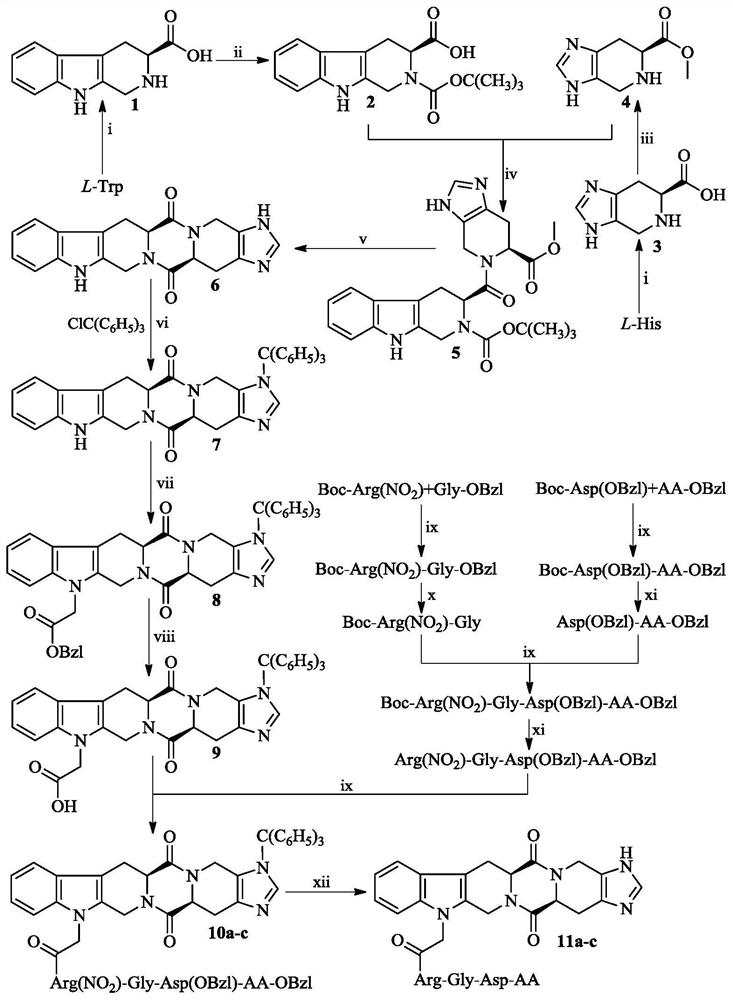
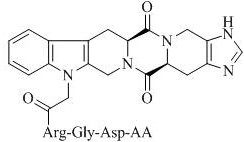
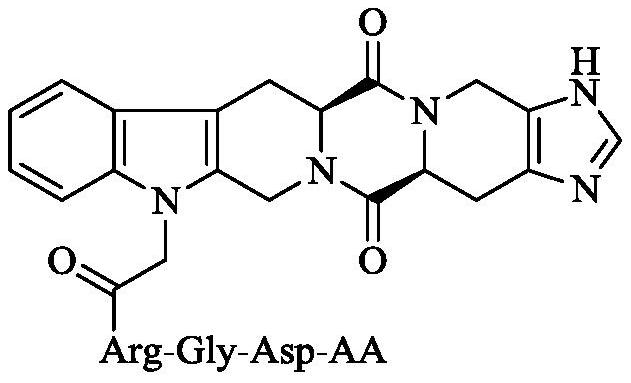
Hexacyclic piperazine dione modified with rgd sequence peptide, its preparation, antitumor activity and application
A technology of piperazine and diketone, applied in the application field of preparing antitumor drugs, can solve the problems of expensive treatment price, troubled cancer treatment, multidrug resistance and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Example 1 Preparation of Boc-Arg (NO 2 )-Gly-OBzl
[0024] 1.595 g (5.0 mmol) of Boc-L-Arg (NO 2 ) was dissolved in 20 mL of tetrahydrofuran, then 0.675 g (5.0 mmol) of 1-hydroxybenzotriazole and 1.236 g (6.0 mmol) of dicyclohexylcarbodiimide were added, and the reaction was carried out for 0.5 h. Then 1.854 g (5.5 mmol) Tos·Gly-OBzl was added, and finally the pH of the reaction solution was adjusted to 8 with N-methylmorpholine. The resulting solution was stirred at room temperature for 6h until TLC showed Boc-L-Arg(NO 2 )disappear completely. The reaction mixture was filtered, and the filtrate was concentrated under reduced pressure. The obtained pale yellow oil was dissolved in 60 mL of ethyl acetate. The obtained solution was washed with saturated aqueous sodium bicarbonate solution (20 mL × 3) and saturated aqueous sodium chloride solution (20 mL × 3) successively. , washed with 5% aqueous potassium hydrogen sulfate (20 mL×3), washed with saturated aqueous sodi...
Embodiment 2
[0025] Example 2 Preparation of Boc-Arg (NO 2 )-Gly
[0026] 2.120 g (5.0 mmol) of Boc-Arg (NO 2 )-Gly-OBzl was dissolved in 15 mL of methanol, added with aqueous sodium hydroxide solution (2M) to adjust the pH to 11, and stirred until TLC showed Boc-Arg(NO). 2 )-Gly-OBzl disappeared completely. The reaction solution was first adjusted to neutral pH with saturated aqueous potassium hydrogen sulfate solution, concentrated under reduced pressure, adjusted to pH 2 with saturated aqueous potassium hydrogen sulfate solution, extracted with ethyl acetate (50 mL×3), combined with the ethyl acetate layers and washed with saturated chlorine Washed with aqueous sodium chloride solution (30 mL×3). The ethyl acetate layer was dried over anhydrous sodium sulfate for 12 h, filtered, and the filtrate was concentrated under reduced pressure to obtain 1.542 g (82%) of the title compound as a colorless oily product. ESI-MS(m / e): 375[M-H] - .
Embodiment 3
[0027] Example 3 Preparation of Boc-Asp(OBzl)-Ser-OBzl
[0028] Using the method of Example 1, 2.520 g (92%) of the title compound was obtained as a colorless oil from 1.615 g (5.0 mmol) Boc-L-Asp(OBzl) and 1.271 g (5.5 mmol) L-Ser-OBzl. ESI-MS(m / e):501[M+H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com