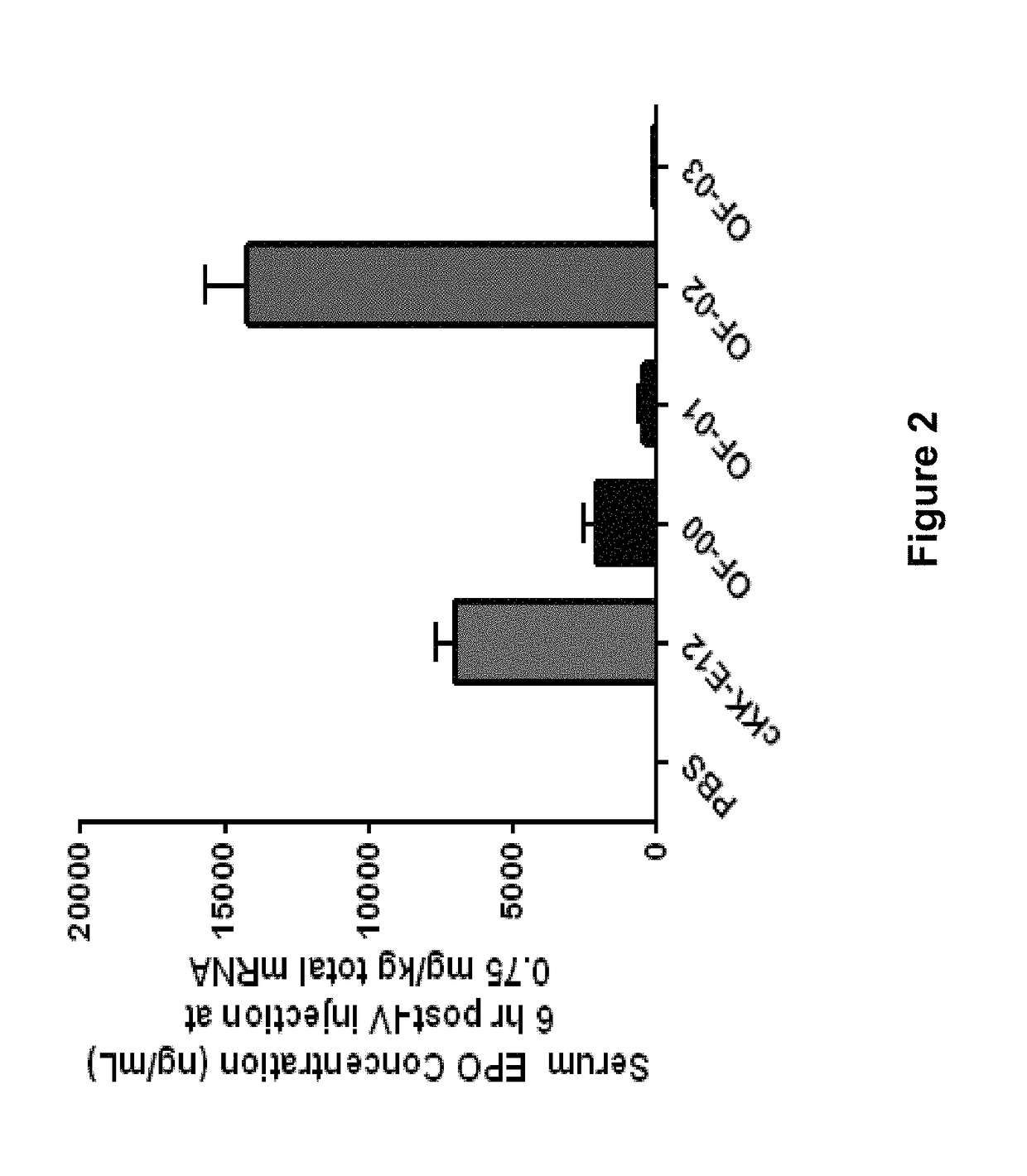
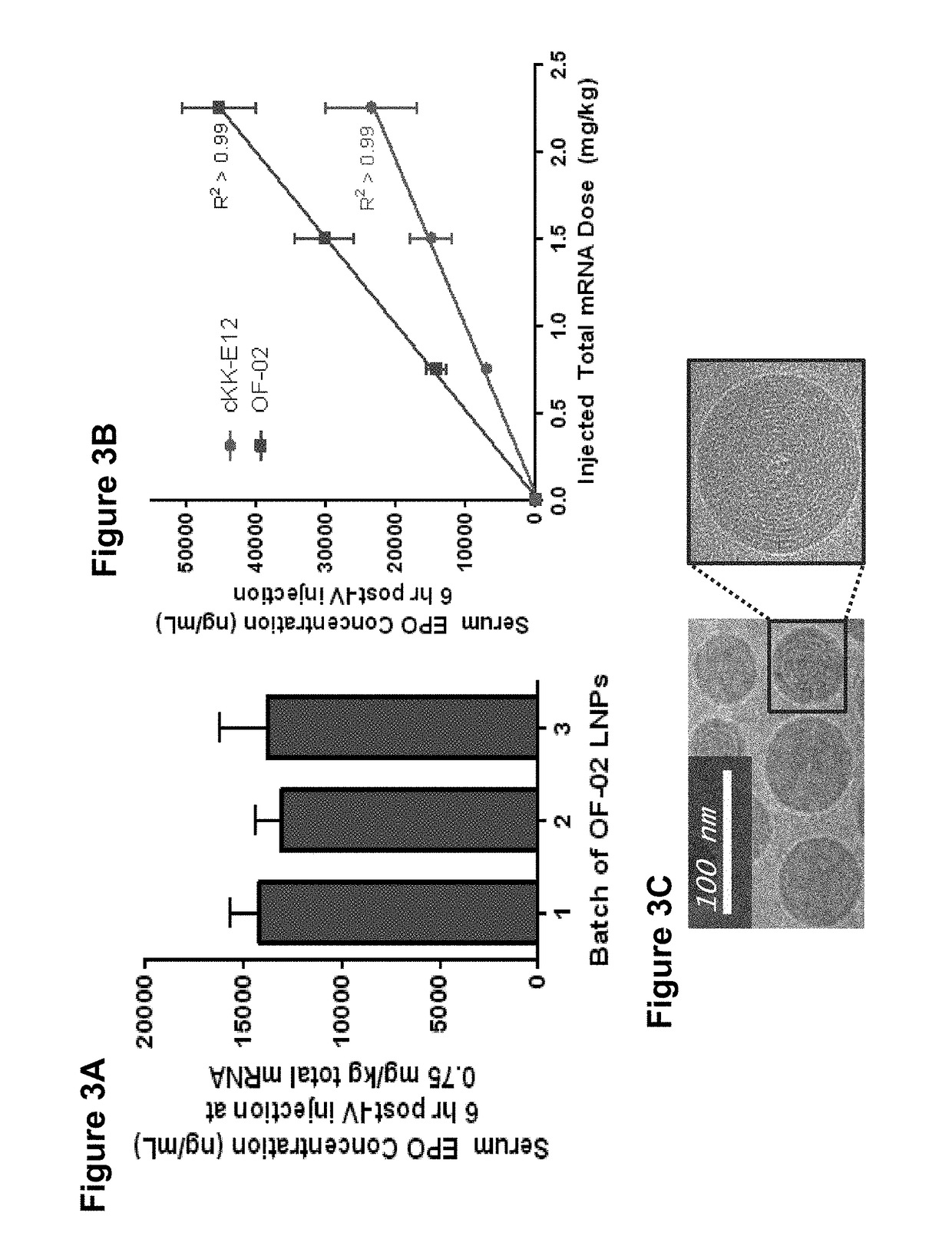
Alkenyl substituted 2,5-piperazinediones, compositions, and uses thereof
ActiveUS20160367686A1Increase exposureImprove concentrationOrganic active ingredientsNervous disorderChemistryPiperazinedione
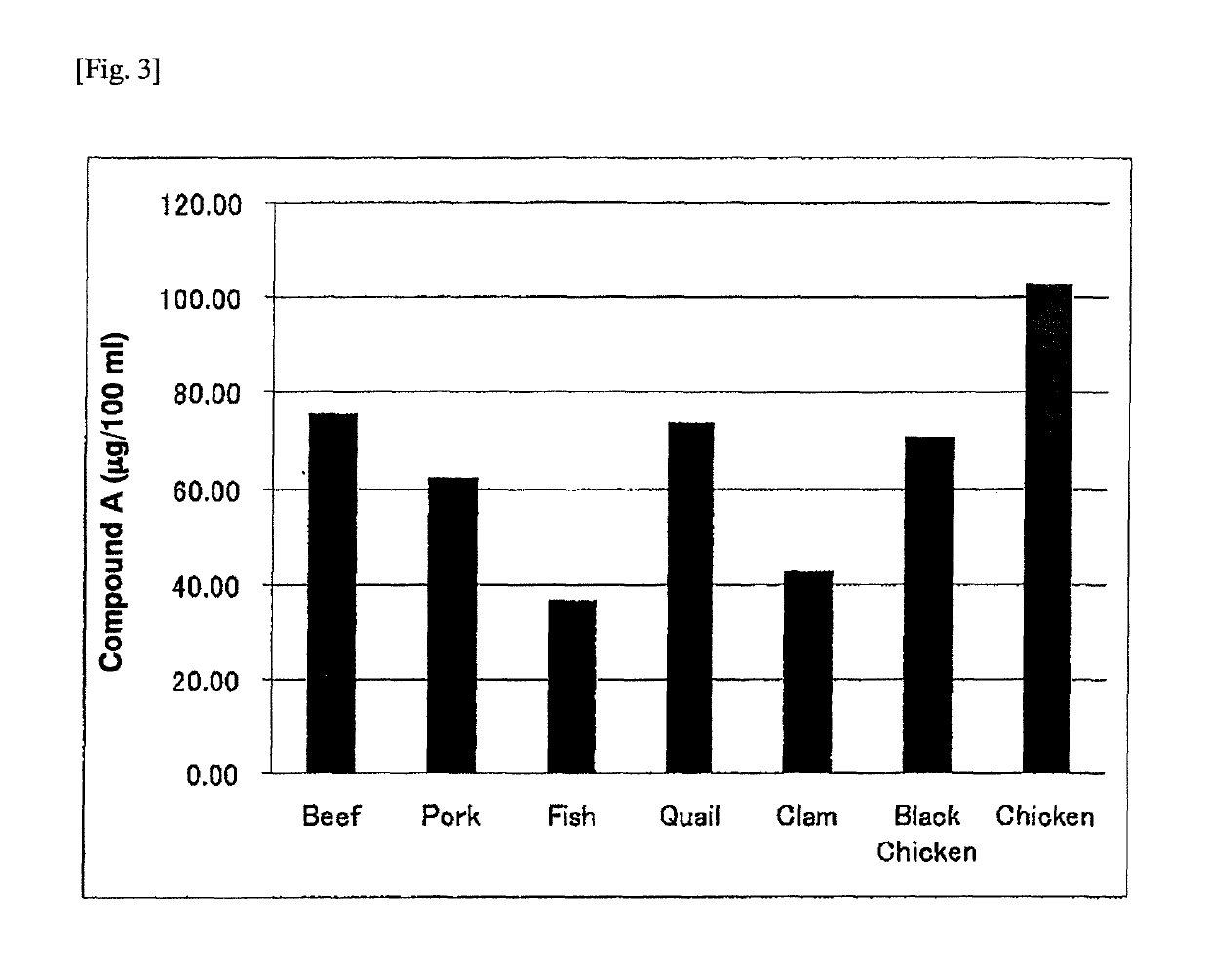
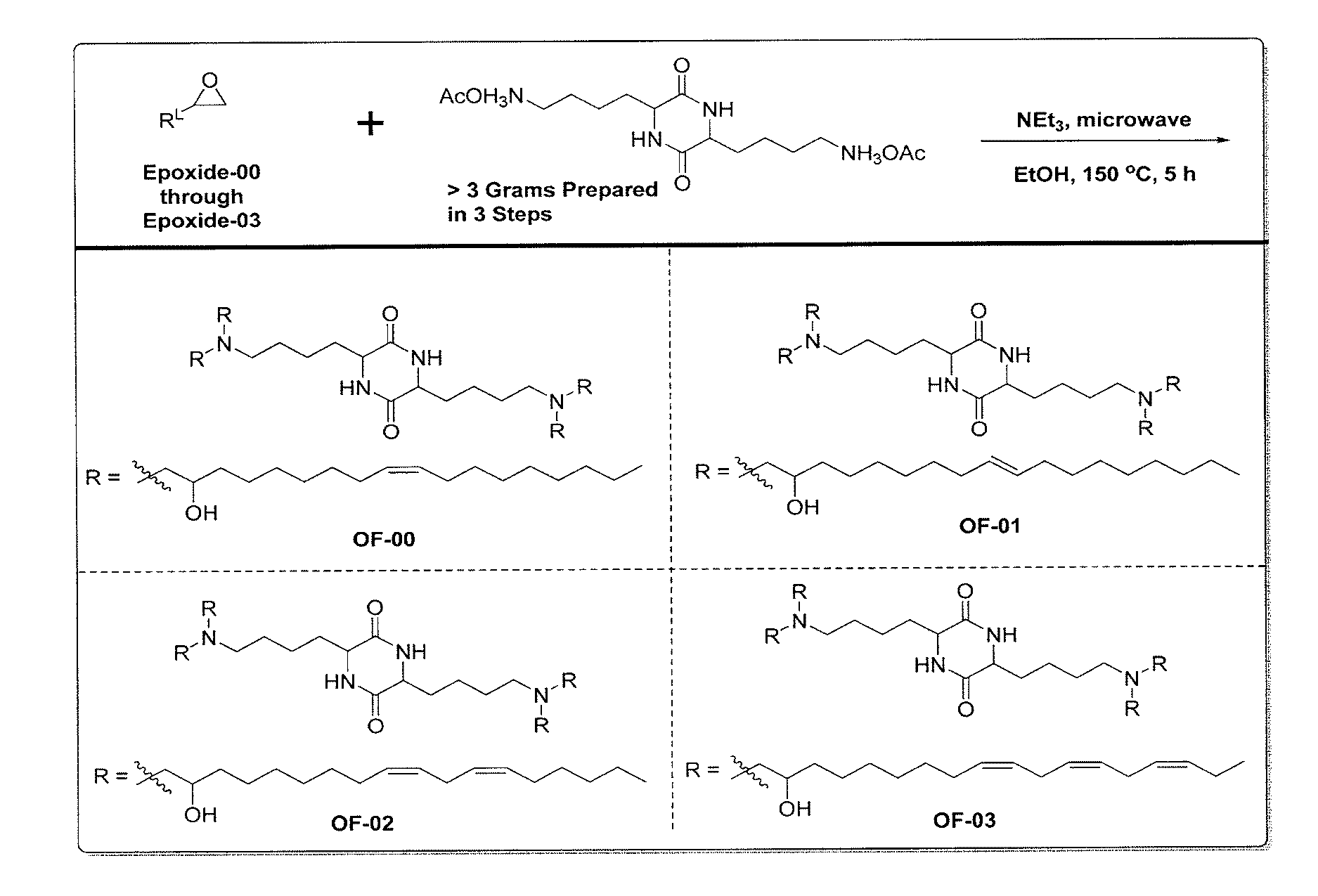
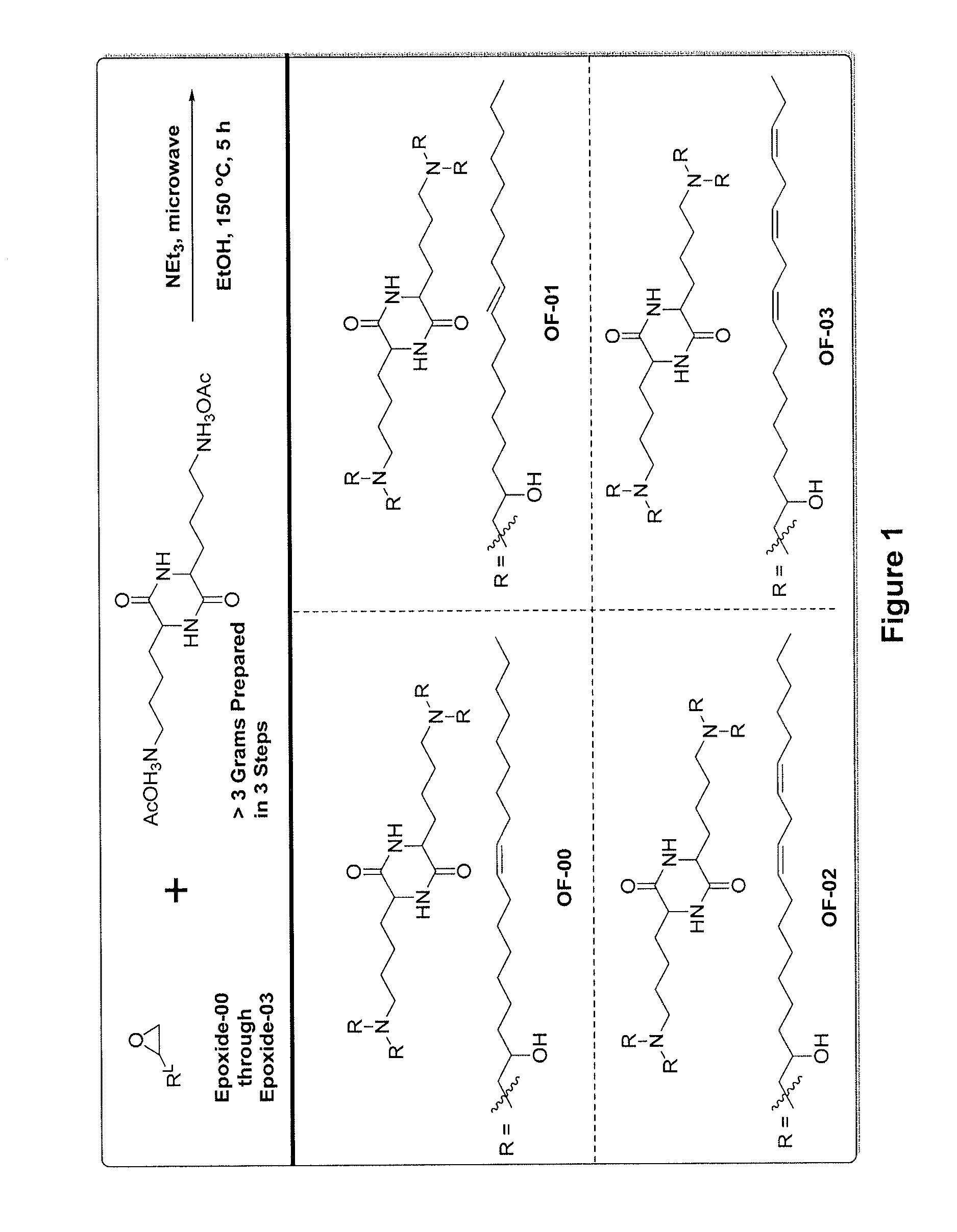
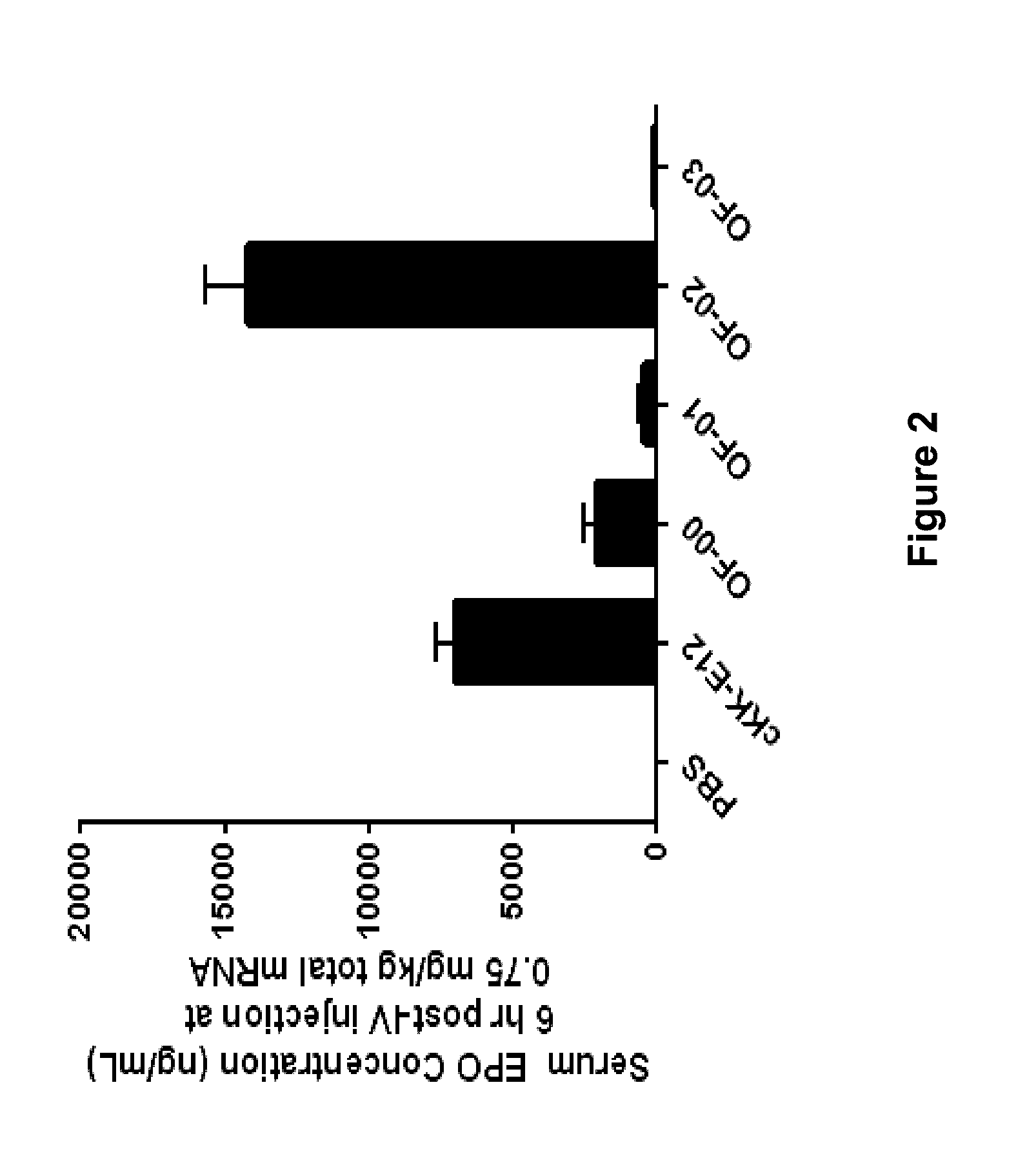
Provided herein are compounds of Formula (I), and salts thereof, wherein each instance of RL is independently optionally substituted C6-C40 alkenyl. Further provided are compositions comprising a compound of Formula (I) and an agent. Further provided are methods and kits using the compositions for delivering an agent to a subject or cell and for treating and / or preventing a range of diseases. Further provided are methods of preparing compounds of Formula (I) and precursors thereof.
Owner:MASSACHUSETTS INST OF TECH
Alkenyl substituted 2,5-piperazinediones, compositions, and uses thereof
ActiveUS10201618B2Increase exposureImprove concentrationOrganic active ingredientsNervous disorderDiseasePiperazinedione
Provided herein are compounds of Formula (I), and salts thereof, wherein each instance of RL is independently optionally substituted C6-C40 alkenyl. Further provided are compositions comprising a compound of Formula (I) and an agent. Further provided are methods and kits using the compositions for delivering an agent to a subject or cell and for treating and / or preventing a range of diseases. Further provided are methods of preparing compounds of Formula (I) and precursors thereof.
Owner:MASSACHUSETTS INST OF TECH
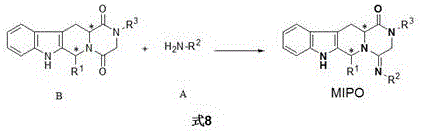
Synthesis and purpose of amidine compound containing two chiral centers
The invention discloses a synthesis method of 1-methyl-5--methylimino-piperazin-2-ketone derivative compound with the general formula being MIPO and containing two chiral centers, and application of the compound. The target compound with the general formula being MIPO is obtained from a compound of H2NR<2> (ammonia or substituted ammonia) with the general formula being A and a compound (1-piperazin-1,4-diketone derivative compound) with the general formula being B through reaction, wherein substitutes of R1, R2 and R3 in the general formulas A and B are identical to those in the general formula MIPO. The compound with the general formula being MIPO can be used as an effective ingredient of a sterilizing agent.
Owner:嘉兴慧泉生物科技有限公司
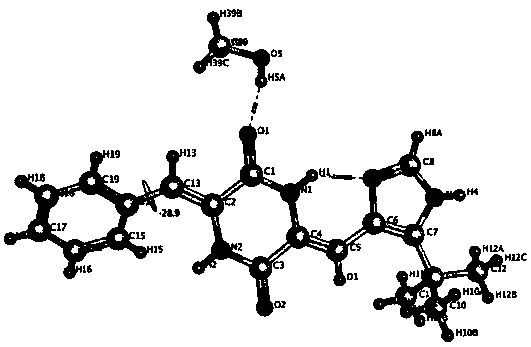
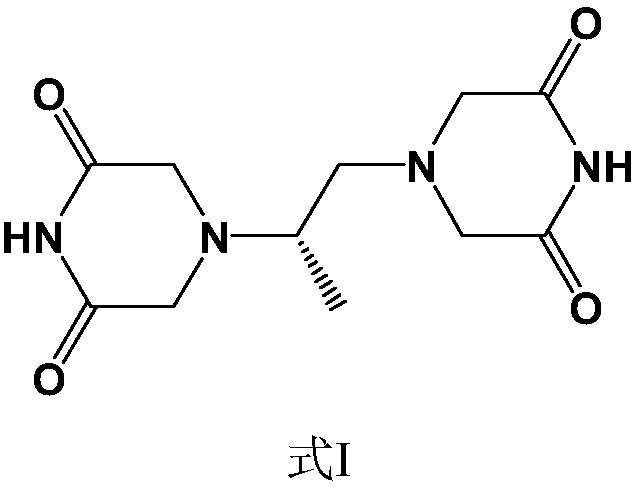
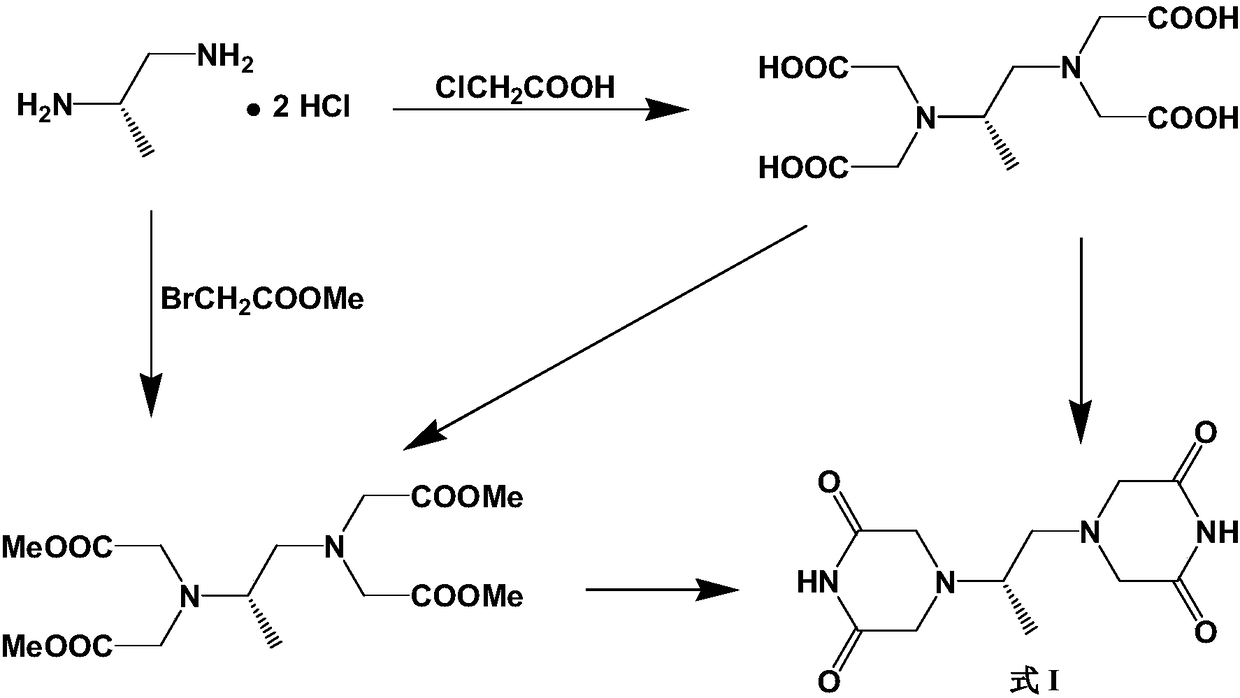
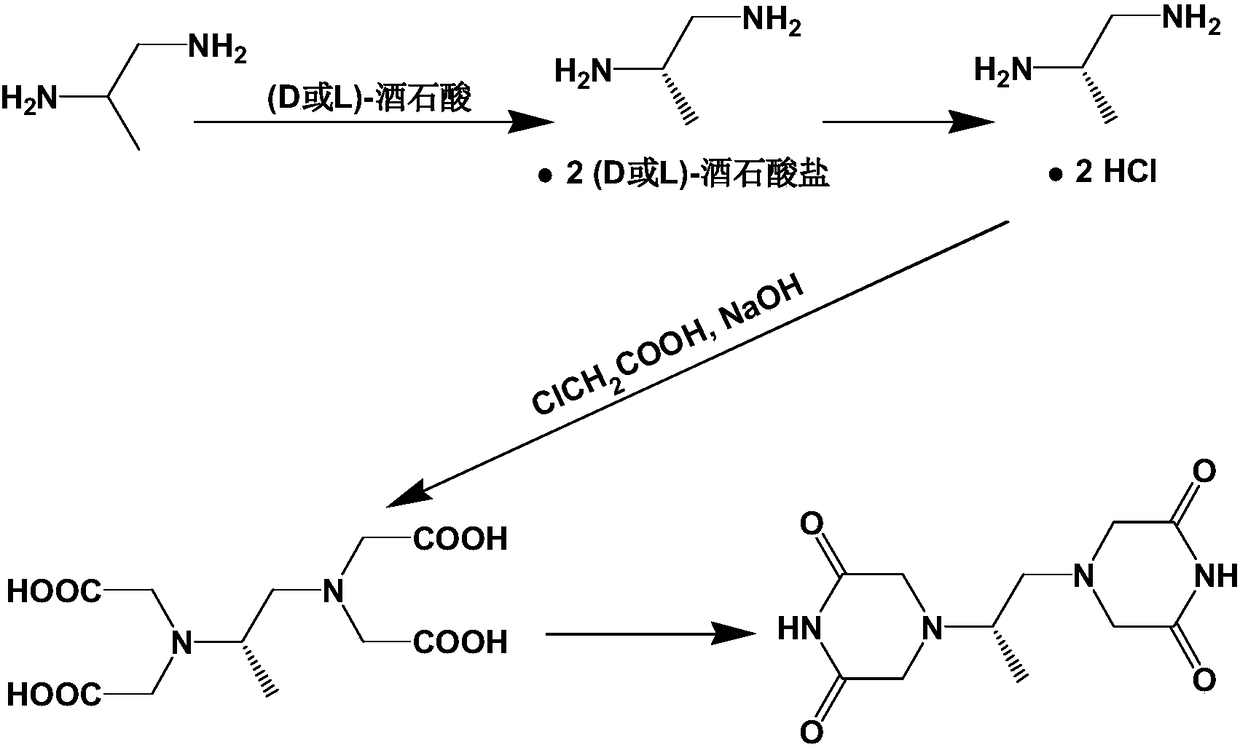
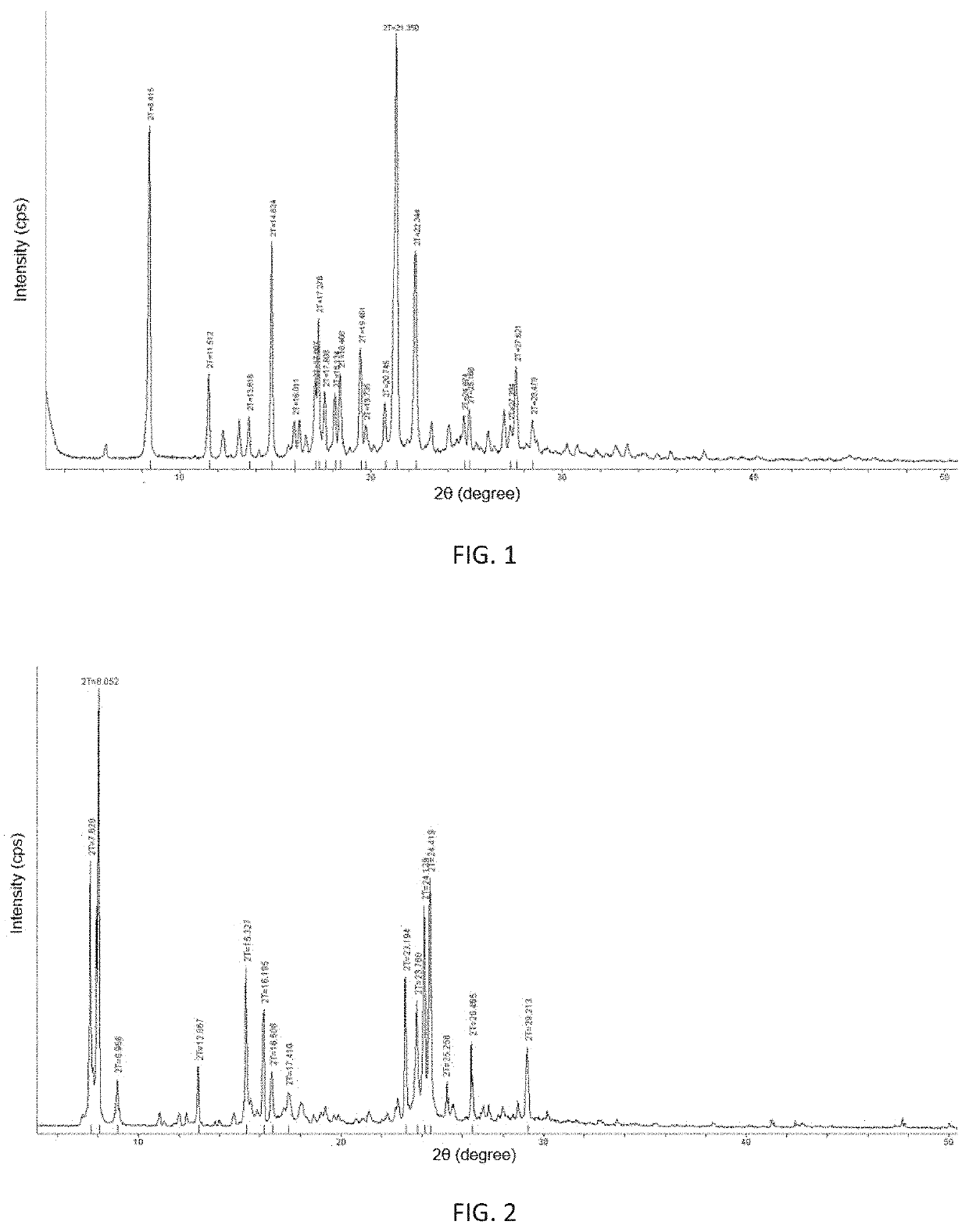
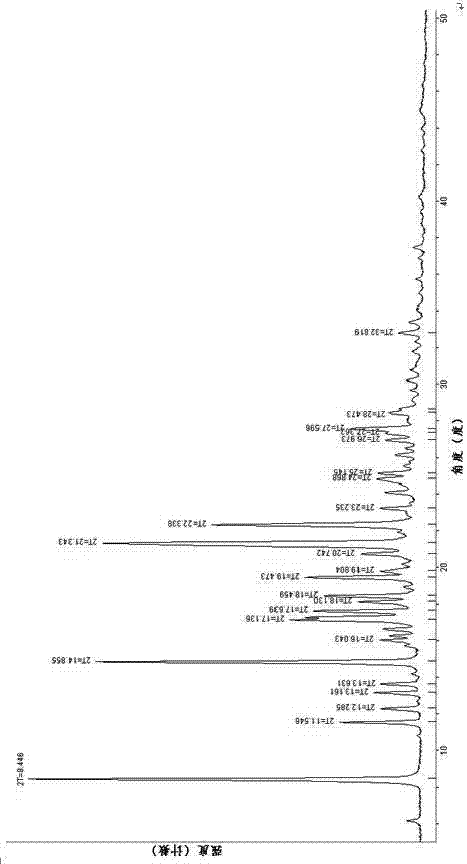
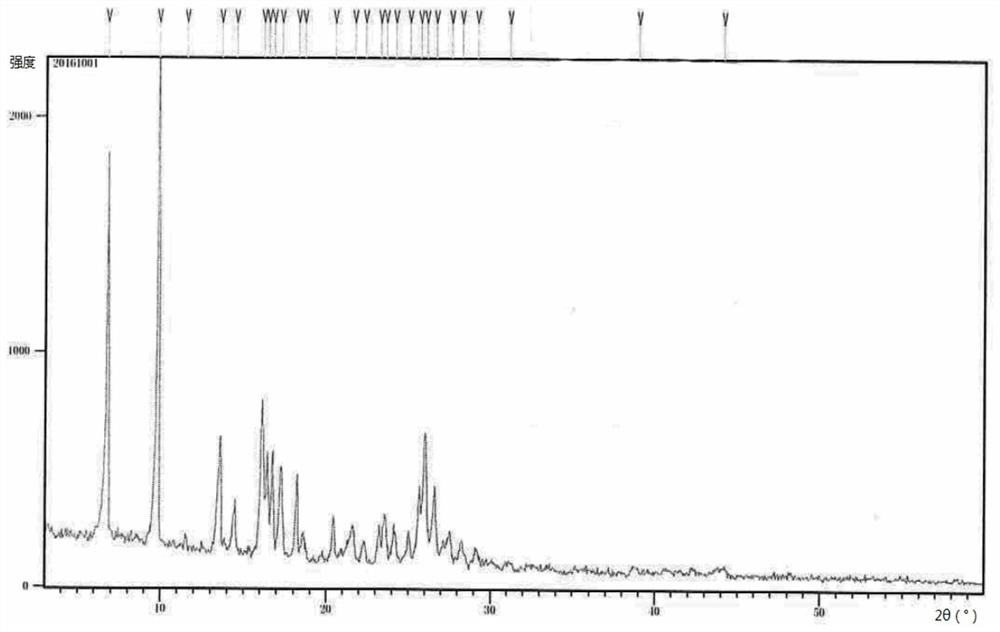
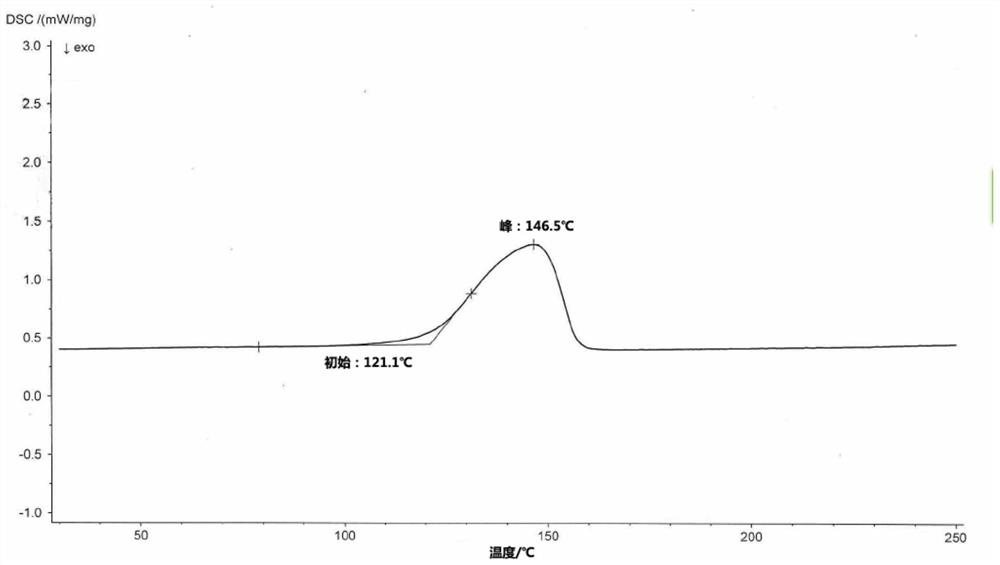
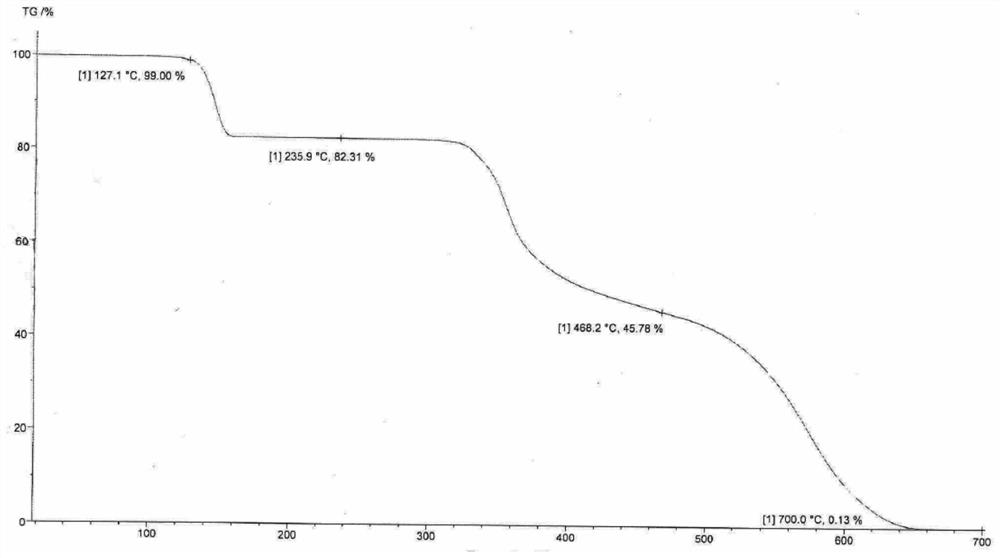
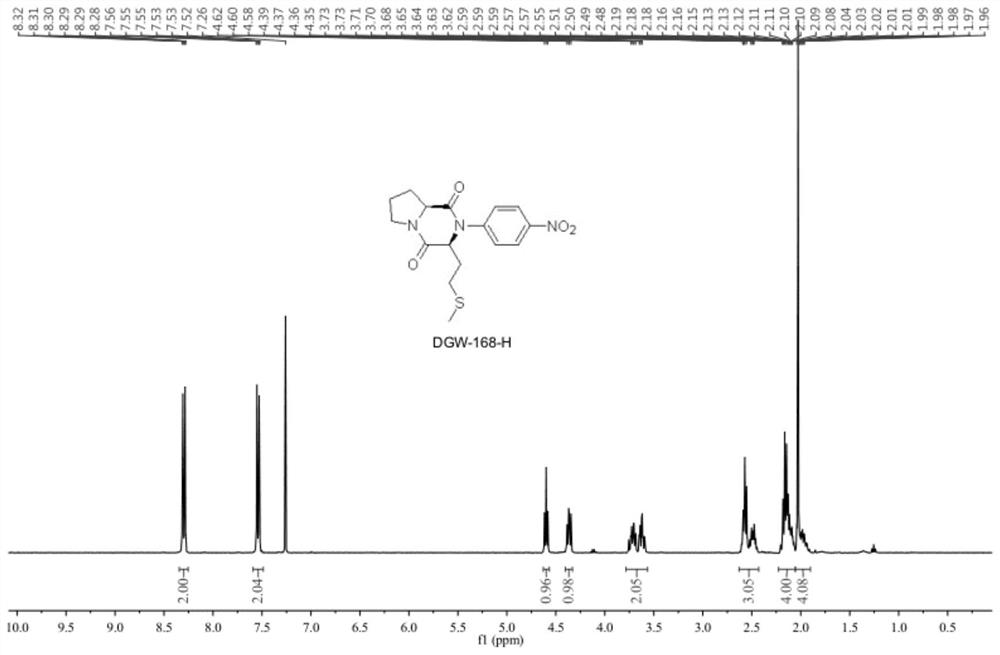
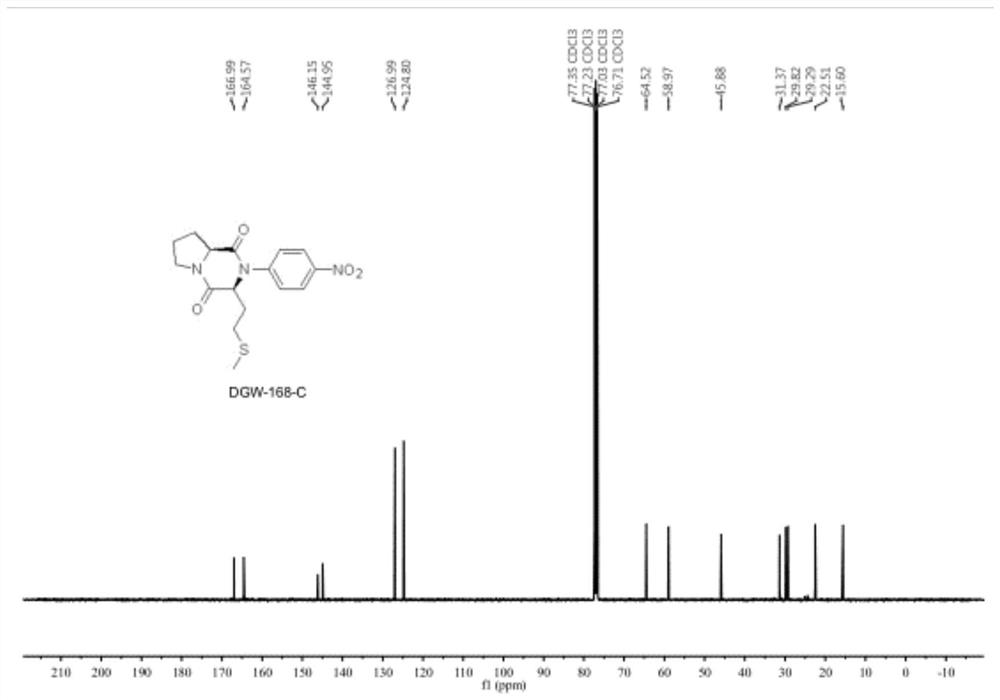
Crystalline forms of (3R, 6R)-3-(2,3-dihydro-1H-inden-2-yl)-1-[(1R)-1-(2,6-dimethyl-3-pyridinyl)-2-(4-morpholinyl)-2-oxoethyl]-6-[(1S)-1-methylpropyl]-2,5-piperazinedione
The present invention relates to crystalline forms of (3R,6R)-3-(2,3-dihydro-1H-inden-2-yl)-1-[(1R)-1-(2,6-dimethyl-3-pyridinyl)-2-(4-morpholinyl)-2-oxoethyl]-6-[(1S)-1-methylpropyl]-2,5-piperazinedione benzenesulfonate salt and pharmaceutical compositions thereof. Also disclosed are processes for the preparation the above compounds and methods for use thereof.
Owner:GLAXO GRP LTD
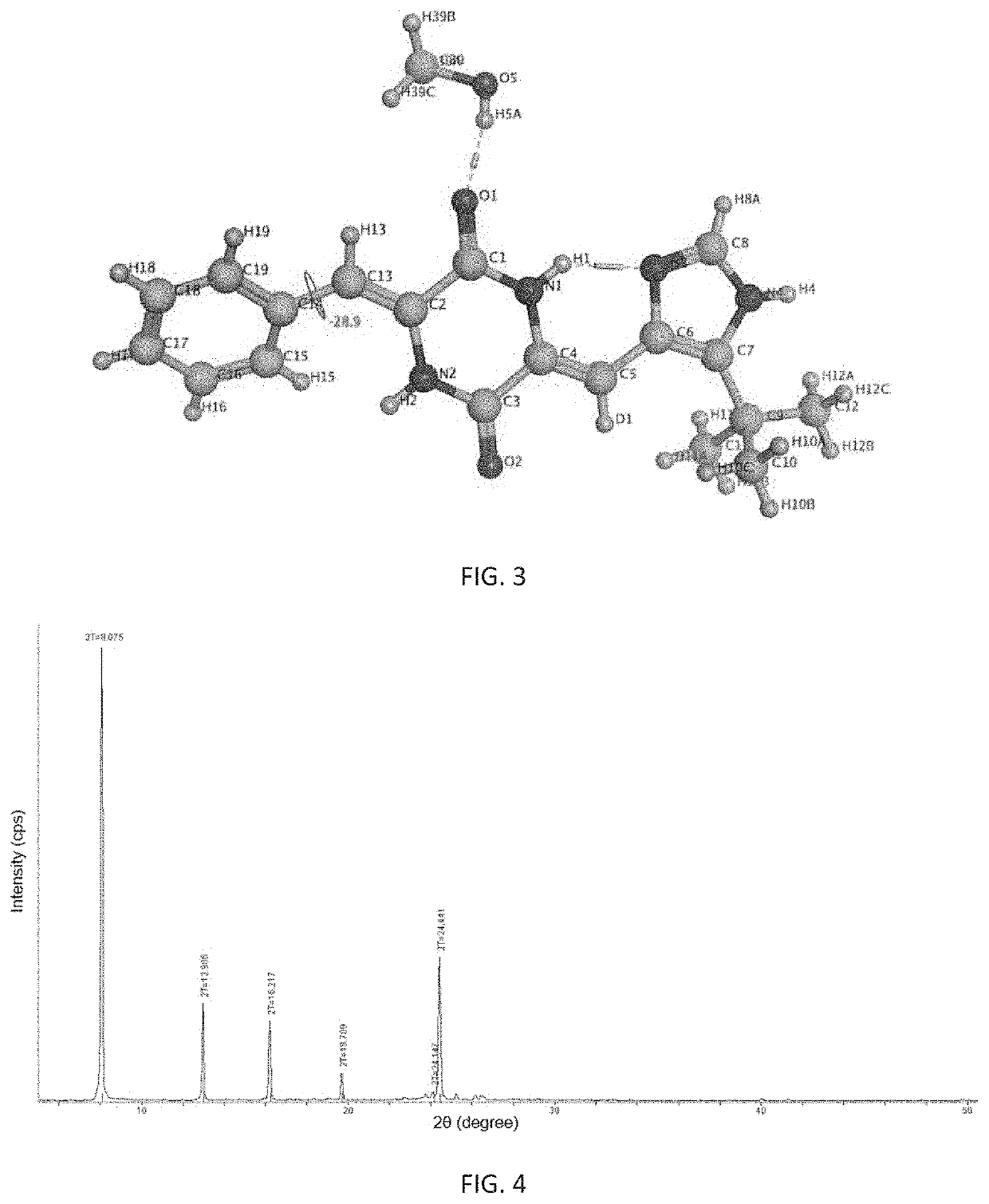
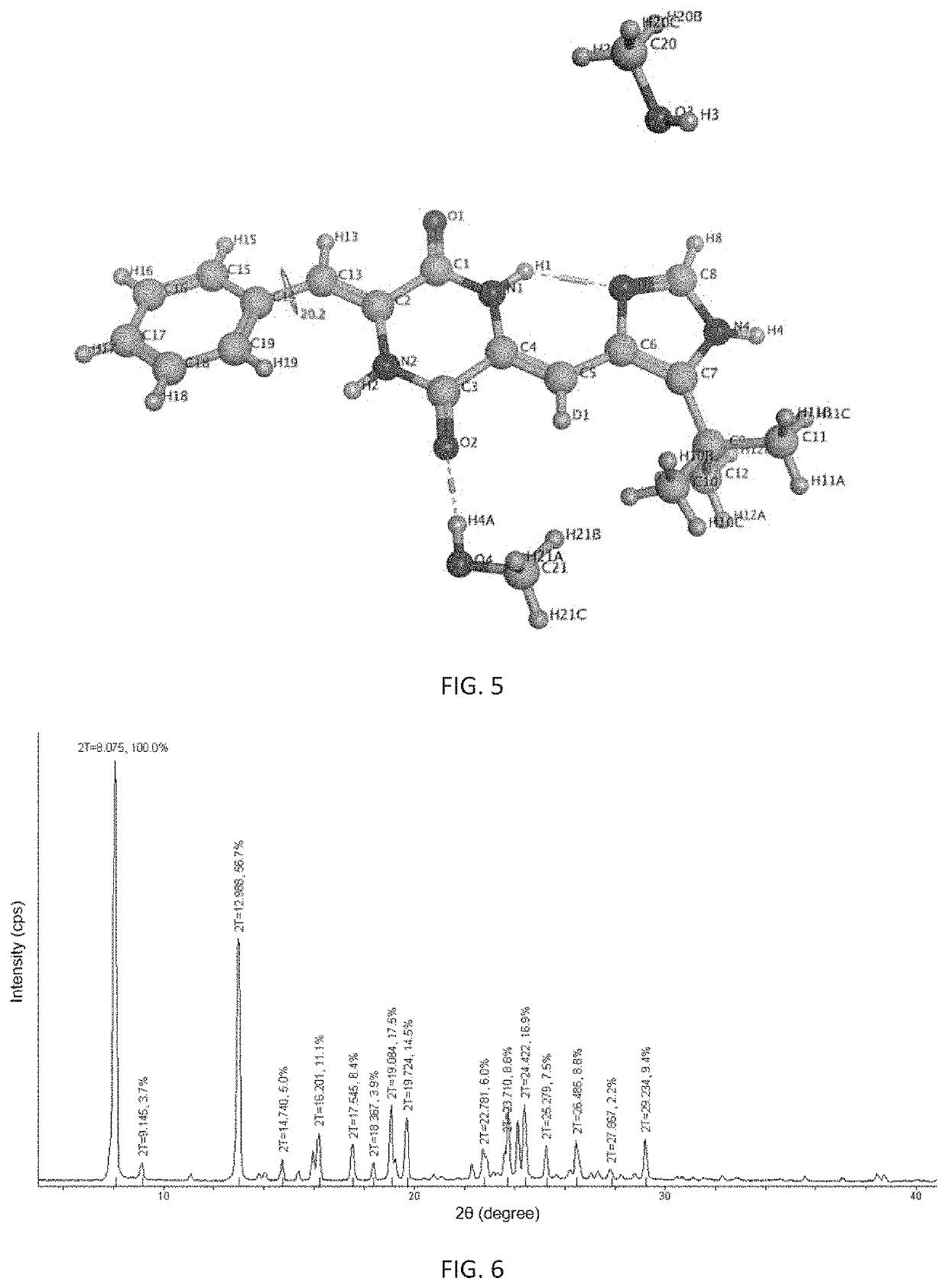
Polymorphism of deuterium-substituted plinabulin compound and preparation method and application of polymorphism
ActiveCN107778297AOrganic active ingredientsIsotope introduction to heterocyclic compoundsPiperazidinePhenyl group
The invention provides polymorphism of a deuterium-substituted plinabulin compound and a preparation method and application of the polymorphism, specifically polymorphism of (3Z,6Z)-3-benzylidene-6-((5-tert-butyl-1H-imidazole-4-yl) deuterium-substituted methylene)piperazidine-2,5-diketone and preparation method and application of the polymorphism. Based on (3Z,6Z)-3-benzylidene-6-((5-tert-butyl-1H-imidazole-4-yl) deuterium-substituted methylene)piperazidine-2,5-diketone, the polymorphism of (3Z,6Z)-3-benzylidene-6-((5-tert-butyl-1H-imidazole-4-yl) deuterium-substituted methylene)piperazidine-2,5-diketone is researched, and alpha, beta, gamma, delta, and epsilon crystal forms are found. The polymorphism and preparation method and application are of great significance on research of pharmaceutical polymorphism and screening of a crystal form with excellent pharmaceutical effect, and alpha, beta, gamma, delta, and epsilon crystal forms are fully developed and applied with respect to curative effect and dosage form selection of antitumor drugs.
Owner:SHENZHEN HUAHONG MARINE BIOMEDICINE CO LTD
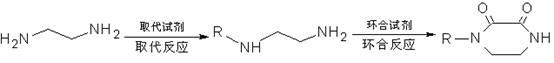
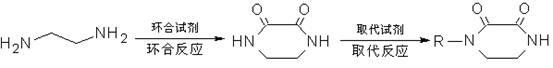
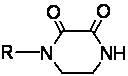
Method for synthesizing N-monosubstituted piperazine-2,3-dione
The invention discloses a method for synthesizing N-monosubstituted piperazine-2,3-dione. The method comprises the following steps of: performing cyclization reaction on a starting material of ethylenediamine by using a cyclizing reagent to obtain an intermediate product of piperazine-2,3-dione; and performing substitution reaction on the intermediate product serving as a raw material by using a substituting reagent to obtain the target product of N-monosubstituted piperazine-2,3-dione. In the reaction process, the intermediate product of piperazine-2,3-dione obtained in the first step can be not required to be separated and directly undergo the substitution reaction in the second step. The synthesis method has the advantages of high yield, mild conditions, simple equipment, simple posttreatment, stable product quality, high efficiency, low energy consumption, environment friendliness and the like, and has great industrialization prospect and promotion value.
Owner:山西新天源药业有限公司
Preparation method of piperazine diketone type compound
ActiveCN108250094AHigh yieldHigh purityOrganic compound preparationOrganic chemistry methodsDiketoneAlcohol
The invention discloses a method for preparing a piperazine diketone type compound and specifically relates to a method for preparing a compound shown as a formula (I) by cyclization after substitution. The method takes (S)-1,2-diaminopropane tartrate, haloacetic acid and low-carbon alcohol as raw materials to prepare the compound shown as the formula (I). Compared with the prior art, a pluralityof disadvantages are improved and overcome by the preparation method; the preparation method has the advantages of simple steps, low cost, convenience for post-treatment, cleanness, high yield and purity, low toxicity and less pollution, and industrial production is facilitated.
Owner:JIANGSU AOSAIKANG PHARMA CO LTD
Modified oligochitosan for aquaculture and preparation method thereof
The invention discloses modified oligochitosan for aquaculture and a preparation method thereof. The preparation method includes: adopting piperazinedione acid to graft C2-NH2 on oligochitosan high in antibacterial performance; grafting quaternary ammonium salt with C6-OH on oligochitosan modified by piperazinedione acid to prepare and synthesize the modified oligochitosan. The preparation method is simple to operate and safe in operation environment, and both antibacterial performance and dissolubility of an oligochitosan derivative obtained are improved obviously.
Owner:宁波科瑞特动物药业有限公司
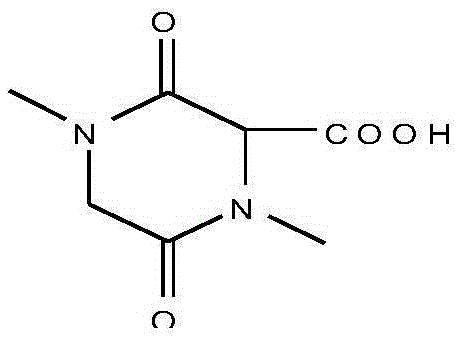
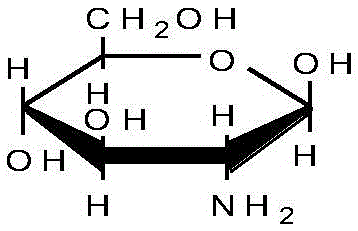
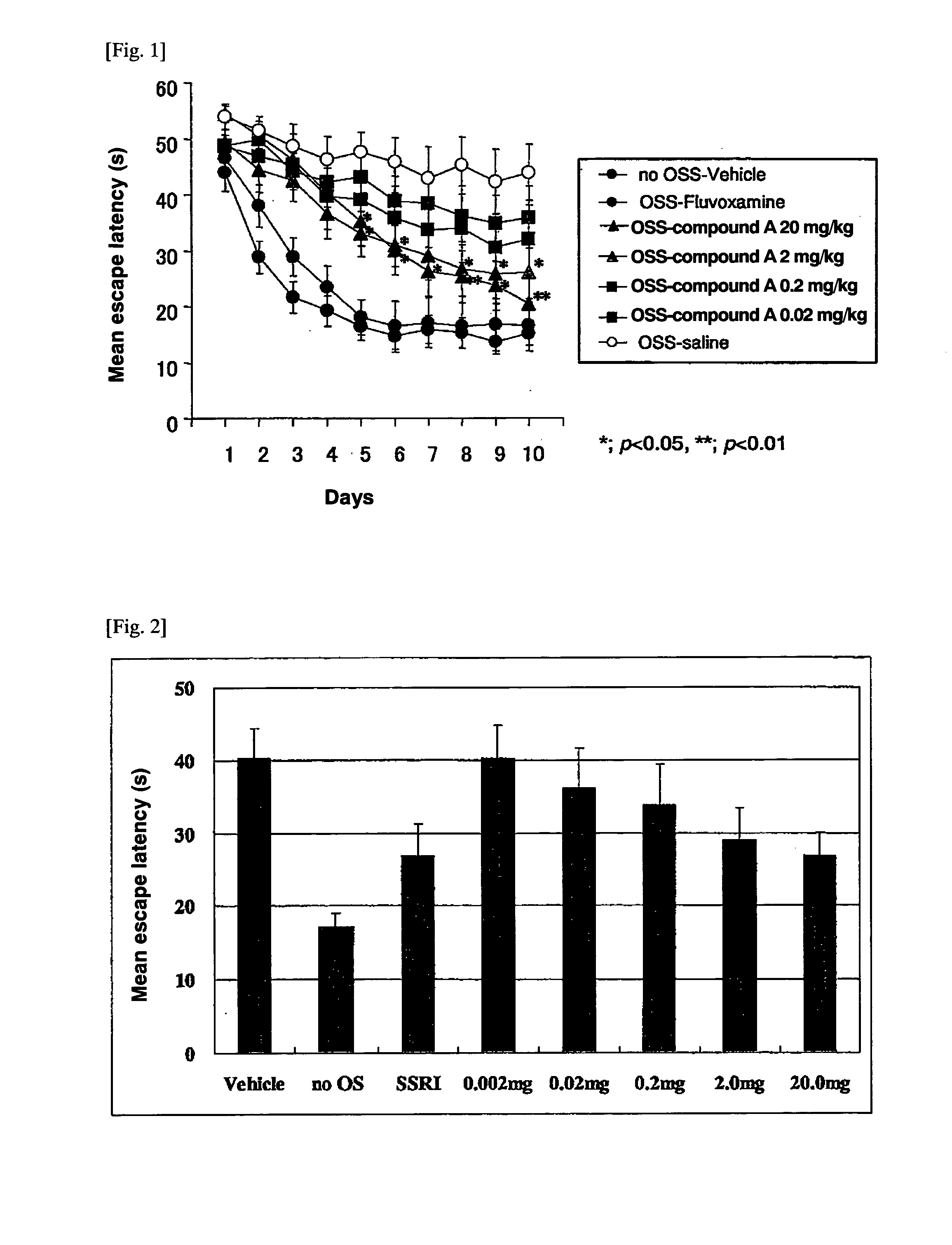
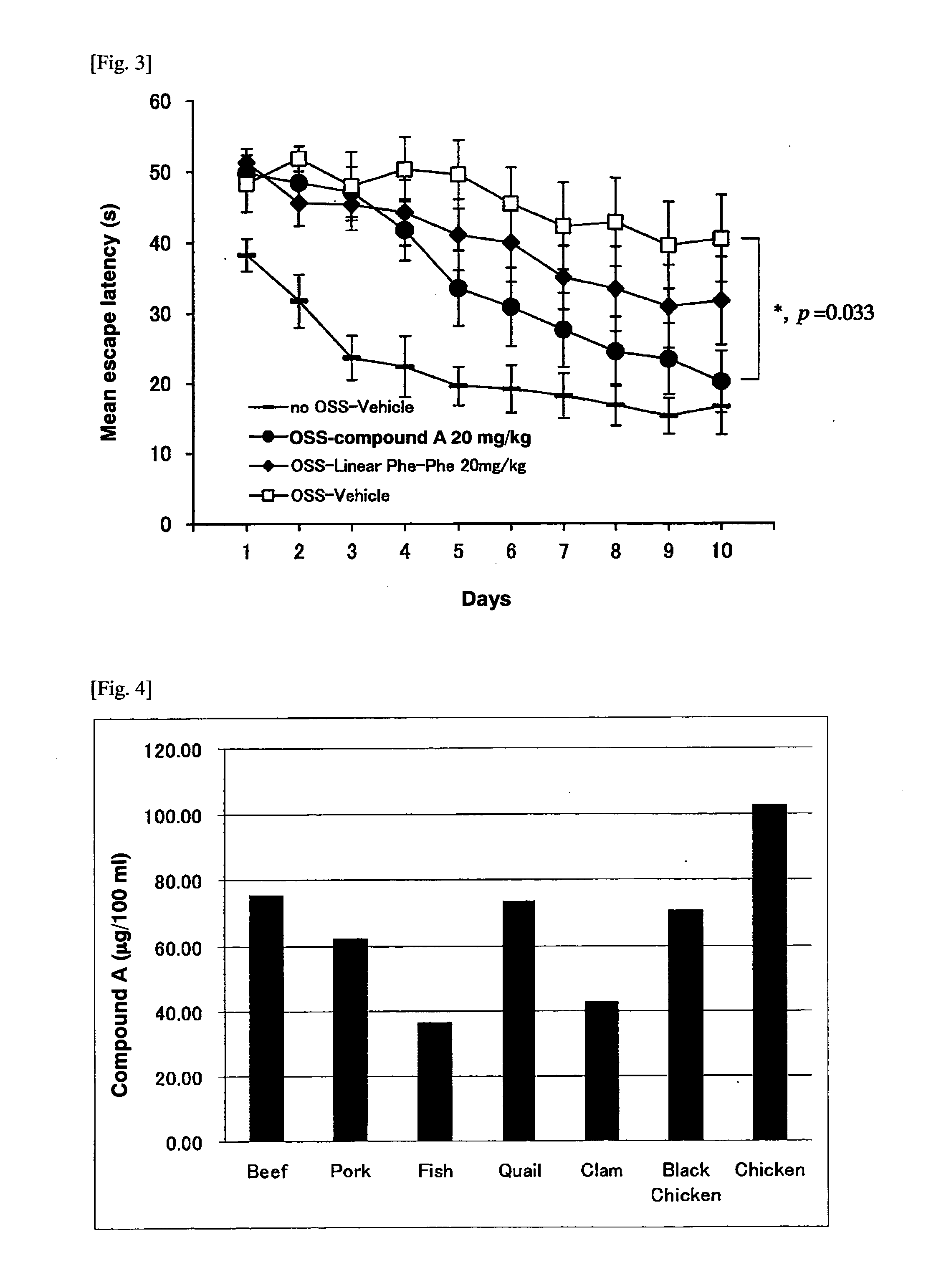
Acidic extracts and beverages containing 2,5-piperazinedione,3,6-bis(phenylmethyl)-(3s,6s)-
ActiveUS20120282387A1Good effectSafe without side effectNervous disorderSolid waste disposalPiperazinedioneSedimentation
The present invention aims to provide extracts available for use in acidic beverage production and containing 2,5-piperazinedione,3,6-bis(phenylmethyl)-,(3S,6S)-, which is a useful substance with an improving effect on learning motivation. When an acid treatment step is included in the production of extracts containing 2,5-piperazinedione,3,6-bis(phenylmethyl)-,(3S,6S)-, it is possible to obtain acidic extracts which cause no sedimentation even when added to beverages. The extracts of the present invention can be added to beverages and so on without impairing the taste inherent to foods and beverages, and can be used for production of acidic beverages preferred by most consumers.
Owner:SUNTORY BEVERAGE & FOOD ASIA PTE LTD +1
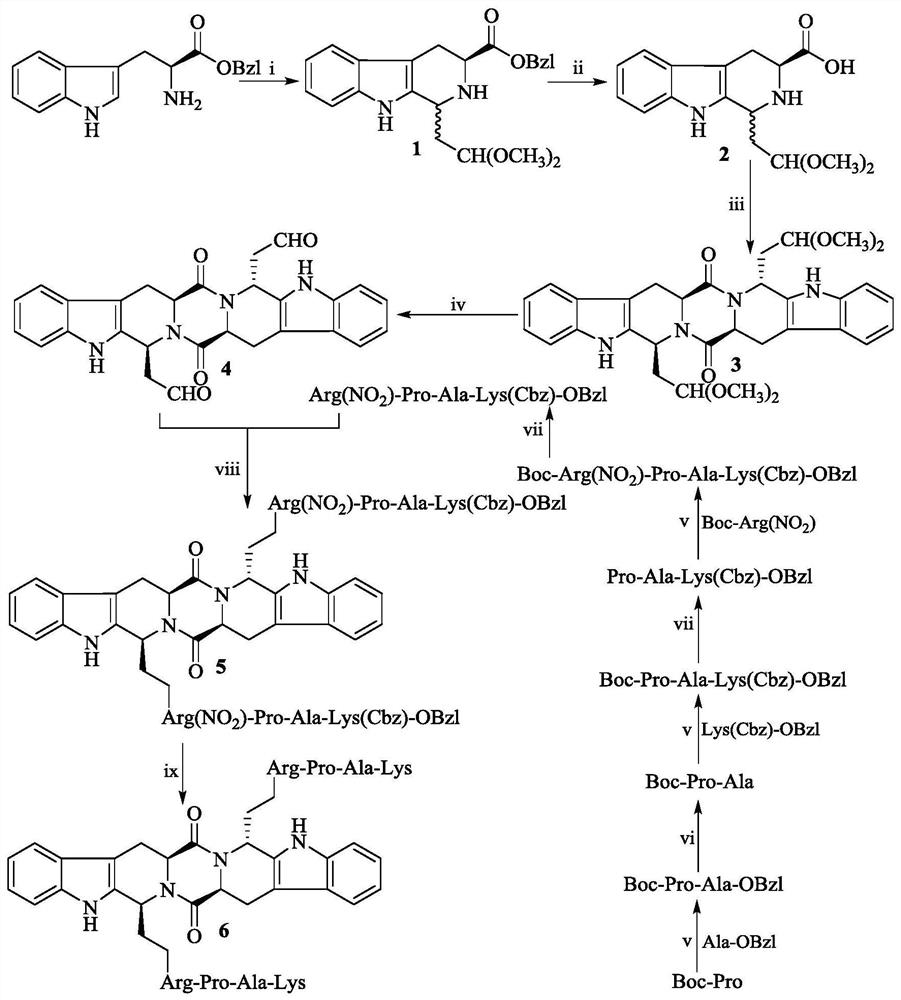
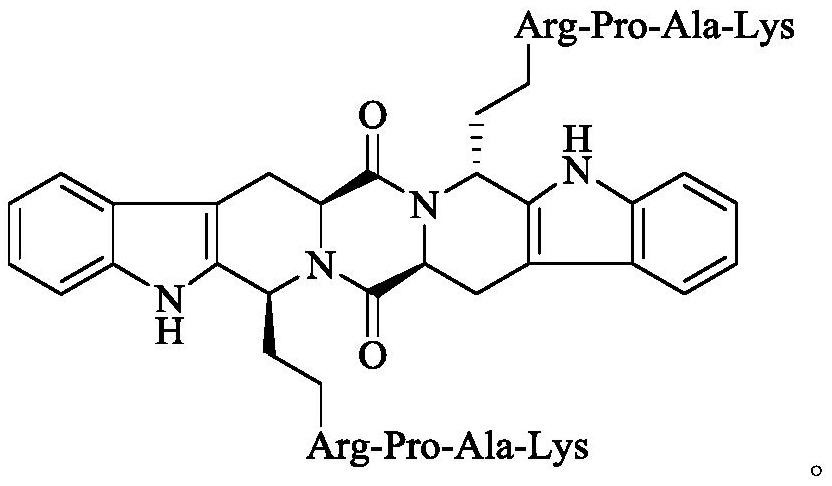
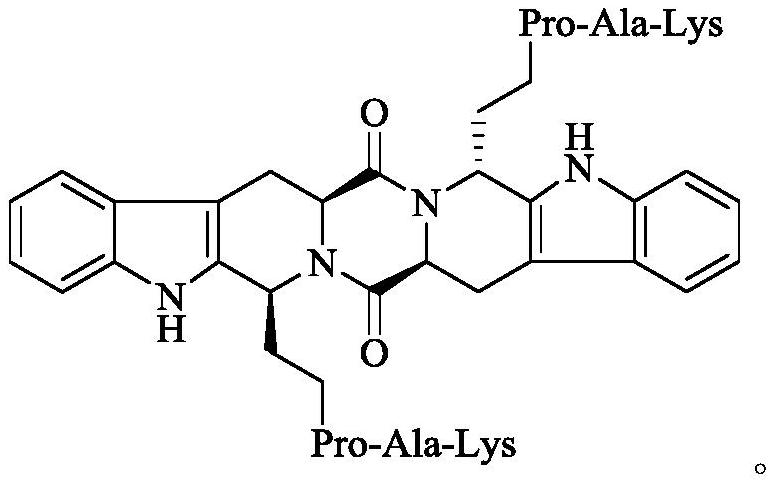
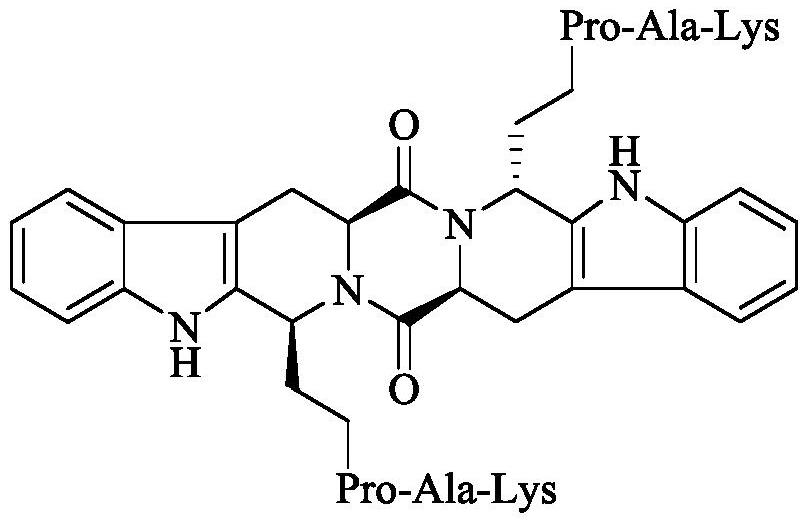
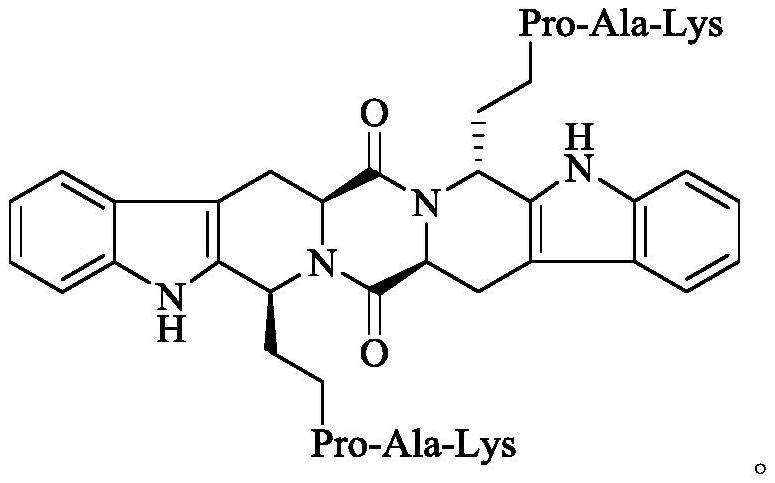
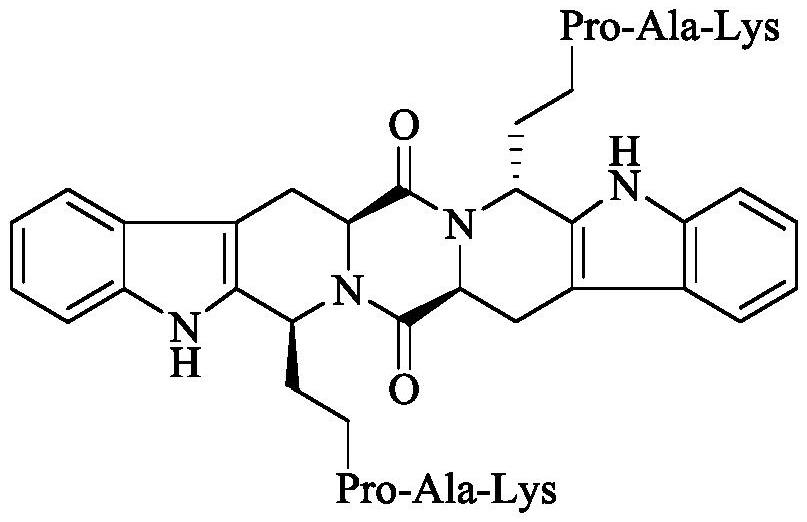
Ethyl RPAK modified biscarbolopiperazinedione as well as preparation, activity and application thereof
The invention discloses (2 'S, 5' S) tetrahydropyrazine [1 ', 2': 1, 6] bis [1S, 1R (1-ethyl-Arg-Pro-Ala-Lys)-2, 3, 4, 9-tetrahydro-1H-pyridine [3, 4-b]oindole] 1 ', 4'-diketone of the following formula, a preparation method thereof, thrombolytic activity thereof and antithrombotic activity thereof, and a characteristic that the compound is still effective 24 hours after onset of ischemic stroke.Therefore, the invention discloses an application of the compound in preparation of thrombolytic drugs, an application of the compound in preparation of antithrombotic drugs, an application of the compound in preparation of drugs which are still effective 24 hours after onset of ischemic stroke, and an application of the compound in preparation of drugs with triple effects of thrombolysis, antithrombosis and being still effective 24 hours after onset of ischemic stroke.
Owner:CAPITAL UNIVERSITY OF MEDICAL SCIENCES
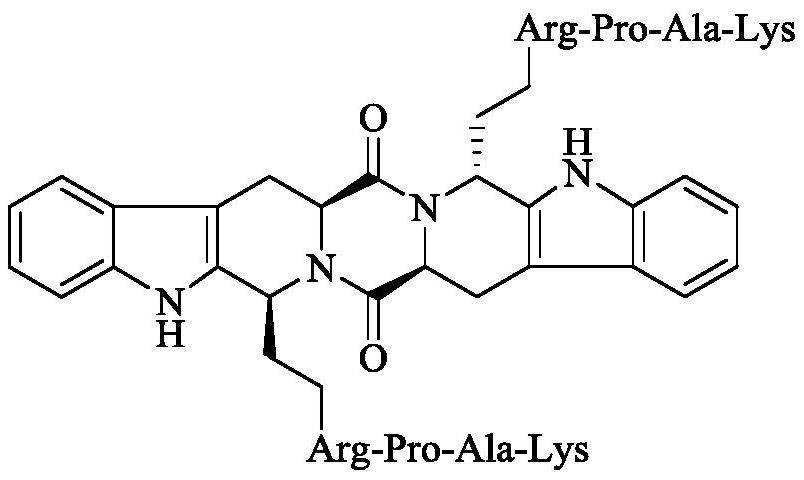
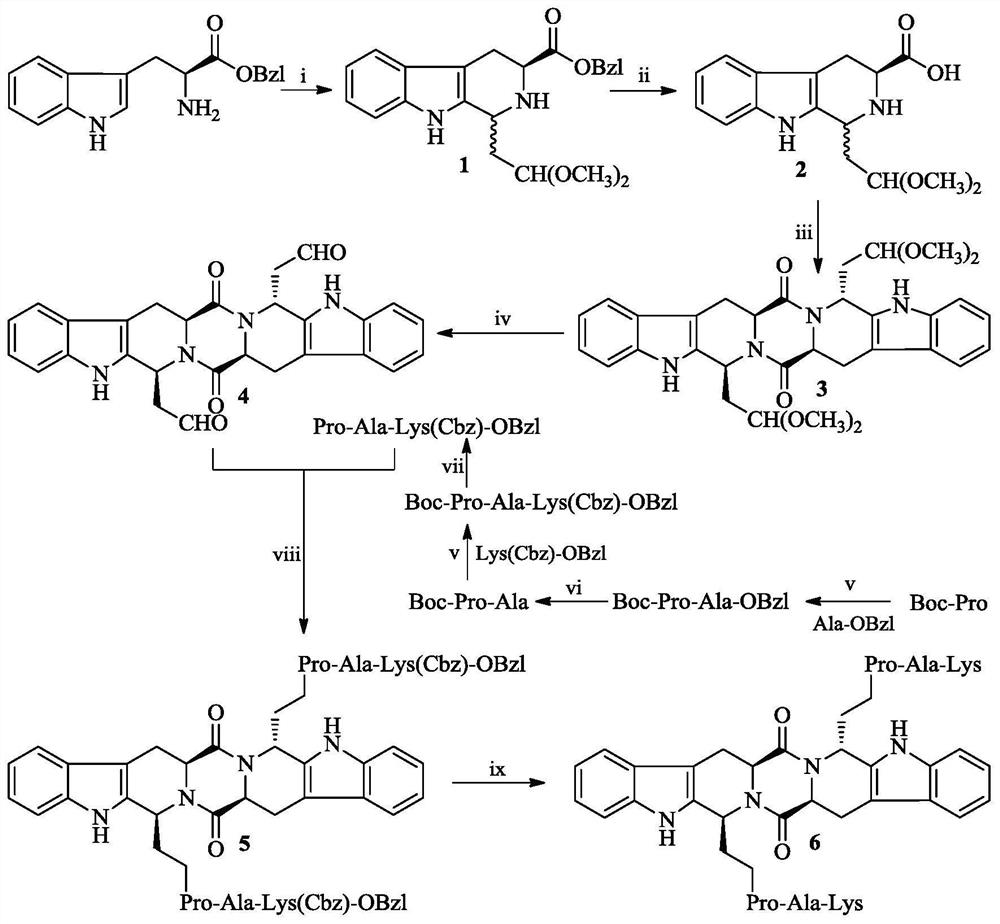
Ethyl PAK modified biscarboline piperazine diketone and preparation, activity and application thereof
The invention discloses (2'S,5'S)-tetrahydropyrazine[1',2':1,6]bis[1S,1R-(1-ethyl-Pro-Ala-Lys)-2,3,4,9-tetrahydro-1H-pyridine[3,4-b]indole]-1',4'-diketone as shown in a formula in the specification, apreparation method of the compound, the thrombolytic activity of the compound, the antithrombotic activity of the compound, and the characteristic that the compound is still effective for ischemic stroke attacking for 24 hours. Therefore, the invention discloses an application of the compound in the preparation of thrombolytic drugs, an application of the compound in the preparation of antithrombotic drugs, an application of the compound in the preparation of drugs which are still effective for ischemic stroke attacking for 24 hours, and an application of the compound in the preparation of drugs which have triple effects of dissolving thrombus, resisting thrombus and being still effective for ischemic stroke attacking for 24 hours.
Owner:CAPITAL UNIVERSITY OF MEDICAL SCIENCES
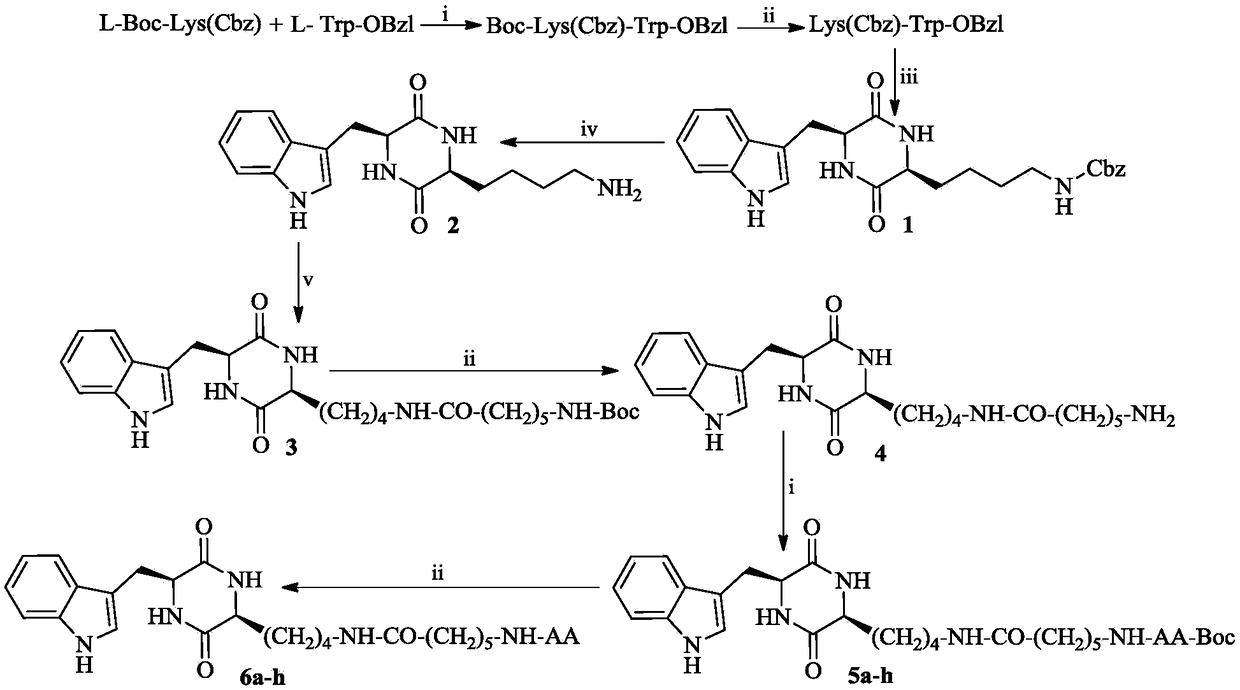
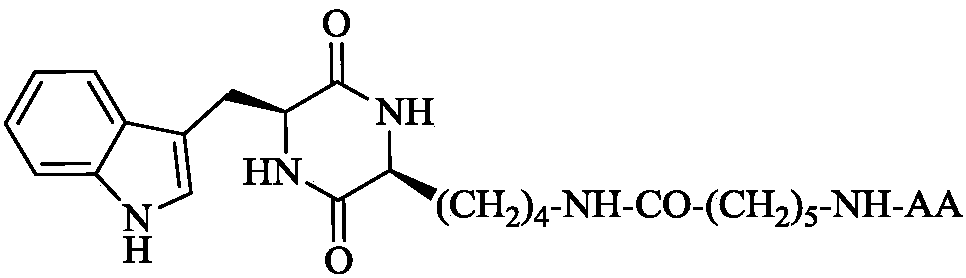
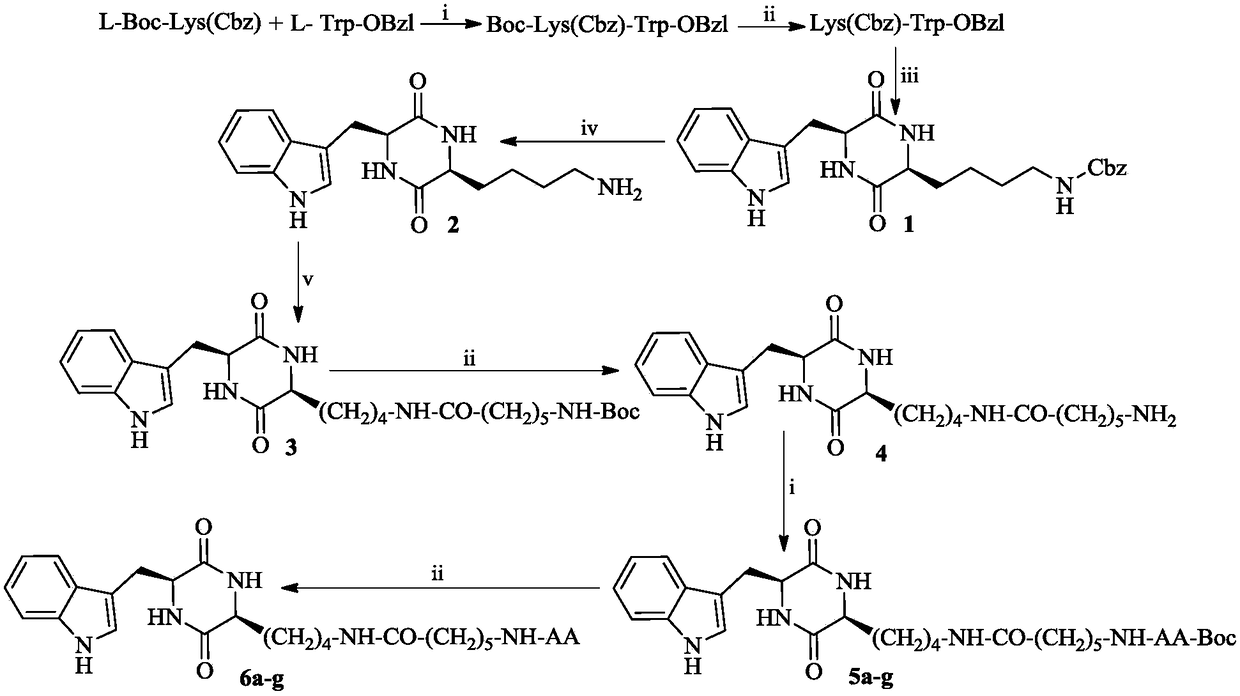
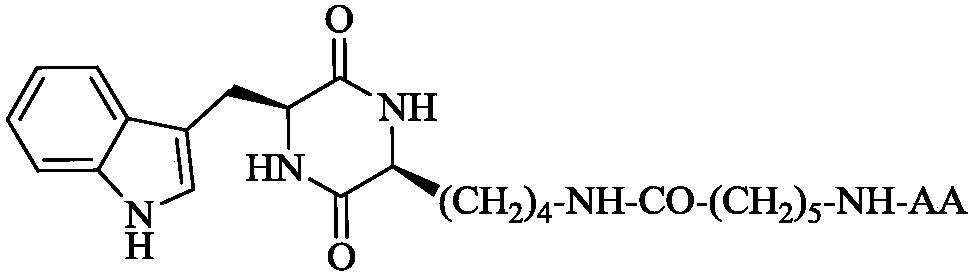
3S-indoleethyl-6S-polar amino acid modified piperazine-2,5-dione, and synthesis, activity and application thereof
The invention discloses (3S,6S)-3-(AA-amino-n-hexanoylamino-n-butyl)-6-(indole-3-ethyl)-piperazine-2,5-dione with a formula which is described in the specification (in the formula, AA is selected fromthe group consisting of L-Asp residues, L-Arg residues, L-Gln residues, L-Glu residues, L-Lys residues, L-Asn residues, L-Ser residues and L-Thr residues). The invention disclose a preparation method, antitumor activity, antitumor metastatic activity and anti-inflammatory activity of the (3S,6S)-3-(AA-amino-n-hexanoylamino-n-butyl)-6-(indole-3-ethyl)-piperazine-2,5-dione. The invention also discloses an application of the (3S,6S)-3-(AA-amino-n-hexanoylamino-n-butyl)-6-(indole-3-ethyl)-piperazine-2,5-dione in preparation of antitumor drugs, antitumor metastatic drugs and anti-inflammatory drugs.
Owner:CAPITAL UNIVERSITY OF MEDICAL SCIENCES
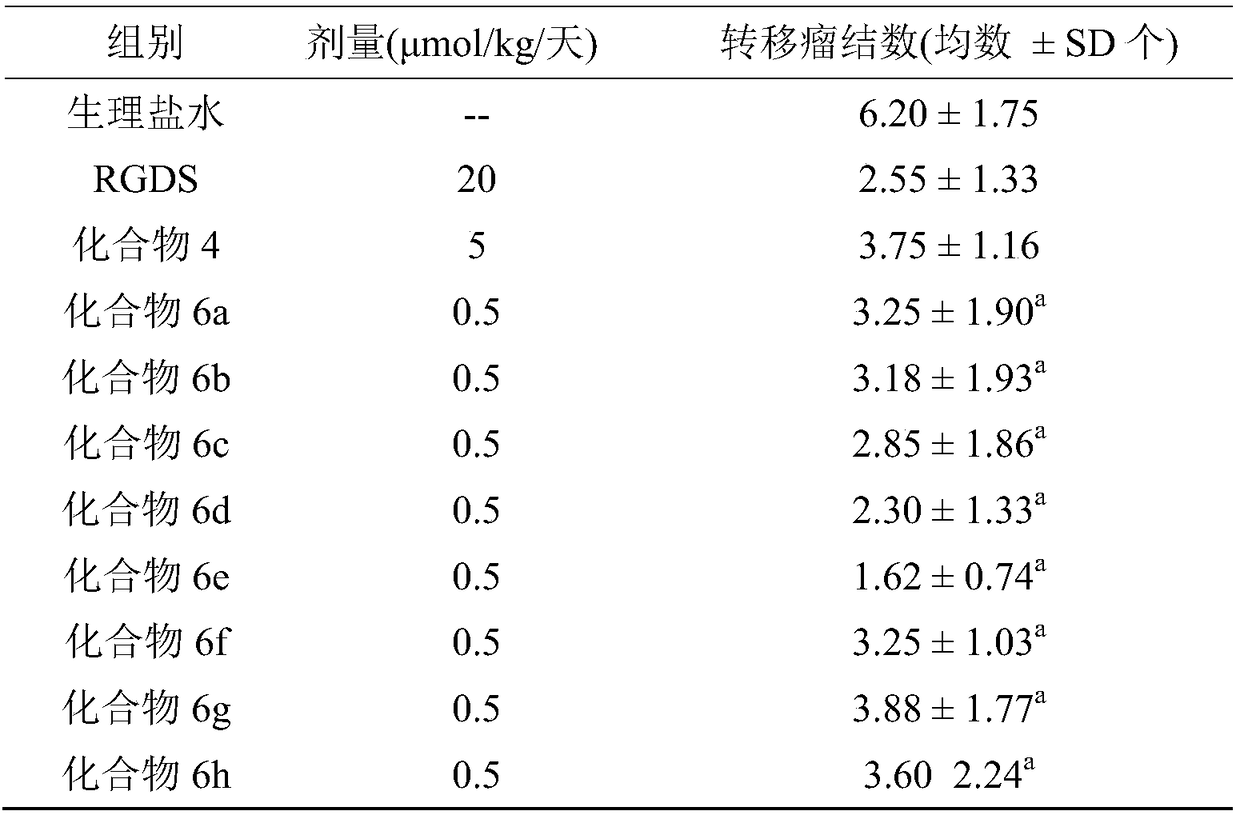
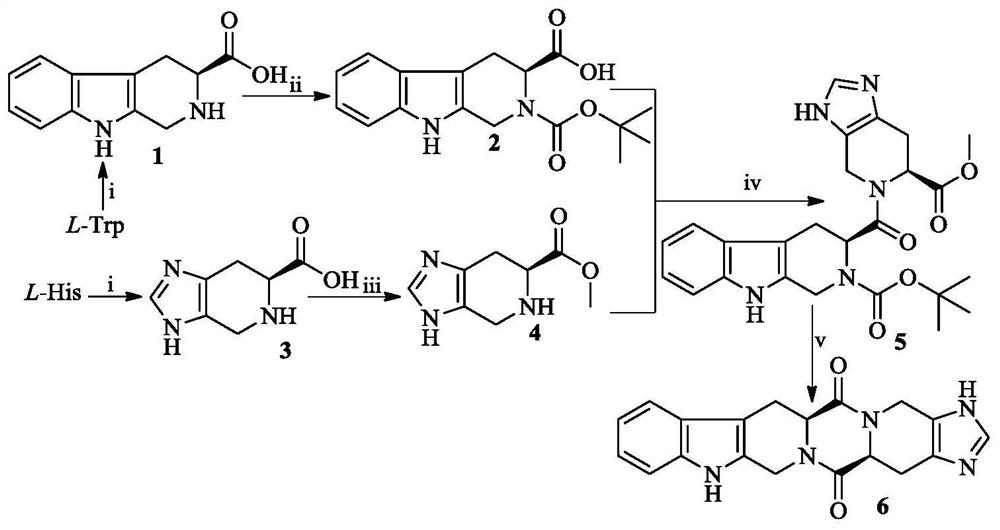
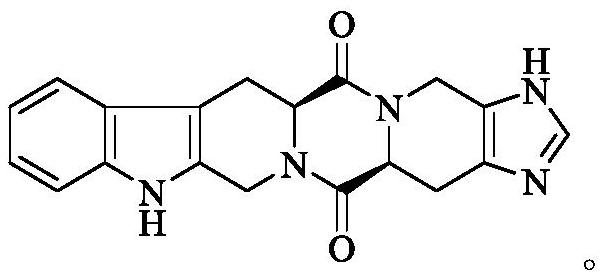
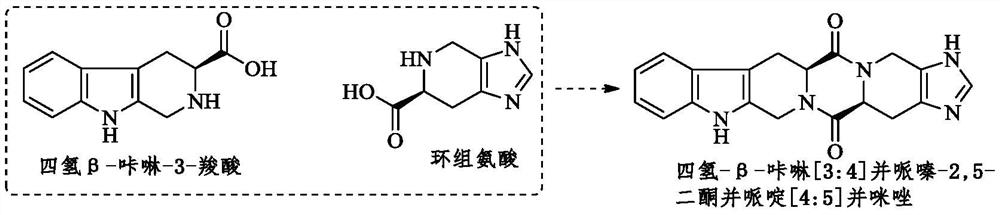
Hexacyclic piperazinedione compound and preparation, biological activity and application thereof
The invention discloses a hexacyclic piperazinedione compound as shown in the following formula: tetrahydro-beta-carboline [3: 4] piperazine-2, 5-diketopiperidine [4: 5] imidazole. The invention discloses a preparation method and application thereof. The compound not only has an anti-tumor proliferation effect, but also can inhibit migration and invasion of tumor cells, and also has an in-vivo anti-metastasis effect.
Owner:CAPITAL UNIVERSITY OF MEDICAL SCIENCES
Polycrystalline form of dehydrophenylahistin-like compound, and manufacturing and purification method and application thereof
ActiveUS20210002259A1Excellent stability and safetyTest stableOrganic active ingredientsAntimycoticsPharmaceutical SubstancesPhenyl group
A polycrystalline form of a dehydrophenylahistin-like compound, and a manufacturing and purification method and application thereof A (3Z,6Z)-3-benzylidene-6-[(5-tert-butyl-1H-imidazol-4-yl)deuteromethylene]piperazine-2,5-dione monohydrate crystal and a (3Z,6Z)-3-benzylidene-6-[(5-tert-butyl-1H-imidazol-4-yl)methylene]piperazine-2,5-dione monohydrate crystal are more competitive crystalline forms with stable quality. The manufacturing and purification method is simple and easy to operate, and can effectively control the generation of a trans-isomer contaminant to obtain a high purity product. The polycrystalline form of the dehydrophenylahistin-like compound has a certain value in an application for manufacturing an antitumor pharmaceutical product.
Owner:SHENZHEN HUAHONG MARINE BIOMEDICINE CO LTD
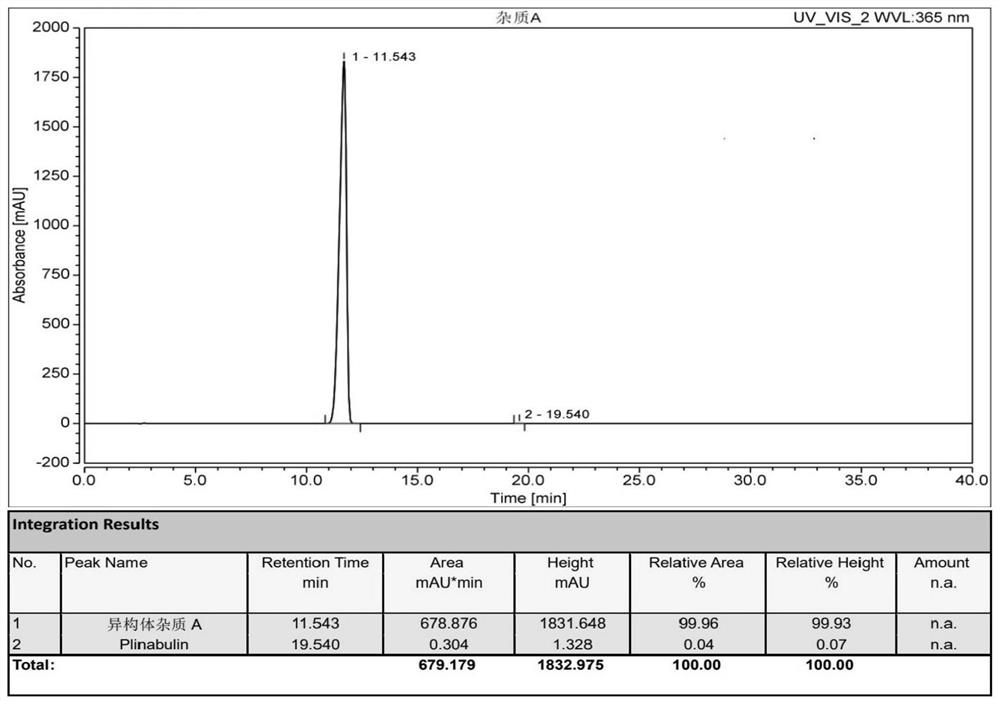
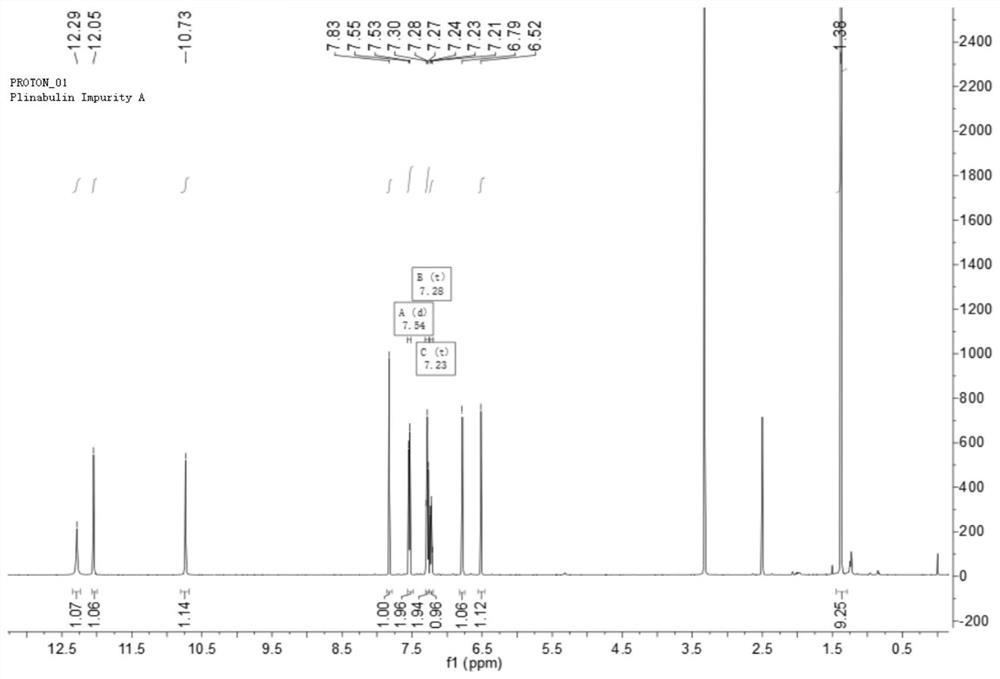
Preparation method and application of tubulin inhibitor plinabulin isomer impurity
The invention discloses a preparation method and application of a tubulin inhibitor plinabulin isomer impurity, and belongs to the technical field of medicinal chemistry. (3Z, 6Z)-3-[(5-tert-butyl-1H-imidazole-4-yl) methylene]-6-(benzylidene)-2, 5-piperazinedione is subjected to illumination, and after the reaction reaches equilibrium, a mixed solvent is recrystallized and purified to obtain the impurity with the 6Z-to-6E configuration. The impurity is very key in quality research on preparation of a medicine plinabulin for treating and resisting tumor diseases and neutropenia, and can be used as an impurity reference substance based on related characteristics of the impurity.
Owner:OCEAN UNIV OF CHINA
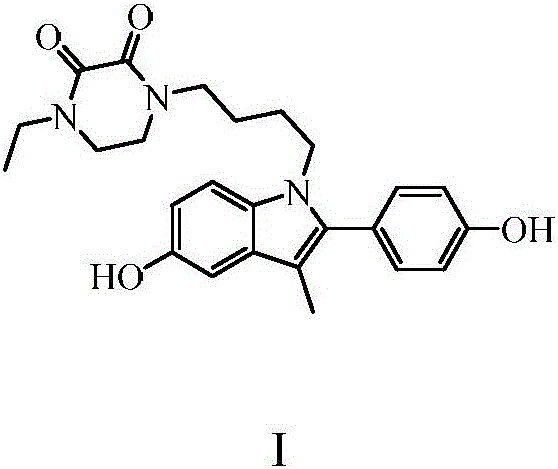
Piperazinone compounds and application thereof
ActiveCN105801564AEasy to prepareHigh yieldOrganic active ingredientsOrganic chemistryHormone Receptor ModulatorsHectic fever
The invention belongs to the technical field of medicine and relates to 1-ethyl-4-[4-[5-hydroxy-2-(4-hydroxyphenyl)-3-methyl-1H-indole-1-yl]butyl]piperazine-2,3-dione as well as a medical application, a stereoisomer and pharmaceutically acceptable salt thereof. The structural formula of 1-ethyl-4-[4-[5-hydroxy-2-(4-hydroxyphenyl)-3-methyl-1H-indole-1-yl] butyl]piperazine-2,3-dione is represented in the specification; 1-ethyl-4-[4-[5-hydroxy-2-(4-hydroxyphenyl)-3-methyl-1H-indole-1-yl]butyl]piperazine-2,3-dione and pharmaceutically acceptable acid addition salt of the compound can be combined with existing drugs or can be independently used as an estrogen receptor modulator for treating or preventing various estrogen function related diseases such as bone loss, fracture, osteoporosis, hectic fever, LDL cholesterol level rise, cardiovascular disease, cognitive impairment, brain degeneration disease and anxiety, as well as depression, sexual dysfunction, hypertension, retinal degeneration and cancer caused by estrogen deficiency, especially osteoporosis.
Owner:SHENYANG PHARMA UNIVERSITY
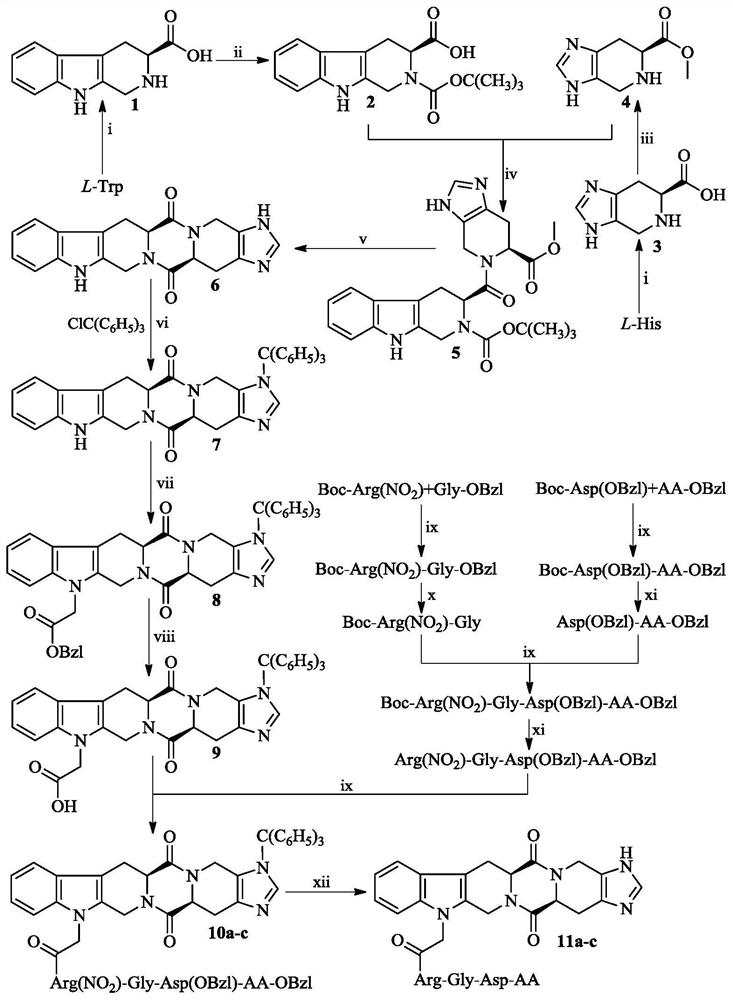
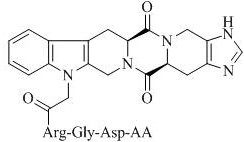
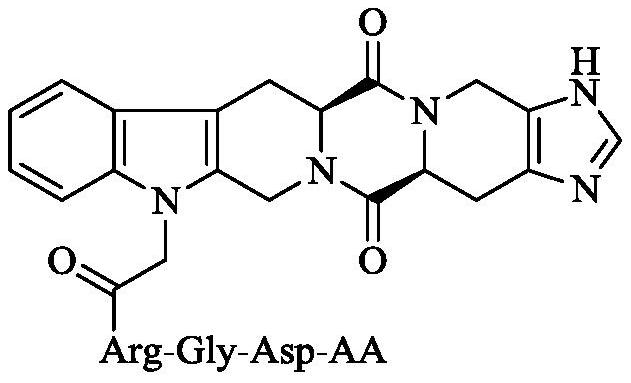
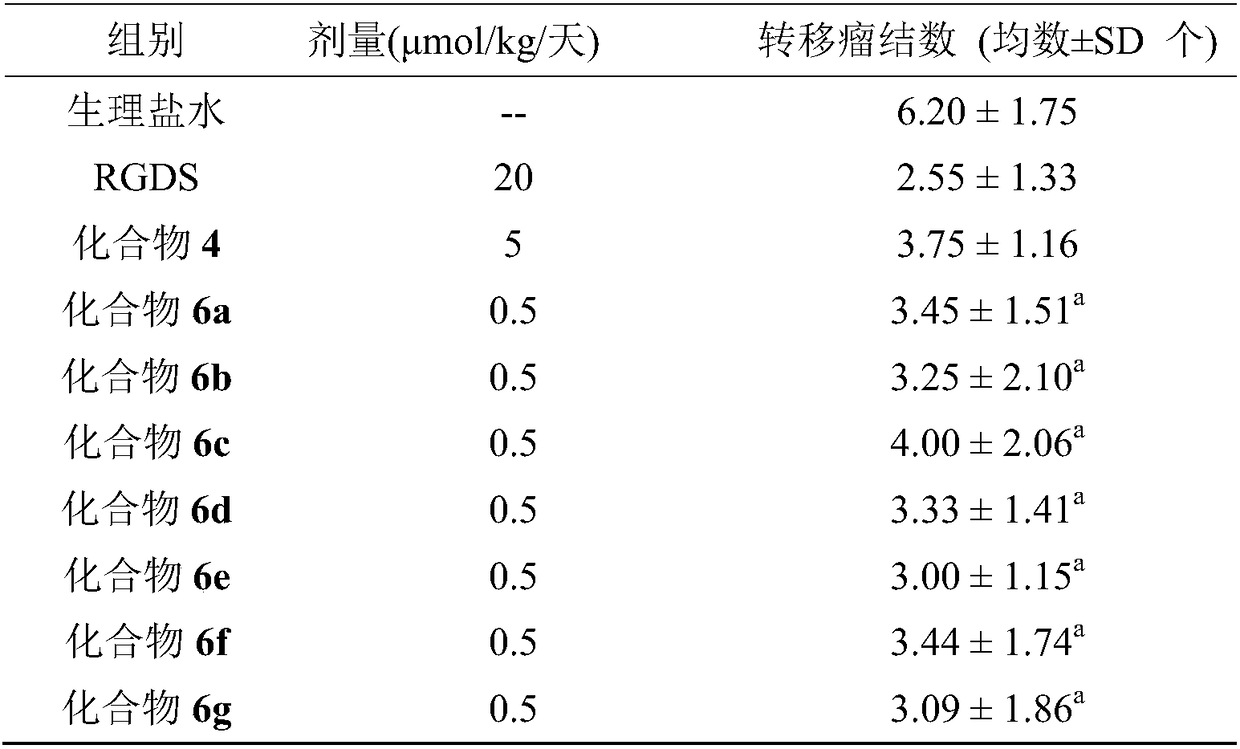
Hexacyclic piperazine dione modified with rgd sequence peptide, its preparation, antitumor activity and application
ActiveCN111978372BTetrapeptide ingredientsPeptide preparation methodsCombinatorial chemistryPharmaceutical Substances
The present invention discloses 9-(CH of the following formula 2 CO-Arg-Gly-Asp-AA)-tetrahydro-β-carboline[3:4]piperazine-2,5-diketopiperidine[4:5]imidazole (where AA is a Ser residue base, Phe residue and Val residue). Their preparation method is disclosed, and their application in the preparation of antitumor drugs is disclosed.
Owner:CAPITAL UNIVERSITY OF MEDICAL SCIENCES
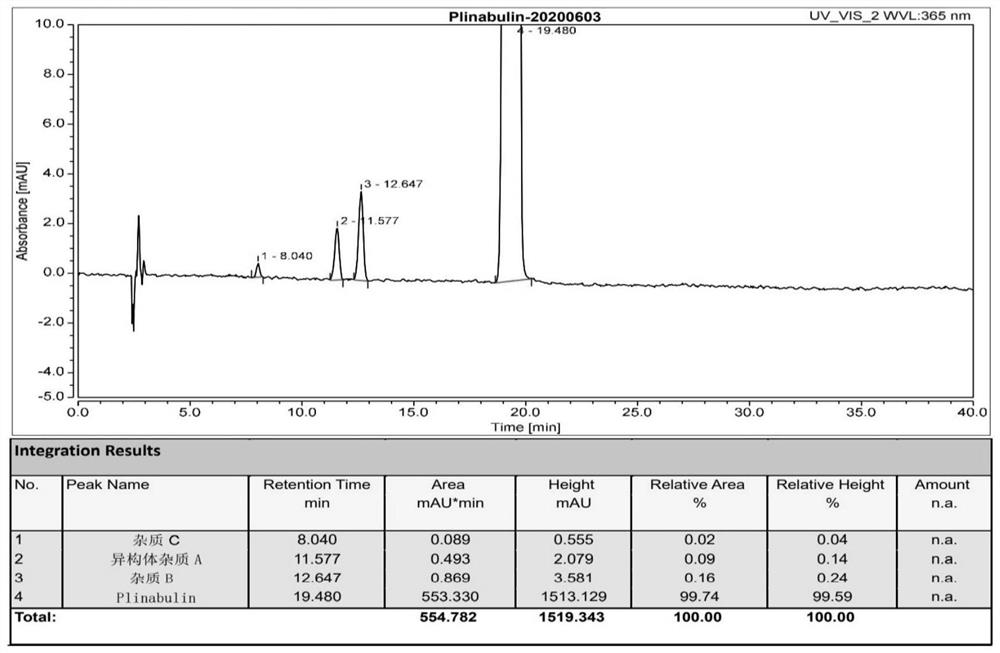
Plinabulin compound polycrystalline type and preparation method thereof
The invention provides a plinabulin compound polycrystalline type and a preparation method thereof, and particularly relates to a polycrystalline type of (3Z,6Z)-3-benzylidene-6-((5-tert-butyl-1H-imidazole-4-yl) methylene)piperazidine-2,5-diketone and a preparation method thereof. Three kinds of crystalline types beta, gamma and delta are developed on the basis of the crystalline type alpha of (3Z,6Z)-3-benzylidene-6-((5-tert-butyl-1H-imidazole-4-yl) methylene)piperazidine-2,5-diketone, wherein the three kinds of crystalline types beta, gamma and delta can be prepared into monocrystallines; the three kinds of crystalline types have the advantages of clear conformation, high purity and high method repeatability; the important significance is realized on implementation of plinabulin biological effectiveness study and dosage form variety development.
Owner:深圳华大海洋科技有限公司
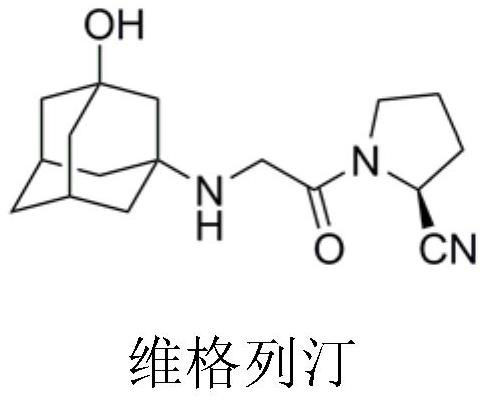
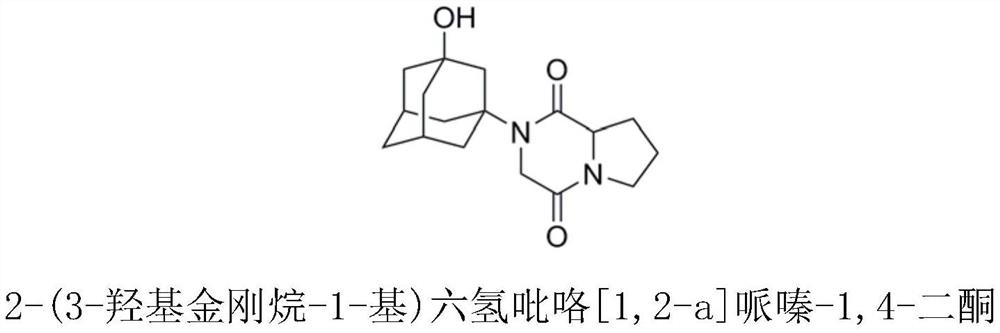
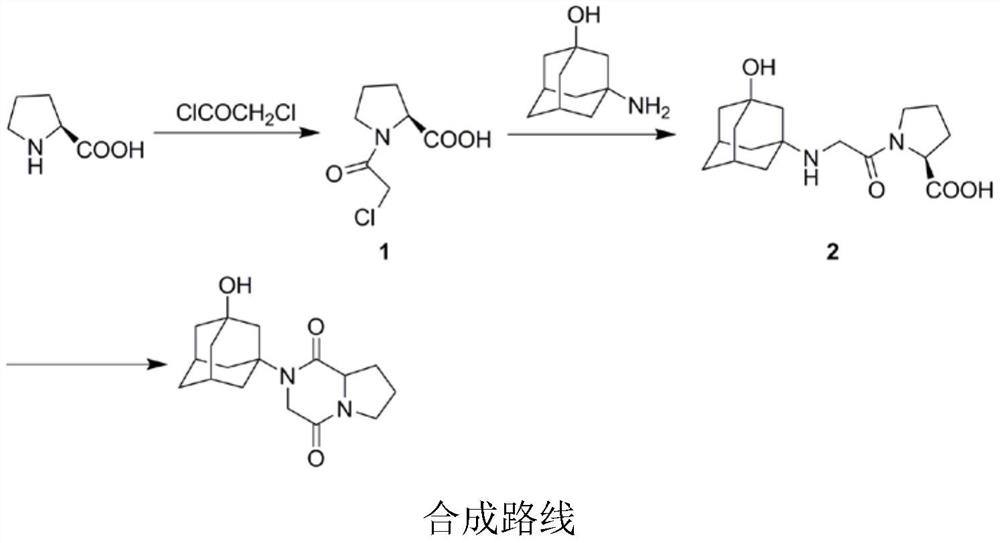
Preparation process of vildagliptin impurity
The invention discloses a preparation process of a vildagliptin impurity, namely 2-(3-hydroxy adamantane-1-yl) hexahydropyrrole [1, 2-a] piperazine-1, 4-diketone. According to the process, L-proline is used as an initial raw material, and 2-(3-hydroxy adamantane- 1-yl) hexahydropyrrole [1, 2-a] piperazine-1, 4-diketone is prepared through secondary N-chloracetylation reaction, amino substitution and intramolecular cyclization. The method is mainly characterized in that reaction steps and time are shortened, and expensive catalysts and tedious operation means are not involved in the whole reaction process. The preparation process has the advantages of easily available raw materials, mild and easily controllable reaction conditions and high product purity, and the vildagliptin impurity can be used as a reference substance for vildagliptin bulk drug quality control.
Owner:CHONGQING MEDICAL UNIVERSITY
3S-indolylethyl-6S-aliphatic amino acid-modified piperazidine-2,5-dione and synthesis, activity and application thereof
The invention discloses (3S,6S)-3-(AA-amino-n-hexanoylamino-n-butyl)-6-(indolyl-3-ethyl)-piperazidine-2,5-dione, and a preparation method, antitumor activity, antitumor metastasis activity and anti-inflammatory activity thereof. The compounds are shown in a following formula, wherein AA is L-Ala residues, Gly residues, L-Met residues, L-IIe residues, L-Pro residues, L-Val residues and L-Leu residues. Thus the invention discloses application of the compounds in preparation of antitumor drugs, antitumor metastasis drugs and anti-inflammatory drugs.
Owner:CAPITAL UNIVERSITY OF MEDICAL SCIENCES
A crystal form of a tubulin inhibitor (vda-1)
ActiveCN108658945BStable formStable melting pointOrganic active ingredientsOrganic chemistry methodsDiseaseDepressant
The present invention provides an A crystal form of (3Z,6Z)-3-[((E)-3-(5-tert-butyl)-1H-imidazol-4-yl)methylene]-6-((E)-3-(3-fluorophenyl)-2-propenylidene)piperazine-2,5-dione (VDA-1). The A crystal form has a stable morphology, a definite melting point, and good chemical stability and is high-temperature resistant and suitable for pharmaceutical uses. The A crystal form can be used to treat a hyperproliferative disease.
Owner:SHENZHEN NEPTUNUS PHARMA RES INST CO LTD
Ethyl pak-modified diketone biscarboline and piperazine, its preparation, activity and application
The invention discloses (2'S,5'S)-tetrahydropyrazine[1',2':1,6]bis[1S,1R-(1-ethyl-Pro-Ala-Lys)-2, 3,4,9-tetrahydro-1H-pyridin[3,4-b]indole]-1',4'-dione discloses its preparation method, discloses its thrombolytic activity, discloses Its antithrombotic activity also discloses that it remains effective for 24 hours after the onset of ischemic stroke. Therefore, the present invention discloses its application in the preparation of thrombolytic drugs, its application in the preparation of antithrombotic drugs, its application in the preparation of drugs that are still effective for ischemic stroke for 24 hours, and its application in the preparation of antithrombotic drugs. Its application in the preparation of a drug with triple effects of thrombolysis, antithrombosis and ischemic stroke still effective for 24 hours.
Owner:CAPITAL UNIVERSITY OF MEDICAL SCIENCES
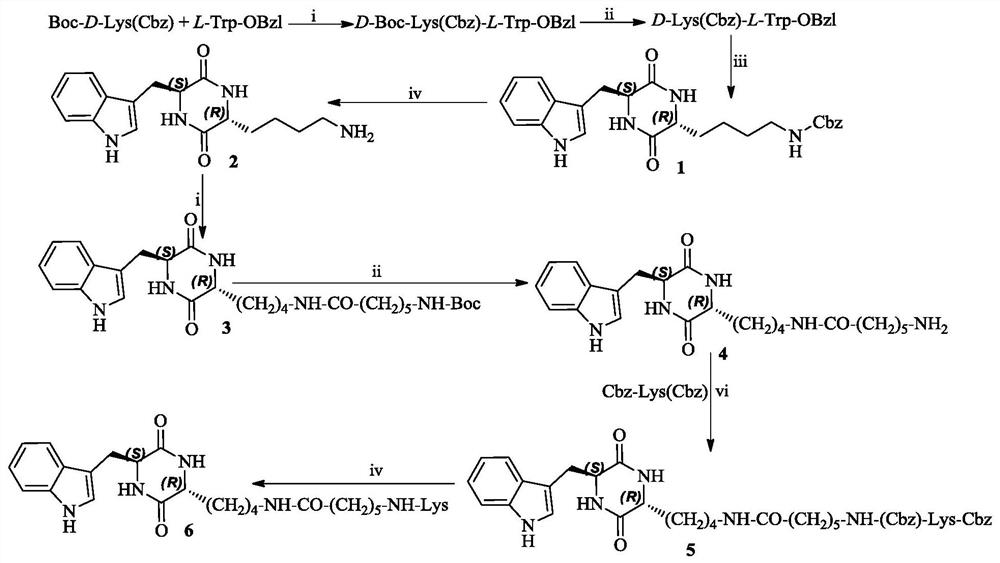
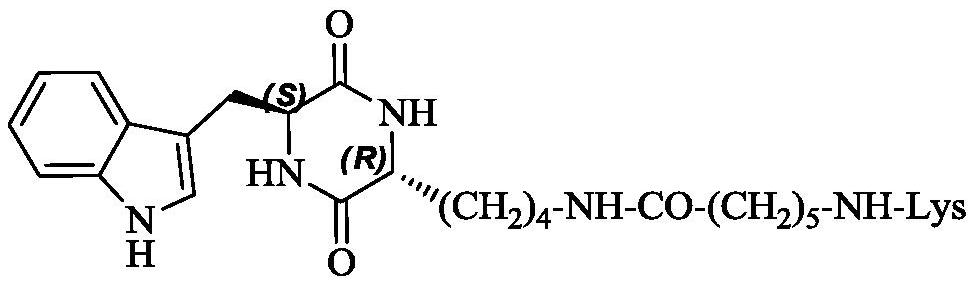
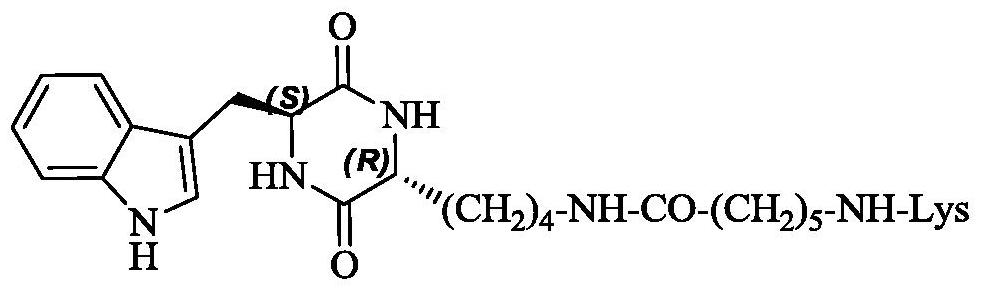
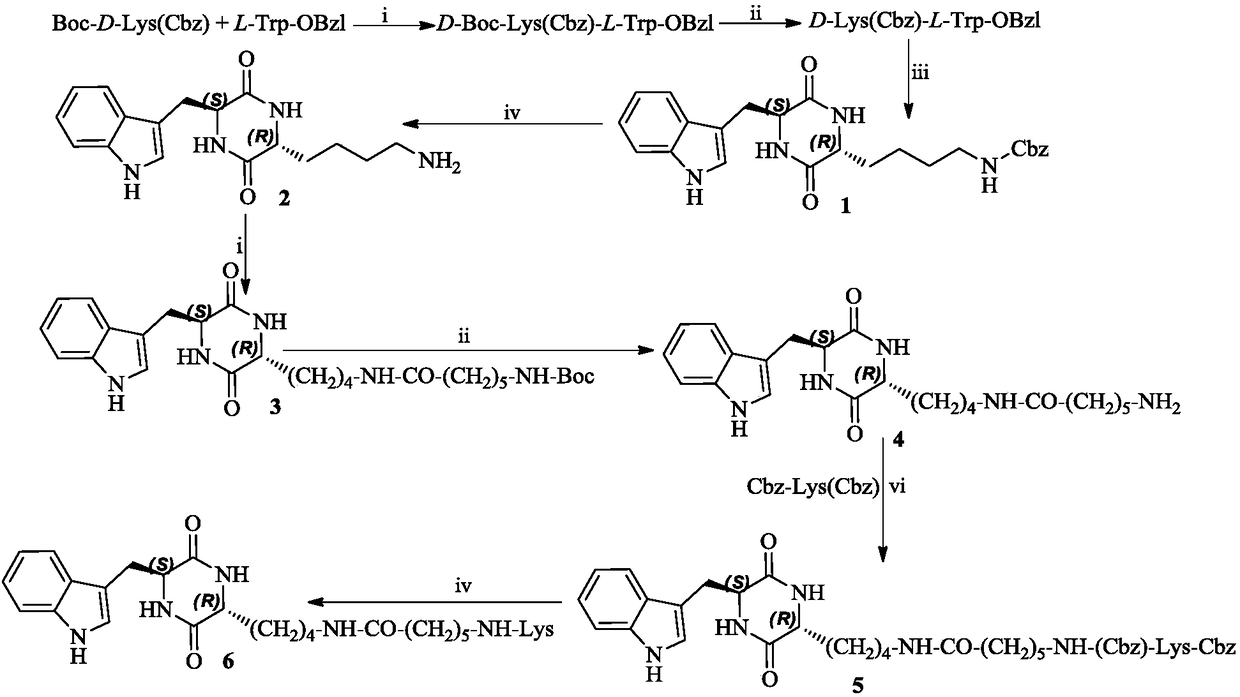
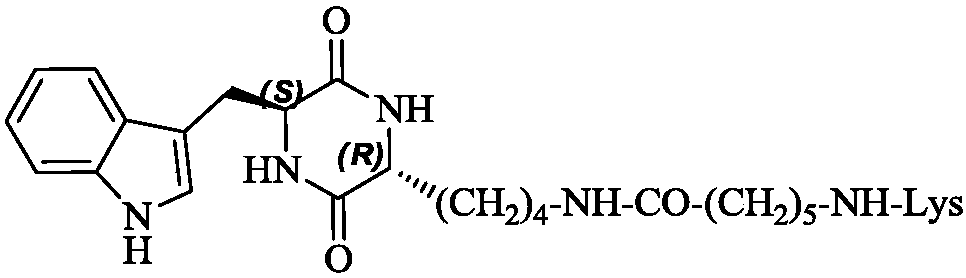
3r-indolemethyl-6s-lys modified piperazine-2,5-dione, its synthesis, activity and application
The present invention discloses (3R,6S)-3-(Lys-amino n-hexanoylamino n-butyl)-6-(indole-3-methyl)-piperazine-2,5-dione of the following formula. Its preparation method, its anti-tumor metastasis activity and its anti-inflammation activity are disclosed, so the present invention discloses its application in the preparation of anti-tumor metastasis medicine and anti-inflammation medicine.
Owner:CAPITAL UNIVERSITY OF MEDICAL SCIENCES
3R-indolemethyl-6S-Lys modified piperazine-2,5-dione, synthesis, activities and applications thereof
The present invention discloses (3R,6S)-3-(Lys-amino-n-hexanoylamino-n-butyl)-6-(indol-3-methyl)-piperazine-2,5-dione represented by the following formula, a preparation method, anti-tumor-metastasisactivity and anti-inflammatory activity thereof, such that the present invention discloses applications of the (3R,6S)-3-(Lys-amino-n-hexanoylamino-n-butyl)-6-(indol-3-methyl)-piperazine-2,5-dione inpreparation of anti-tumor-metastasis drugs and anti-inflammatory drugs. The formula is defined in the specification.
Owner:CAPITAL UNIVERSITY OF MEDICAL SCIENCES
Ring-fused compound of tetrahydropyrrole and piperazinedione as well as preparation and pharmaceutical application of ring-fused compound of tetrahydropyrrole and piperazinedione
ActiveCN114805364AAvoid infectionOrganic active ingredientsOrganic chemistry methodsPharmaceutical drugPharmaceutical medicine
The invention is applicable to the field of medicinal chemistry, and provides a fused ring compound of tetrahydropyrrole and piperazinedione as well as preparation and pharmaceutical application of the fused ring compound, and the compound is a compound shown in the formula or other pharmaceutically acceptable salts. The compound disclosed by the invention has the functions of inhibiting infection and replication of the dengue virus, so that the compound disclosed by the invention can be used as a medicine for preventing and treating diseases caused by the dengue virus.
Owner:CHANGZHOU VOCATIONAL INST OF ENG
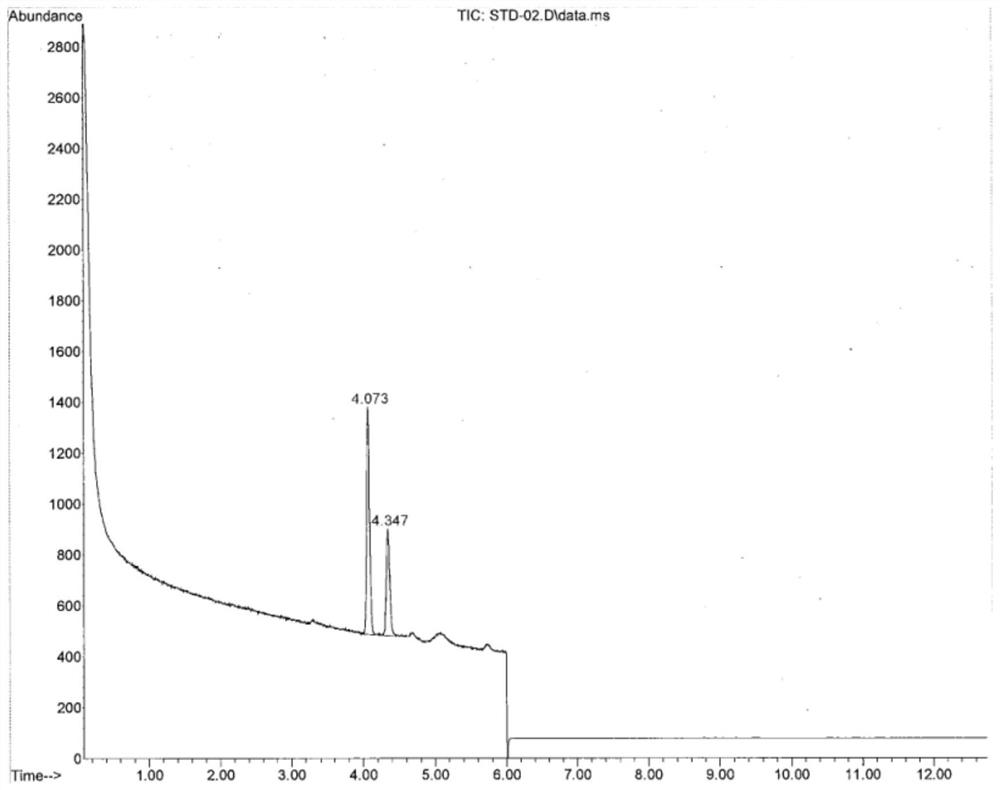
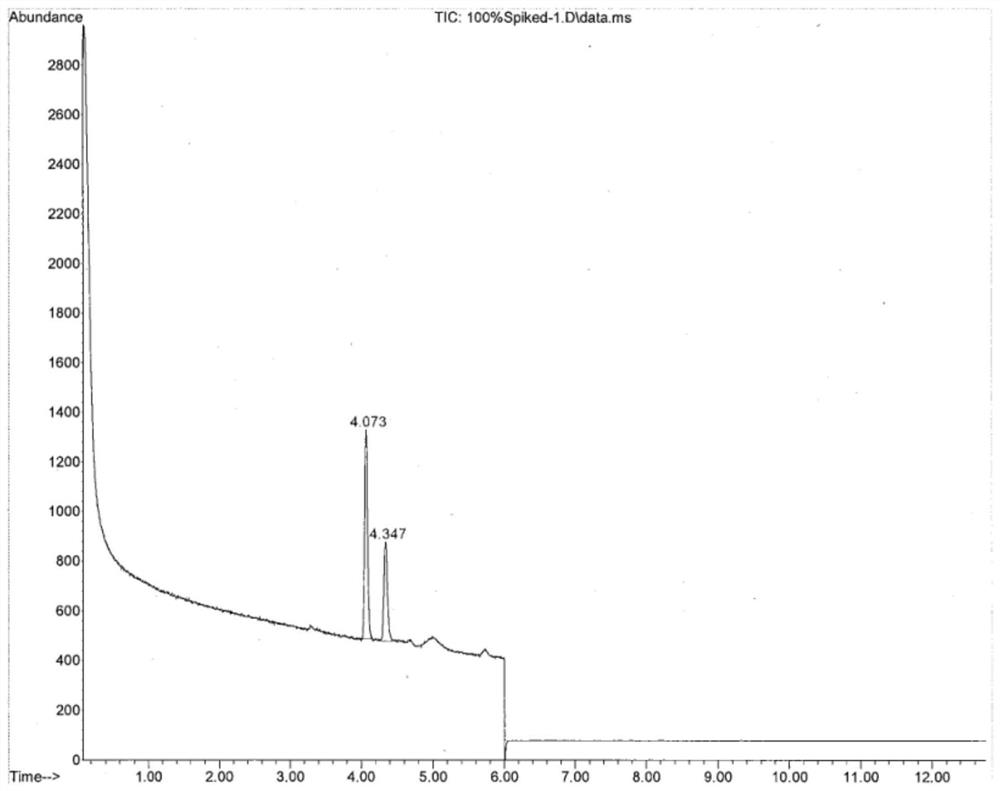
Method for simultaneously detecting methyl trifluoromethanesulfonate and ethyl trifluoromethanesulfonate in tubulin inhibitor bulk drug
ActiveCN112730642AQuality is easy to controlImprove securityComponent separationBulk chemical productionGas liquid chromatographicPolyethylene glycol
The invention discloses a method for simultaneously detecting genotoxic impurities including methyl trifluoromethanesulfonate and ethyl trifluoromethanesulfonate in tubulin inhibitor bulk drugs, and the bulk drugs are (3Z, 6Z)-3-[(E)-3-(5-tert-butyl)-1H-imidazolyl-4-yl) methylene]-6-((E)-3-(3-fluorophenyl)-2-propylene subunit)piperazine-2, 5-diketone. The method comprises the following steps: (1) taking a test sample of the raw material medicine, adding isopropanol, uniformly shaking, carrying out ultrasonic treatment, filtering, taking the subsequent filtrate, and introducing a sample to determine trifluoromethanesulfonic acid methyl ester and trifluoromethanesulfonic acid ethyl ester; wherein methyl trifluoromethanesulfonate reacts in isopropanol to generate methyl isopropyl ether, and ethyl trifluoromethanesulfonate reacts in isopropanol to generate ethyl isopropyl ether; and (2) detection conditions: taking polyethylene glycol modified by nitro terephthalic acid as a capillary column of a stationary phase, and determining by adopting a gas chromatograph-mass spectrometer. According to the method, the methyl trifluoromethanesulfonate and the ethyl trifluoromethanesulfonate in the tubulin inhibitor bulk drug can be qualitatively and quantitatively detected simply, quickly and efficiently at the same time.
Owner:SHENZHEN NEPTUNUS PHARMA RES INST CO LTD
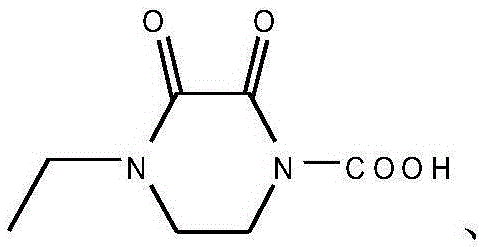
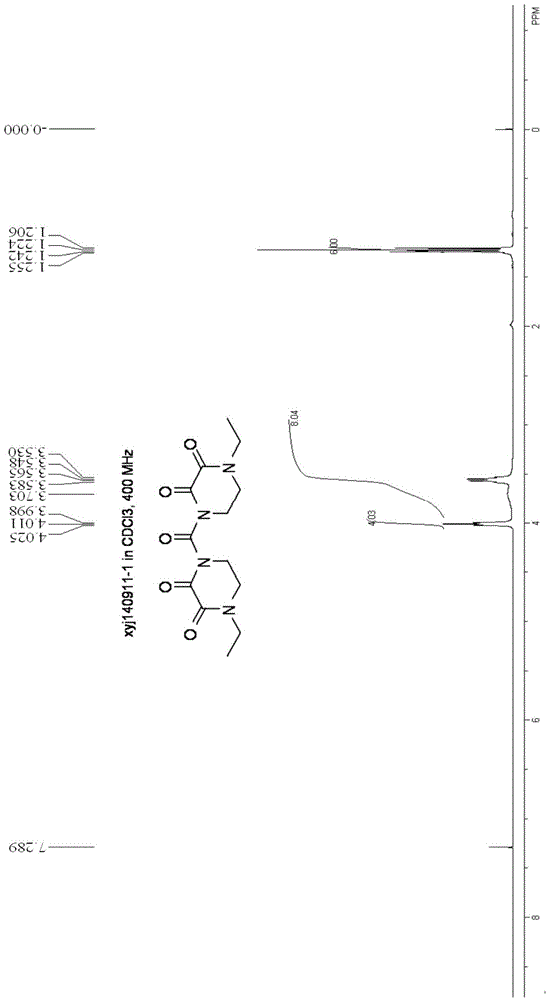
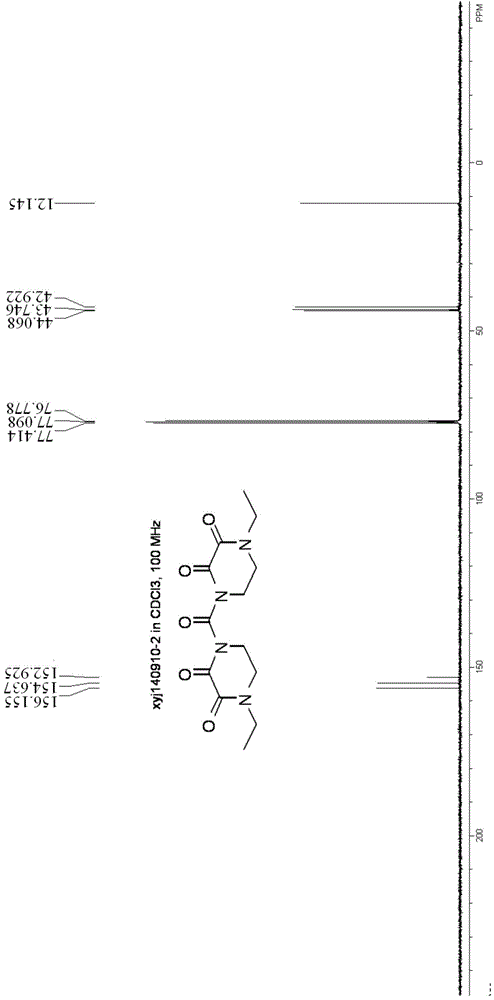
Synthesizing and purifying methods of 4, 4'-carbonyl di-1-ethyl piperazine-2, 3-dione
The invention discloses a synthesizing method of 4, 4'-carbonyl di-1-ethyl piperazine-2, 3-dione. The method comprises the following steps: firstly carrying out reaction on 4-ethyl-2, 3-dioxopiperazine and a silylating reagent in an organic solvent; and then carrying out condensation reaction with 4-ethyl-2, 3-dioxo-piperazine acyl chloride under an alkaline condition to obtain 4, 4'-carbonyl di-1-ethyl piperazine-2, 3-dione. The invention further provides a refining method of 4, 4'-carbonyl di-1-ethyl piperazine-2, 3-dione. The methods disclosed by the invention are high in product purity, simple in process, high in yield and suitable for industrial production. The 4, 4'-carbonyl di-1-ethyl piperazine-2, 3-dione obtained provides a high purity reference substance (the purity is over 98%) for production of 4-ethyl-2, 3-dioxo-piperazine acyl chloride or piperacillin and meanwhile, the 4, 4'-carbonyl di-1-ethyl piperazine-2, 3-dione can be used as a commercial organic chemical intermediate to be sold.
Owner:JIANGXI FUSHINE PHARMA CO LTD
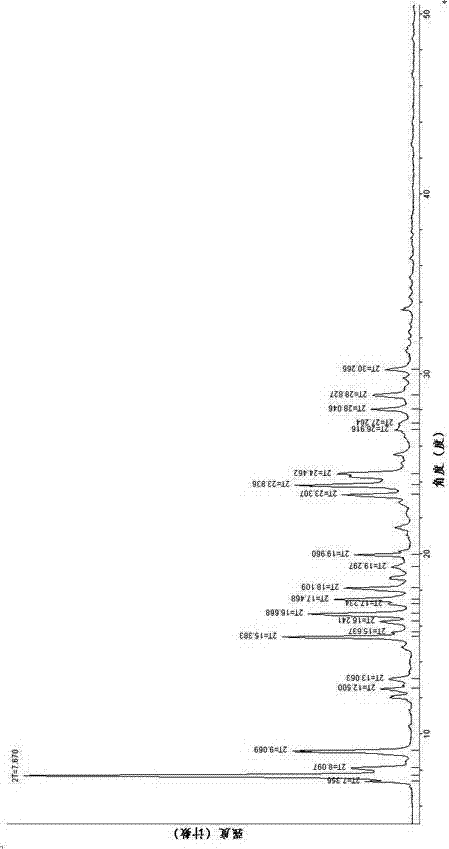
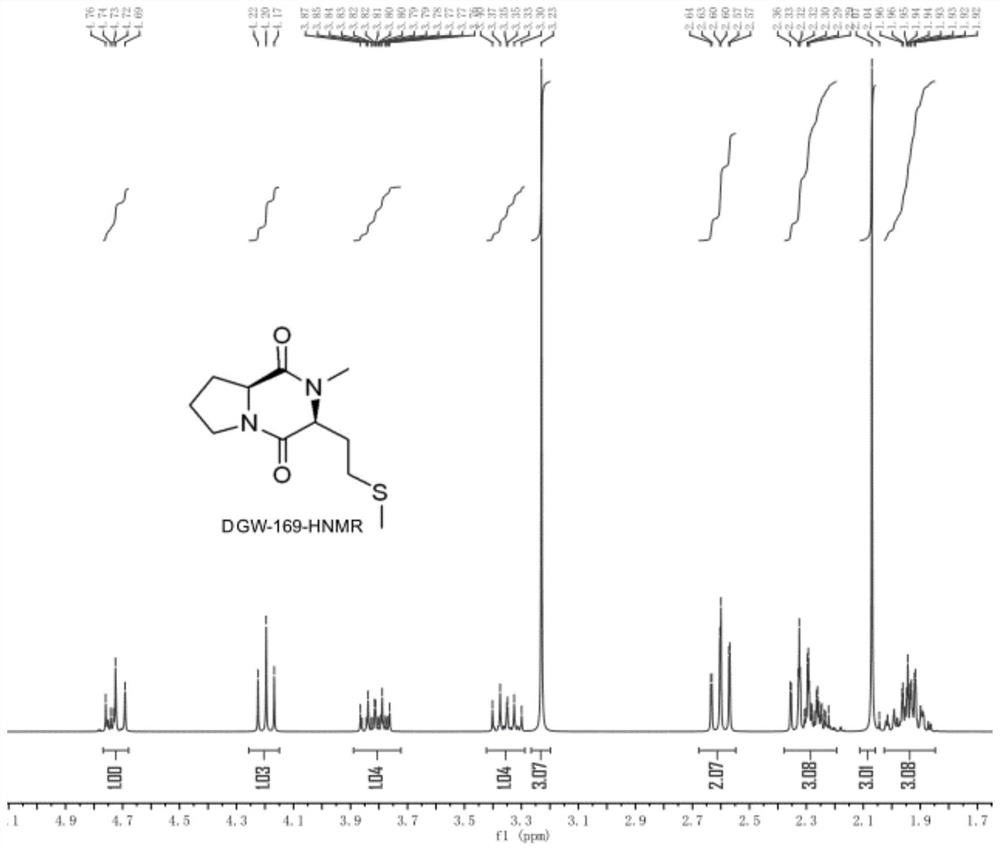
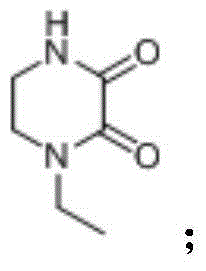
Novel crystalline forms of (3r, 6r)-3-(2,3-dihydro-1h-inden-2-yl)-1-[(1R)-1-(2,6-dimethyl-3-pyridinyl)-2-(4-morpholinyl)-2-oxoethyl]-6-[(1S)-1-methylpropyl]-2,5-piperazinedione
The present invention relates to crystalline forms of (3R,6R)-3-(2,3-dihydro-1H-inden-2-yl)-1-[(1R)-1-(2,6-dimethyl-3-pyridinyl)-2-(4-morpholinyl)-2-oxoethyl]-6-[(1S)-1-methylpropyl]-2,5-piperazinedione benzenesulfonate salt and pharmaceutical compositions thereof. Also disclosed are processes for the preparation the above compounds and methods for use thereof.
Owner:GLAXO GROUP LTD
3,4-methylenedioxyphenyl substituted tetrahydro-β-carboline piperazine diketone derivatives and uses thereof
The present invention relates to a 3,4-methylenedioxyphenyl substituted tetrahydro-beta-carboline piperazine dione derivative and uses thereof, and specifically discloses a compound represented by a formula I, or a stereoisomer thereof, or a pharmaceutically acceptable salt thereof, wherein each group is defined in the specification. According to the present invention, the compound has dual inhibitory activity on acetylcholinesterase and phosphodiesterase 5, and has good blood-brain barrier permeability, such that the compound can be used for preparing drugs for treatment and / or prevention ofAlzheimer's diseases. The formula I is defined in the specification.
Owner:EAST CHINA UNIV OF SCI & TECH +1
Extracts and beverages containing 2,5-piperazinedione, 3,6-bis(phenylmethyl)-, (3S,6S)-
ActiveUS10532079B2Without impairing the taste inherentGood effectNervous disorderDispersion deliveryBrixPiperazinedione
Extracts or beverages whose ratio between the content of 2,5-piperazinedione,3,6-bis(phenylmethyl)-,(3S,6S)-(unit: μg / 100 g) and Brix (Bx) is 6 (μg / 100 g) / Bx or more are good in flavor and feeling on the tongue and further have a good appearance.
Owner:SUNTORY BEVERAGE & FOOD ASIA PTE LTD +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![Crystalline forms of (3R, 6R)-3-(2,3-dihydro-1H-inden-2-yl)-1-[(1R)-1-(2,6-dimethyl-3-pyridinyl)-2-(4-morpholinyl)-2-oxoethyl]-6-[(1S)-1-methylpropyl]-2,5-piperazinedione Crystalline forms of (3R, 6R)-3-(2,3-dihydro-1H-inden-2-yl)-1-[(1R)-1-(2,6-dimethyl-3-pyridinyl)-2-(4-morpholinyl)-2-oxoethyl]-6-[(1S)-1-methylpropyl]-2,5-piperazinedione](https://images-eureka.patsnap.com/patent_img/e2a917ec-9ec7-48de-ba08-cc4b3458c342/US08716286-20140506-D00001.png)
![Crystalline forms of (3R, 6R)-3-(2,3-dihydro-1H-inden-2-yl)-1-[(1R)-1-(2,6-dimethyl-3-pyridinyl)-2-(4-morpholinyl)-2-oxoethyl]-6-[(1S)-1-methylpropyl]-2,5-piperazinedione Crystalline forms of (3R, 6R)-3-(2,3-dihydro-1H-inden-2-yl)-1-[(1R)-1-(2,6-dimethyl-3-pyridinyl)-2-(4-morpholinyl)-2-oxoethyl]-6-[(1S)-1-methylpropyl]-2,5-piperazinedione](https://images-eureka.patsnap.com/patent_img/e2a917ec-9ec7-48de-ba08-cc4b3458c342/US08716286-20140506-D00002.png)
![Crystalline forms of (3R, 6R)-3-(2,3-dihydro-1H-inden-2-yl)-1-[(1R)-1-(2,6-dimethyl-3-pyridinyl)-2-(4-morpholinyl)-2-oxoethyl]-6-[(1S)-1-methylpropyl]-2,5-piperazinedione Crystalline forms of (3R, 6R)-3-(2,3-dihydro-1H-inden-2-yl)-1-[(1R)-1-(2,6-dimethyl-3-pyridinyl)-2-(4-morpholinyl)-2-oxoethyl]-6-[(1S)-1-methylpropyl]-2,5-piperazinedione](https://images-eureka.patsnap.com/patent_img/e2a917ec-9ec7-48de-ba08-cc4b3458c342/US08716286-20140506-D00003.png)

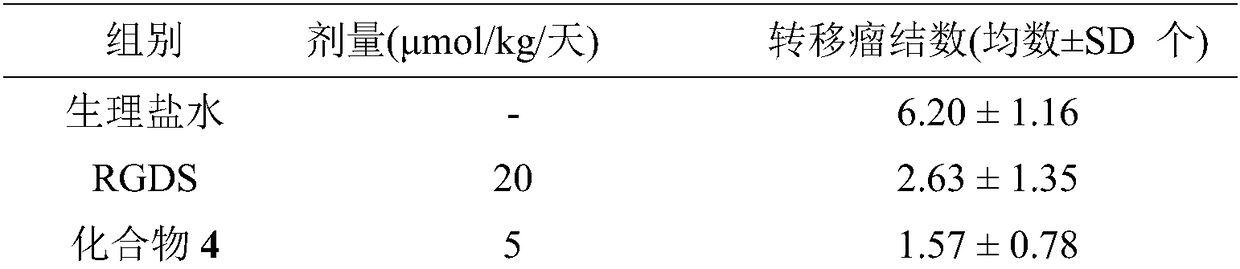
![Novel crystalline forms of (3r, 6r)-3-(2,3-dihydro-1h-inden-2-yl)-1-[(1R)-1-(2,6-dimethyl-3-pyridinyl)-2-(4-morpholinyl)-2-oxoethyl]-6-[(1S)-1-methylpropyl]-2,5-piperazinedione Novel crystalline forms of (3r, 6r)-3-(2,3-dihydro-1h-inden-2-yl)-1-[(1R)-1-(2,6-dimethyl-3-pyridinyl)-2-(4-morpholinyl)-2-oxoethyl]-6-[(1S)-1-methylpropyl]-2,5-piperazinedione](https://images-eureka.patsnap.com/patent_img/1f835e39-03df-4330-bba0-db795cf0fd28/US20130109690A1-20130502-D00001.png)
![Novel crystalline forms of (3r, 6r)-3-(2,3-dihydro-1h-inden-2-yl)-1-[(1R)-1-(2,6-dimethyl-3-pyridinyl)-2-(4-morpholinyl)-2-oxoethyl]-6-[(1S)-1-methylpropyl]-2,5-piperazinedione Novel crystalline forms of (3r, 6r)-3-(2,3-dihydro-1h-inden-2-yl)-1-[(1R)-1-(2,6-dimethyl-3-pyridinyl)-2-(4-morpholinyl)-2-oxoethyl]-6-[(1S)-1-methylpropyl]-2,5-piperazinedione](https://images-eureka.patsnap.com/patent_img/1f835e39-03df-4330-bba0-db795cf0fd28/US20130109690A1-20130502-D00002.png)
![Novel crystalline forms of (3r, 6r)-3-(2,3-dihydro-1h-inden-2-yl)-1-[(1R)-1-(2,6-dimethyl-3-pyridinyl)-2-(4-morpholinyl)-2-oxoethyl]-6-[(1S)-1-methylpropyl]-2,5-piperazinedione Novel crystalline forms of (3r, 6r)-3-(2,3-dihydro-1h-inden-2-yl)-1-[(1R)-1-(2,6-dimethyl-3-pyridinyl)-2-(4-morpholinyl)-2-oxoethyl]-6-[(1S)-1-methylpropyl]-2,5-piperazinedione](https://images-eureka.patsnap.com/patent_img/1f835e39-03df-4330-bba0-db795cf0fd28/US20130109690A1-20130502-D00003.png)