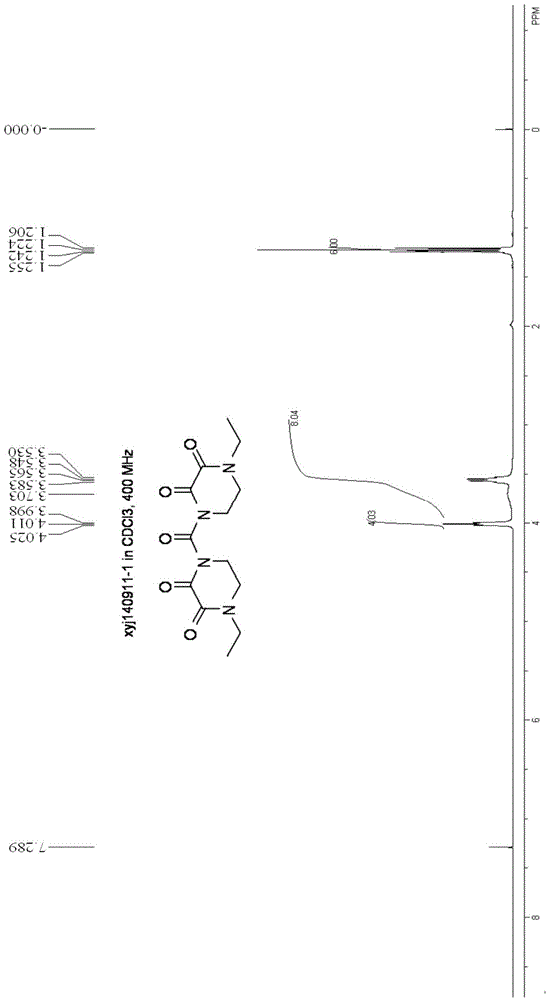
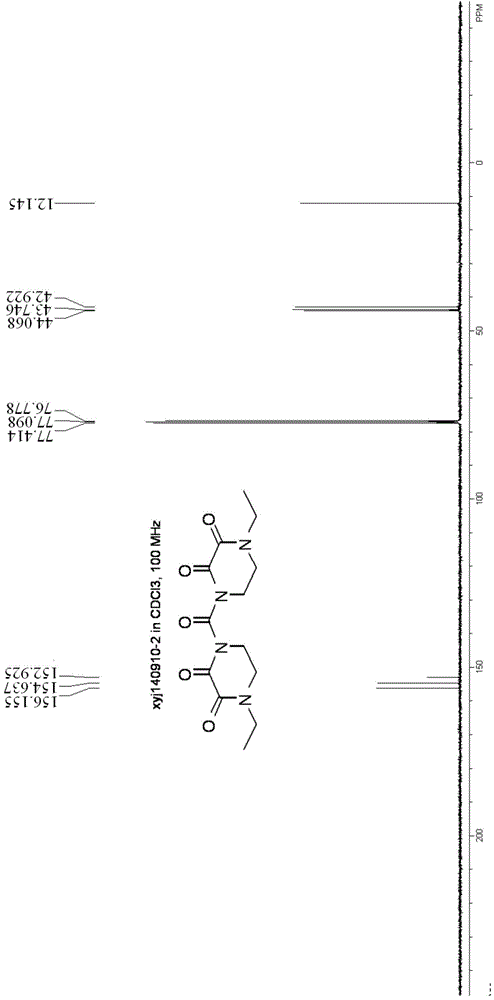
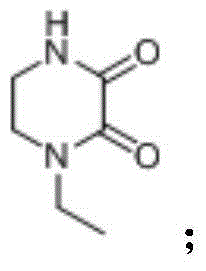
Synthesizing and purifying methods of 4, 4'-carbonyl di-1-ethyl piperazine-2, 3-dione
A technology of ethylpiperazine and synthesis method, which is applied in the field of drug synthesis, can solve the problems of synthesis, separation and characterization, and achieve the effects of high purity, simple process and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] (1) Preparation of 4,4'-carbonyldi-1-ethylpiperazine-2,3-dione
[0034] Add 4-ethyl-2,3-dioxypiperazine (14.2g, 0.1mol), dichloromethane (60ml), trimethylchlorosilane (13.0g, 0.12mol) to the four-necked flask, and control the temperature- 10~-20℃, dropwise addition of triethylamine (25.5g, 0.25mol) was completed, raised to -5~0, stirred for 12min; ) was dissolved in 20ml of dichloromethane, controlled temperature 0 ℃ ~ -5 ℃ and added dropwise for 40 minutes, heated to 25 ℃ and kept for 8 hours, and cooled to -15 ℃ after the reaction, filtered, and the filter cake was cooled with pre-cooled dichloromethane (8ml) was rinsed, the mother liquors were combined, and concentrated (30°C-40°C, vacuum <0.1MPa) to obtain 30.4g of crude product (98.6% yield, 95% purity).
[0035] (2) Purification of 4,4'-carbonyldi-1-ethylpiperazine-2,3-dione
[0036] Add 10g of crude 4,4'-carbonyldi-1-ethylpiperazine-2,3-dione and dichloromethane (50ml) into a four-neck flask, heat and reflux fo...
Embodiment 2
[0038] (1) Preparation of 4,4'-carbonyldi-1-ethylpiperazine-2,3-dione
[0039]Add 4-ethyl-2,3-dioxypiperazine (14.2g, 0.1mol), 1,2-dichloroethane (60ml), trimethylbromosilane (18.2g, 0.12mol) into the four-necked flask ), control the temperature at -10~-20°C, add triethylamine (25.5g, 0.25mol) dropwise, rise to -5~0 and stir for 12min; 4-ethyl-2,3-dioxypiperazine chloride (22.4 g, 0.11mol) was dissolved in 20ml of 1,2-dichloroethane, controlled temperature 0℃~-5℃, added dropwise for 40min, raised the temperature to 25℃ and kept the reaction for 6h, cooled to -15℃ after the reaction, filtered, The filter cake was rinsed with pre-cooled 1,2-dichloroethane (10ml), the mother liquors were combined, and concentrated (40°C-50°C, vacuum <0.1MPa) to obtain 28.5g of crude product (92.0% yield, 89% purity). %).
[0040] (2) Purification of 4,4'-carbonyldi-1-ethylpiperazine-2,3-dione
[0041] Add 10 g of crude 4,4'-carbonyldi-1-ethylpiperazine-2,3-dione and 1,2-dichloroethane (50 ml) ...
Embodiment 3
[0043] (1) Preparation of 4,4'-carbonyldi-1-ethylpiperazine-2,3-dione
[0044] Add 4-ethyl-2,3-dioxypiperazine (14.2g, 0.1mol), chloroform (50ml), tert-butyldiphenylchlorosilane (32.9.0g, 0.12mol) to the four-necked flask, control Temperature -10~-20°C, after adding pyridine (19.8g, 0.25mol) dropwise, rise to -5~0 and stir for 12min; 4-ethyl-2,3-dioxypiperazine chloride (22.4g, 0.11mol) Dissolve in 20ml of chloroform, add dropwise at a temperature of 0°C to -5°C for 40min, heat up to 25°C and keep the temperature for 6h, cool down to -15°C after the reaction, filter, and rinse the filter cake with pre-cooled chloroform (5ml) , combined the mother liquors, concentrated (40°C-50°C, vacuum <0.1MPa) to obtain 27.9g of crude product (80.0% yield, 95.2% purity).
[0045] (2) Purification of 4,4'-carbonyldi-1-ethylpiperazine-2,3-dione
[0046] Add 10g of crude product of 4,4'-carbonyldi-1-ethylpiperazine-2,3-dione and chloroform (45ml) into a four-neck flask, heat and reflux for 30...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com