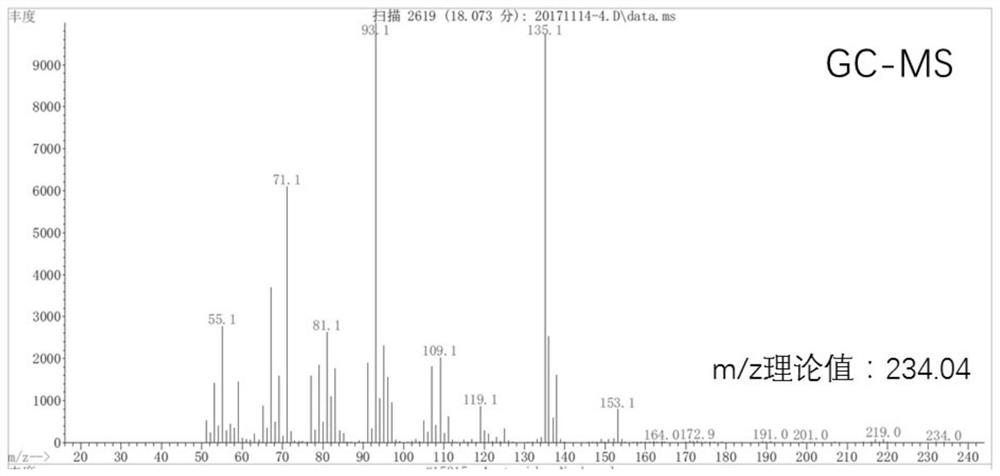
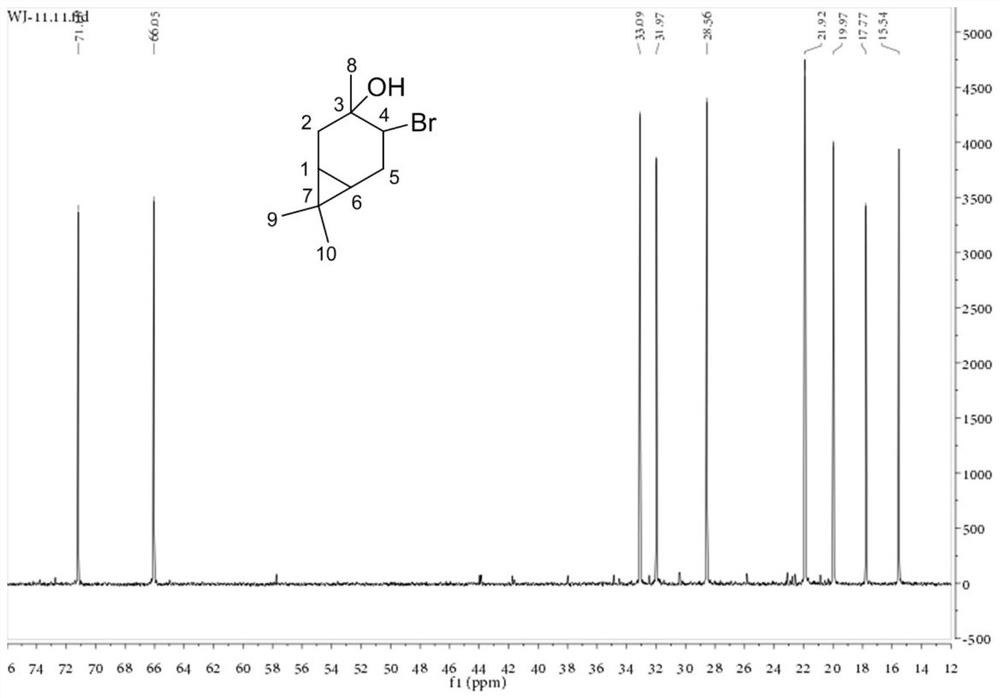
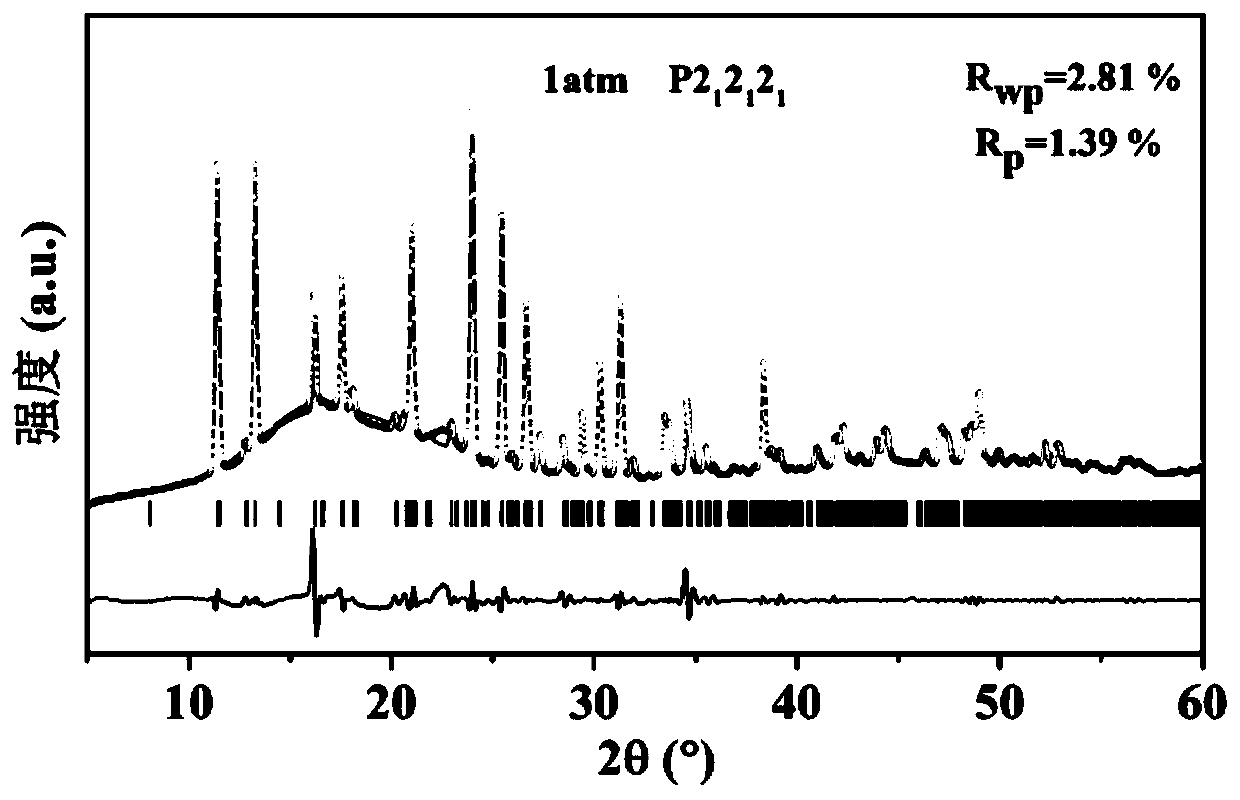
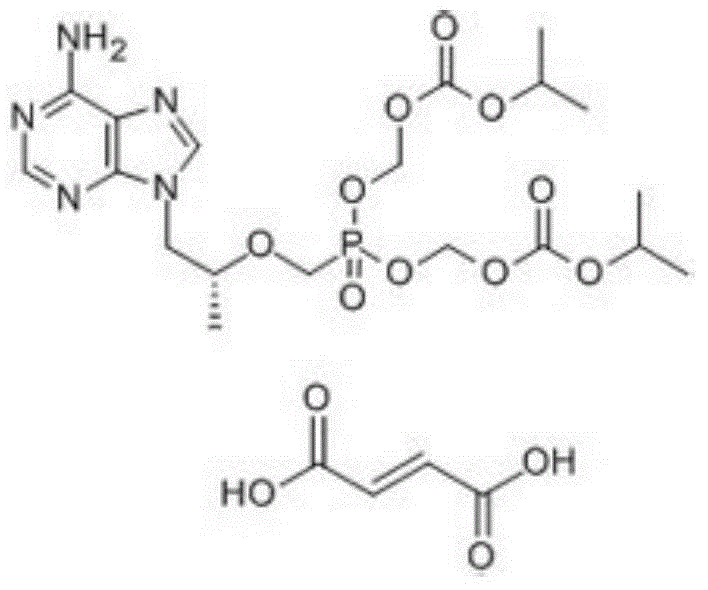
Patents
Literature
39 results about "Trimethylbromosilane" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Synonym: TMBS, Trimethylbromosilane, Trimethylsilyl bromide CAS Number 2857-97-8. Linear Formula (CH 3) 3 SiBr . Molecular Weight 153.09 . Beilstein Registry Number 1731135 . EC Number 220-672-0. MDL number MFCD00000048. PubChem Substance ID 24851651
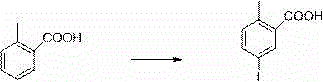
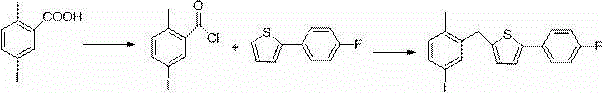
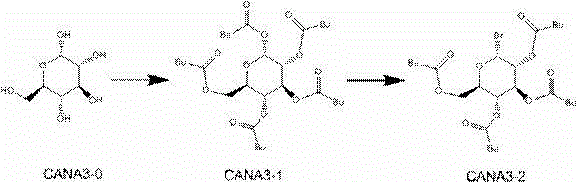
New synthesis process of canagliflozin
The present invention discloses a new synthesis process of canagliflozin. The new synthesis process comprises: adopting 2-methyl benzoic acid as a starting raw material, and adopting a self-made catalyst, iodic acid and iodine to carry out a reaction to produce an intermediate 1, or adopting 2-methyl benzoic acid as a starting raw material, and adding liquid bromine under effects of a metal reagent and a catalyst to synthesize an intermediate 2; optionally selecting the intermediate 1 or 2 to carry out an acylation reaction with thionyl chloride, and then carrying out a Friedel-Crafts reaction to produce an intermediate 3; adopting ALPHA-D-glucose as a raw material, carrying out a reaction with pivaloyl chloride to protect all hydroxyl, and carrying out a reaction with zinc bromide and bromotrimethylsilane to produce an intermediate 4; linking the intermediate 3 and the intermediate 4 to produce an intermediate 5; and finally under an acid condition, removing the pivaloyl to produce the target compound. The new synthesis process has characteristics of high yield, mild condition, safety, reliability, cheap and easily available raw material, and easy production cost control, and is suitable for industrial production.
Owner:HAIMEN RUIYI MEDICAL TECH
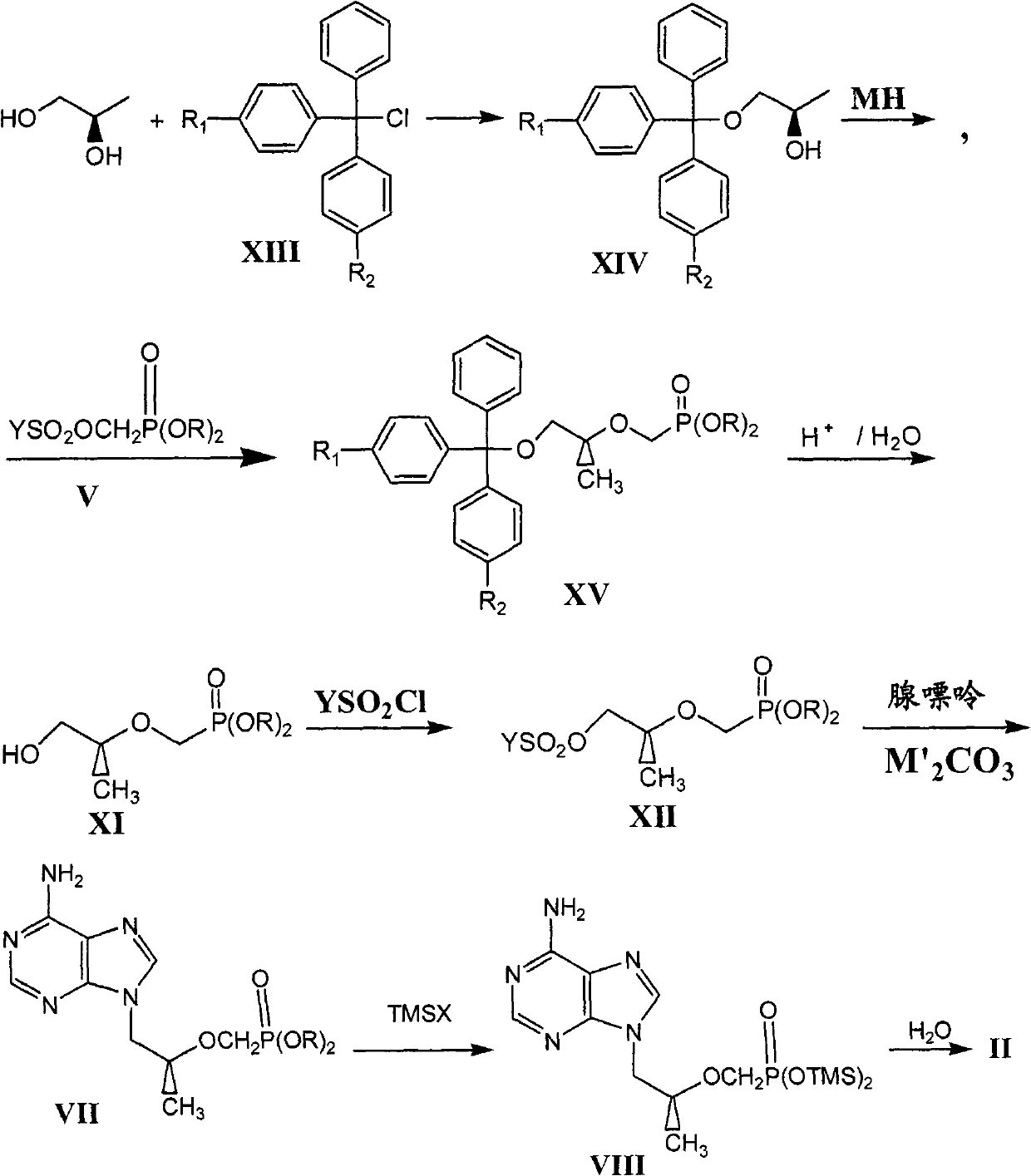
Novel method for preparing tenofovir
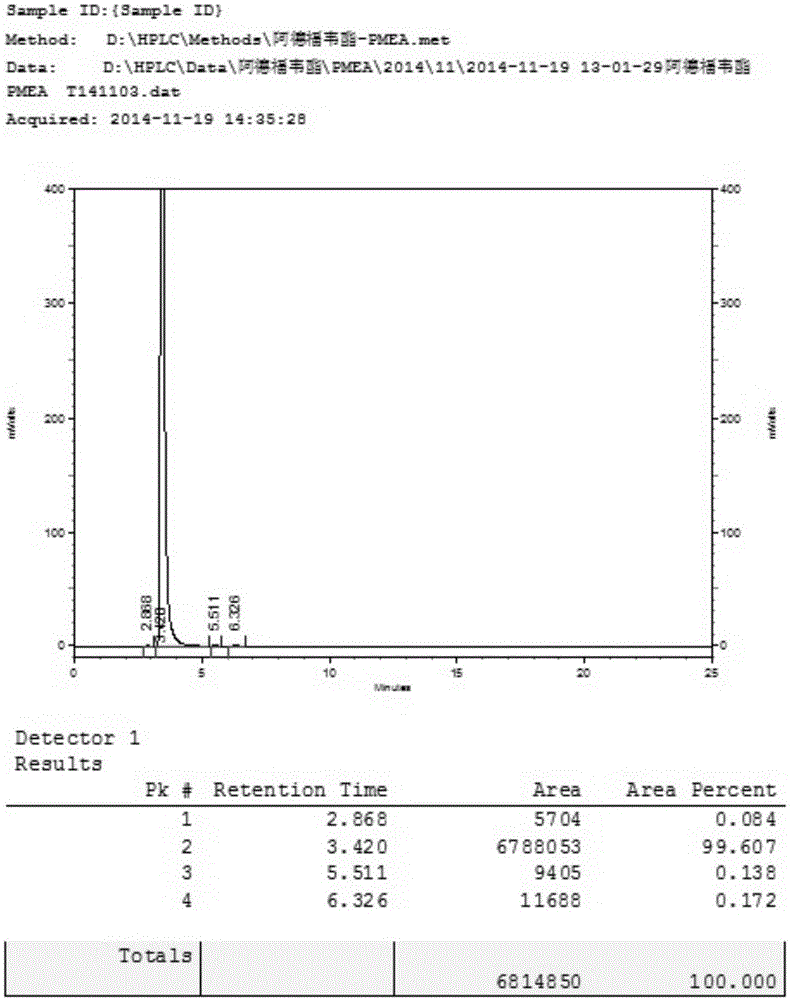
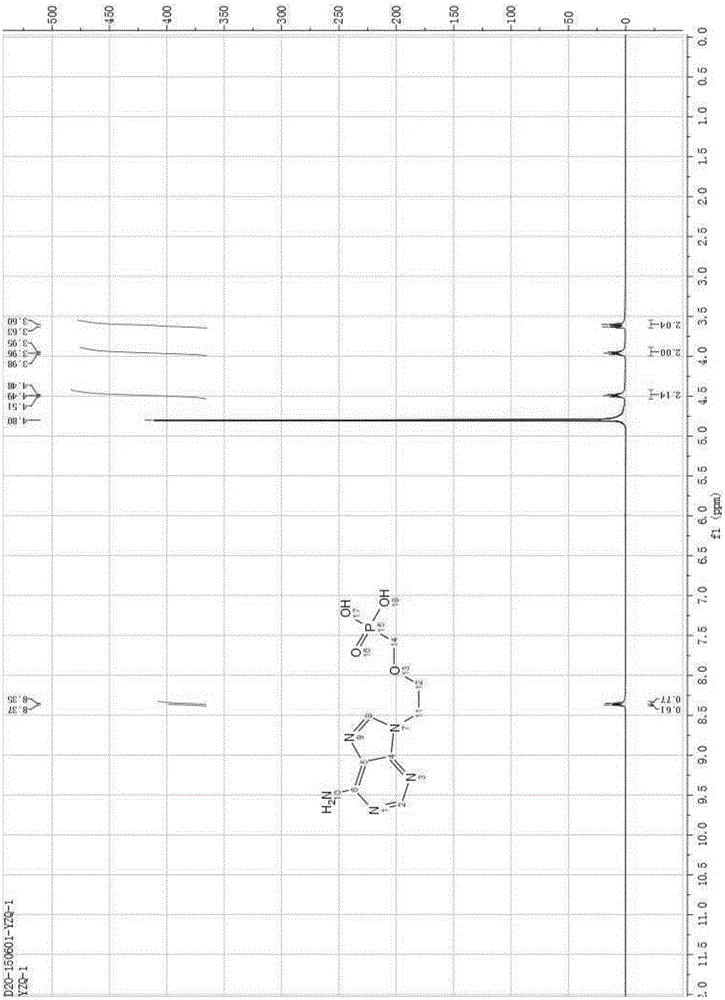
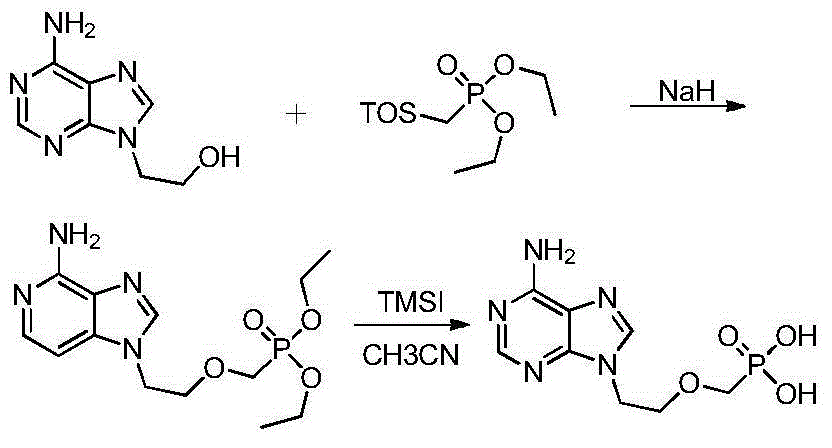
InactiveCN101906119AOrganic active ingredientsGroup 5/15 element organic compoundsTrimethylsilyl bromideHuman immunodeficiency
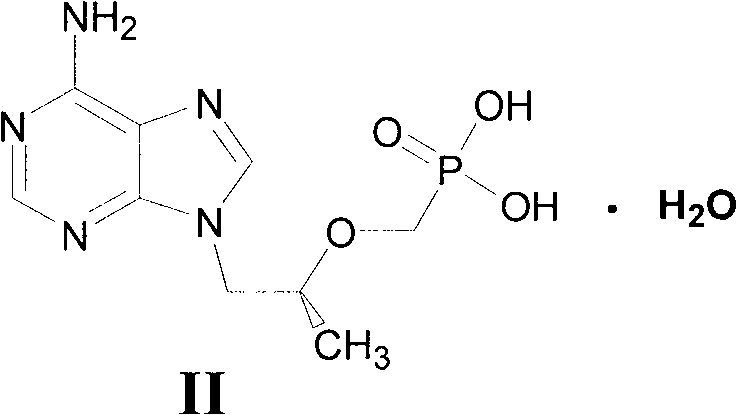
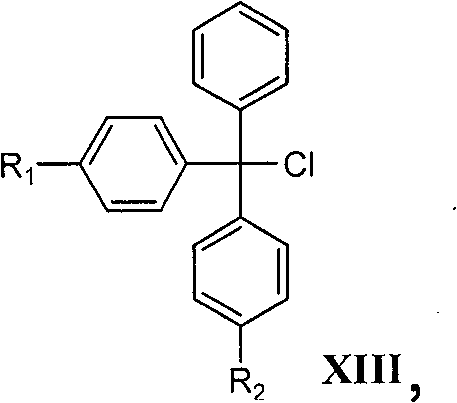
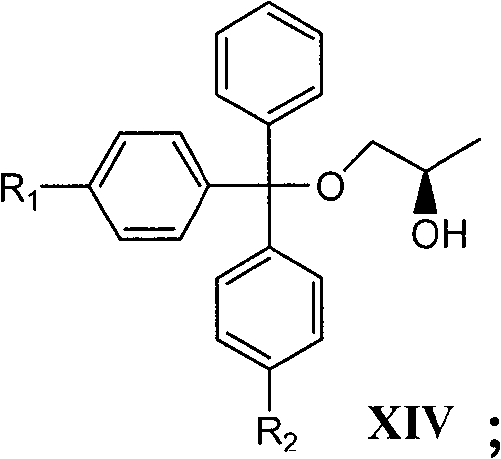
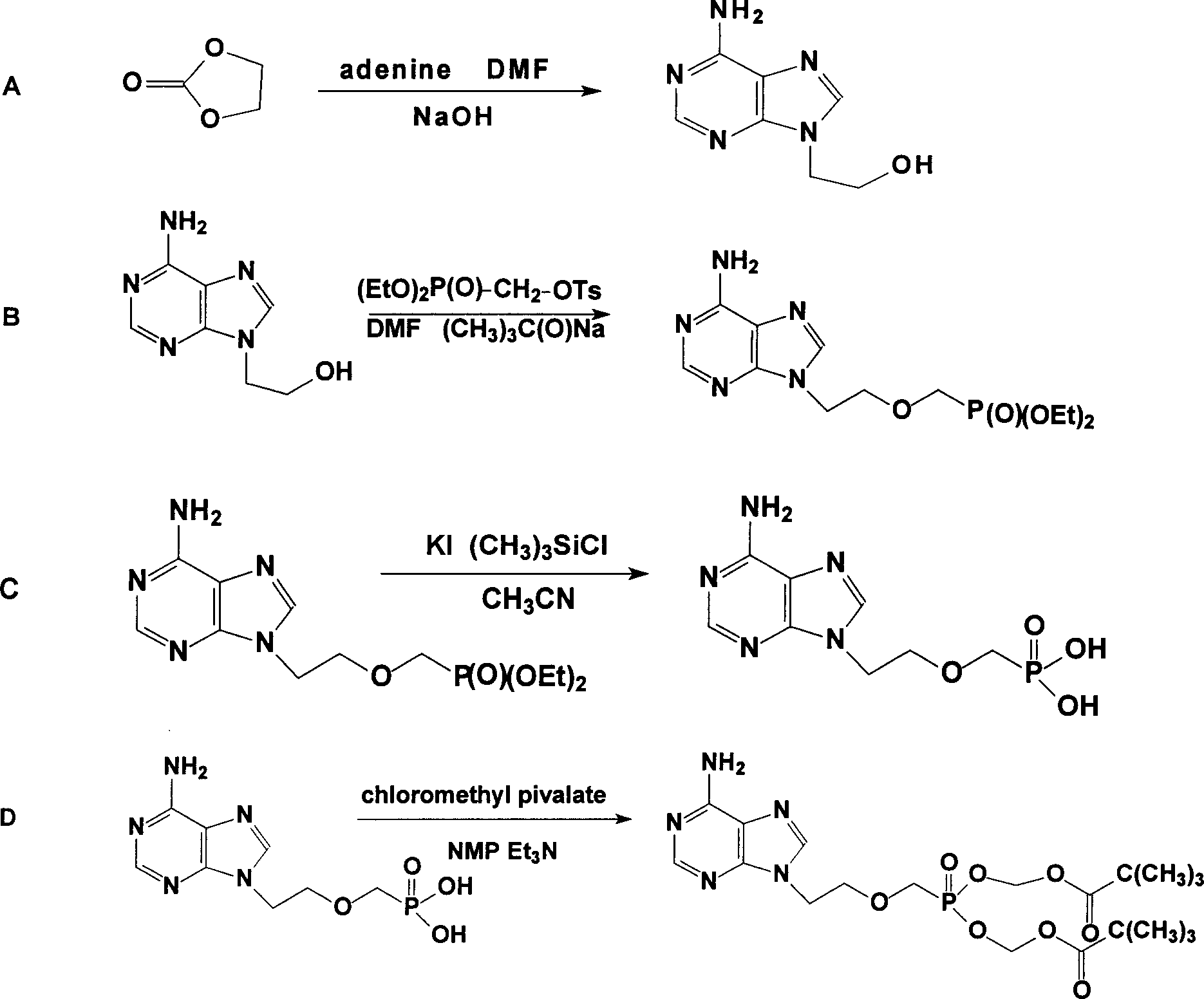
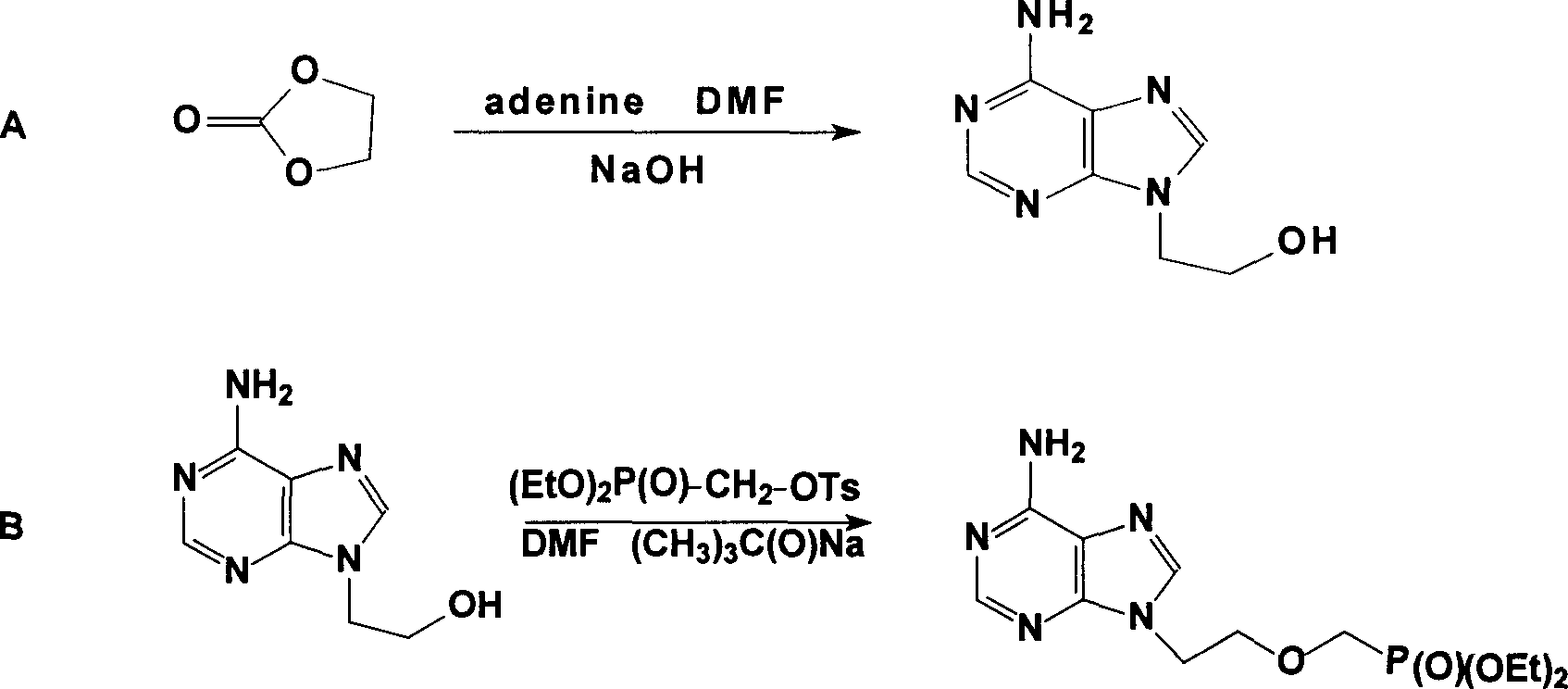
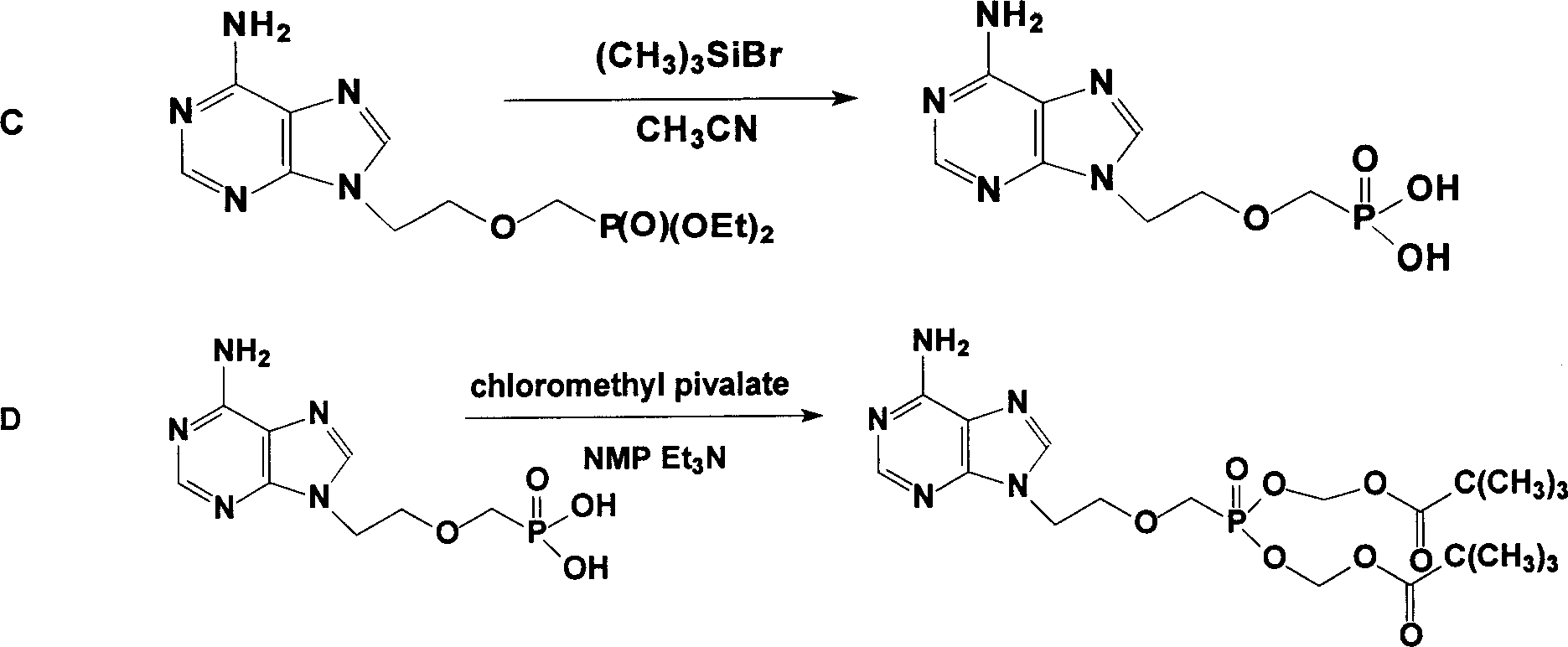
The invention relates to a novel method for preparing tenofovir, in particular to a method for preparing a tenofovir compound, in particular the monohydrate of the tenofovir for resisting hepatitis B viruses and human immunodeficiency viruses. The method comprises the following steps of: using (substituted) triphenylchloromethane XIII to protect hydroxy in the end position of (R)-1,2-propanediol,condensing the obtained product with dialkyl sulfonyloxy methyl phosphonate V to obtain XV, hydrolyzing the XV in the aqueous solution of an organic acid to remove the protective group of the hydroxyl at the end position of XV to obtain an intermediate XI, condensing sulfonate XII of the intermediate XI with adenine to obtain an intermediate VII, transforming the intermediate VII into the corresponding trimethylsilyl phosphonate VIII by trimethylsilyl bromide (TMSBr), and hydrolyzing the VIII to obtain the target product. The method has the advantages of short process route, convenient operation, low cost and easy access of raw materials and favorability for large scale production.
Owner:INST OF PHARMACOLOGY & TOXICOLOGY ACAD OF MILITARY MEDICAL SCI P L A
Prepn process of Adefovir dipivalate
InactiveCN1506370ASuitable for industrializationReduce manufacturing costGroup 5/15 element organic compoundsAntiviralsTrimethylsilyl chloridePurine
The present invention discloses one improved preparation process of Adefovir dipivalate. In the synthesis of the mother compound 9-[2-(phosphinocarboxyl methoxy) ethyl adenine, trimethyl chlorsilane, rather than expensive trimethyl bromosilane, as material and potassium iodide as nucleophilic reagentare used to reduce cost while the product yield and quality is maintained. The production process of the present invention may be used in industrial production.
Owner:HANGZHOU HUADONG MEDICINE GRP PHARMA RES INST
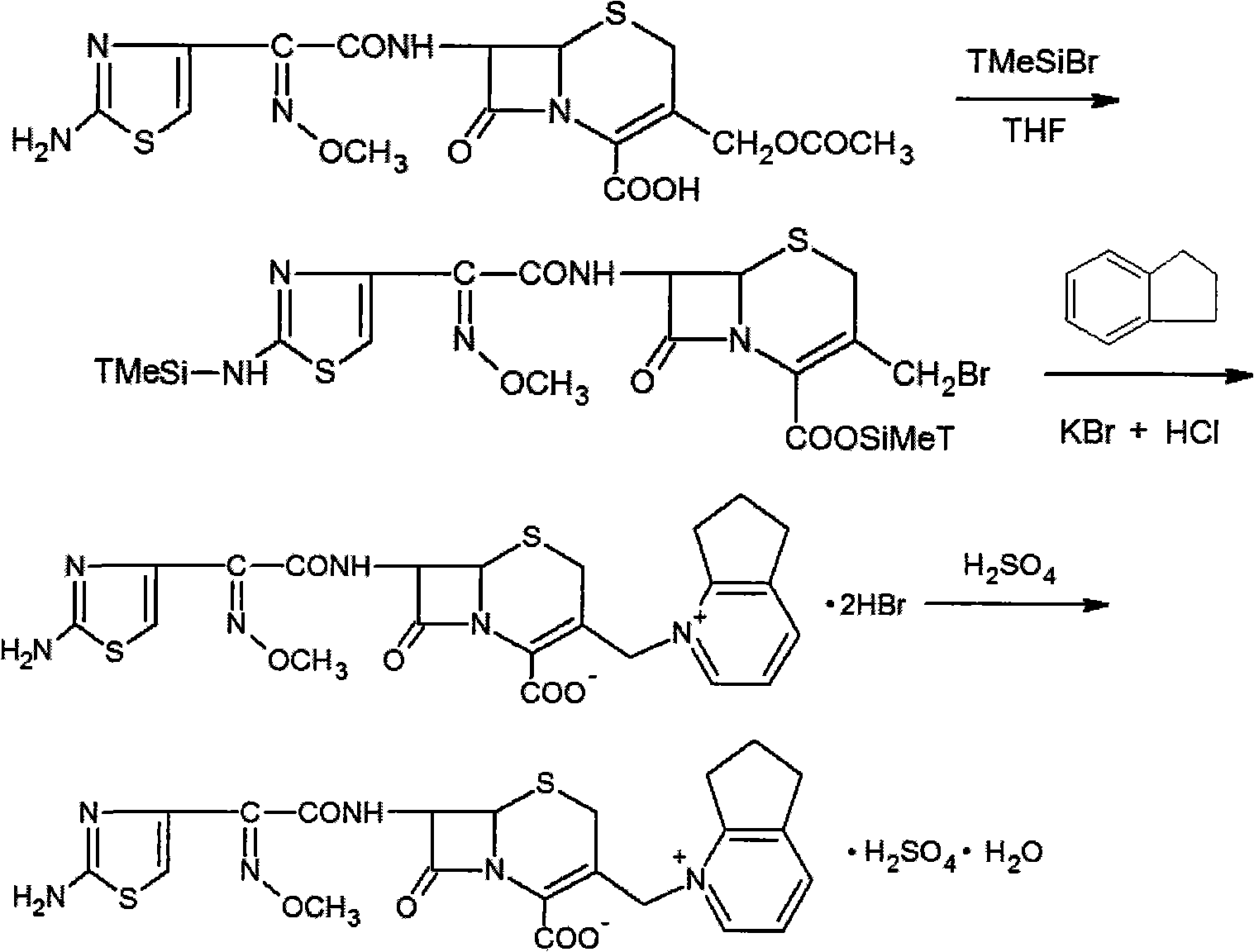
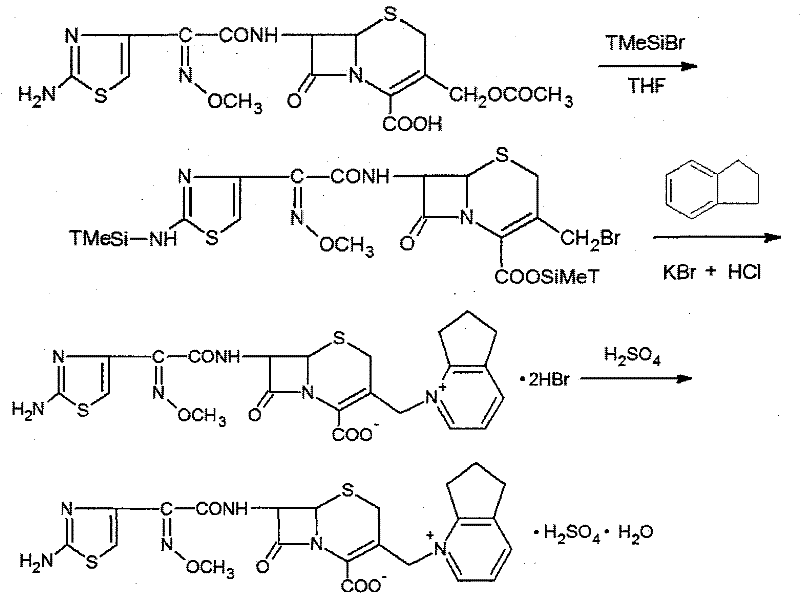
Synthetic method of cefpirome sulfate
ActiveCN101284840ALow costHigh yieldAntibacterial agentsOrganic chemistryTrimethylsilyl chlorideCefotaxime
The invention relates to a synthesis method for making cefpirome sulfate. The method comprises the following steps that: cefotaxime and 2, 3-cyclopenopyridine, with the molar ratio of between 1:7 and 1:12, are mixed with trimethyl bromosilicane used as protecting agent inside organic solvent to carry out substitution reaction, thereby generating cefpirome dihydrobromide; and the salt-forming reaction of the cefpirome dihydrobromide is carried out to make cefpirome sulfate. According to the synthesis method of cefpirome sulfate provided by the technical proposal of the invention, cefotaxime and trimethyl bromosilicane are respectively used as raw material and protecting agent to make cefpirome sulfate. Because enough supply and technical maturity of cefotaxime are realized in China and trimethyl bromosilicane is more active than trimethylchlorosilane and is cheaper than trimethyliodosiliane, the synthesis method has the advantages of low cost of raw material, high yield, simple synthetic route and convenient operation.
Owner:GUANGDONG JIUMING PHARMA
Tenofovir preparation method suitable for industrialized production
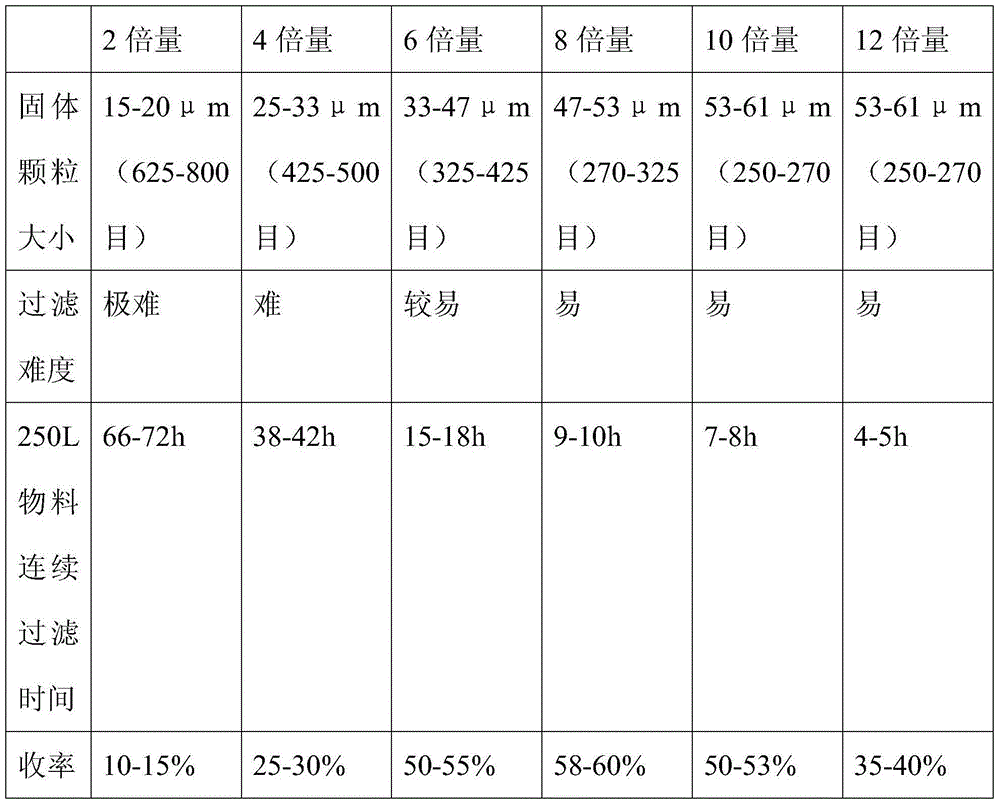
ActiveCN104098605AMild conditions for crystallizationMild reaction conditionsGroup 5/15 element organic compoundsLithiumFiltration
The invention provides a tenofovir preparation method suitable for industrialized production. According to the method, tenofovir is obtained through catalyzing and hydrolyzing a raw material intermediate (R)-9-[2-(diethylphosphonomethoxy)propyl]adenine via trimethylbromosilane, wherein the raw material intermediate (R)-9-[2-(diethylphosphonomethoxy)propyl]adenine is obtained through the following steps: firstly, obtaining (R)-9-(2-hydroxypropyl)adenine via the ring-opening condensation of adenine and (R)-epoxypropane under an alkaline condition; secondly, catalyzing the (R)-9-(2-hydroxypropyl)adenine through lithium t-butoxide; thirdly, conducting etherification on the catalyzed (R)-9-(2-hydroxypropyl)adenine and p-benzenesulfonyloxymethyl phosphoric acid diethylester, so as to obtain the (R)-9-[2-(diethylphosphonomethoxy)propyl]adenine. The method is characterized in that purified water of appropriate proportion is added in a mixed product obtained through catalyzing and hydrolyzing the (R)-9-[2-(diethylphosphonomethoxy)propyl] adenine via trimethylbromosilane; the tenofovir is obtained through spontaneous crystallization at an appropriate temperature and cooling rate, as well as at an appropriate stirring speed. According to the invention, the obtained tenofovir product has the characteristics of high yield, strong indissolubility in operation under room temperature, simplicity in filtration and collection due to large particles, short production time, low energy consumption, and the like.
Owner:FUJIAN COSUNTER PHARMA CO LTD
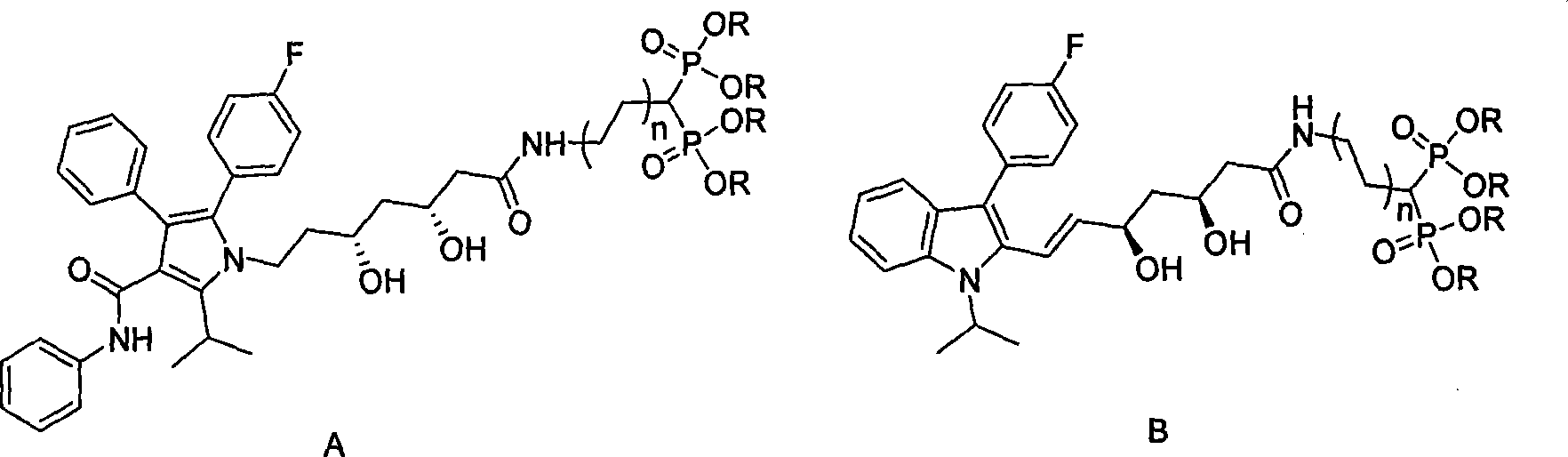
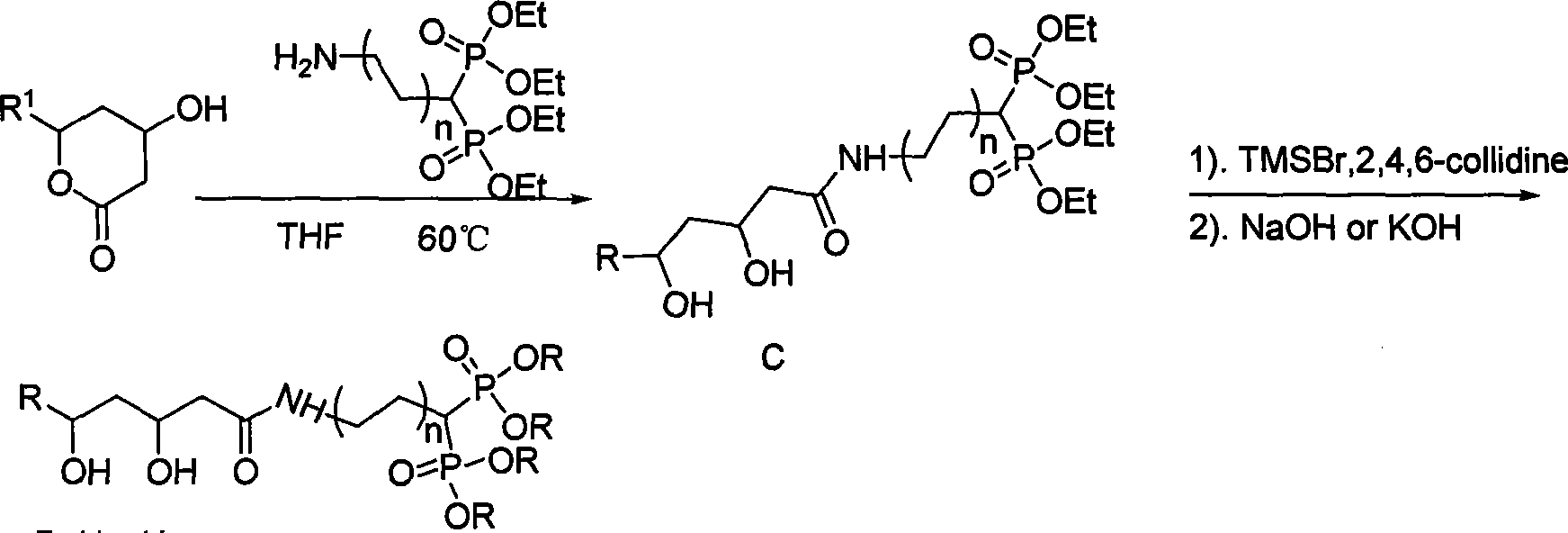
Statin-diphosphonic acid conjugates, preparation method and application thereof
InactiveCN101215297AGood bone affinityThe synthesis method is simpleOrganic active ingredientsMetabolism disorderReaction temperatureSolvent
The invention relates to a statin-double phosphonate coupling compound for curing osteoporosis and other bone calcium metabolic diseases and a preparation process and an application, in particular to an atorvastain or fluvastatin-coupling compound and a synthesis and an application. The invention comprises mixing fluvastatin lactone or atorvastain lactone and double phosphonate according to statin lactone: double phosphonate ester= 1:1-3, obtaining statin double phosphonate coupling compound, and then obtaining salt through the reaction of the 1:4-20:4-20 proportion of double phosphonate coupling compound: 2, 4, 6-trimethyl pyridine: trimethyl bromo-silicane in solution with below 5-10 DEG C of reaction temperature. The synthesis process of the invention is simple, which can synthesize eight new compounds, namely that four new intermediate compounds and four target compounds, and provides an effective medicament for preventing and curing osteoporosis and other bone calcium metabolic diseases.
Owner:HUNAN NORMAL UNIVERSITY
Synthesis method of 5-chloro-7-azaindole
The invention provides a synthesis method of 5-chloro-7-azaindole. The synthesis method comprises the following steps: (1) reacting a dilithium initiator and trimethylbromosilane to prepare silicon-containing organic lithium; (2) reacting 2-amino-3-methylpyridine and di-tert-butyl dicarbonate to prepare 2-N-BOC-amino-3-methylpyridine; (3) performing lithiation on the 2-N-BOC-amino-3-methylpyridine through the silicon-containing organic lithium, and performing delithiation activation, cyclization and dehydration to prepare 7-azaindole; (4) performing hydrogenation reduction reaction on the 7-azaindole to generate 2,3-dihydro-7-azaindole; (5) performing chlorination reaction on the 2,3-dihydro-7-azaindole through liquid chlorine to generate 5-chloro-2,3-dihydro-7-azaindole; and (6) performing dehydrogenation reaction on the 5-chloro-2,3-dihydro-7-azaindole to obtain 5-chloro-7-azaindole. The synthesis method provided by the invention has the advantages of mild conditions and high yield.
Owner:叶芳
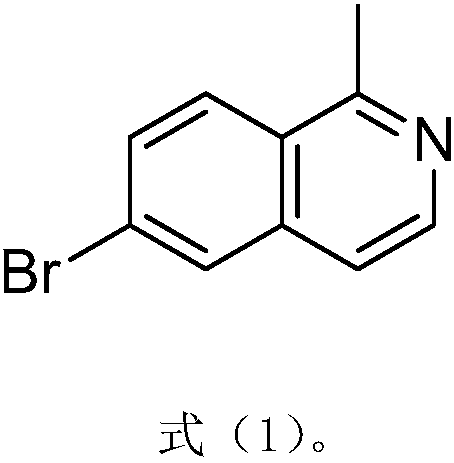
Method for synthesizing pharmaceutical intermediate nitrogen heterocyclic bromo-compound
ActiveCN108084090AFew synthetic stepsStrong invention ideaOrganic chemistryGrignard reagentEthyl acetate
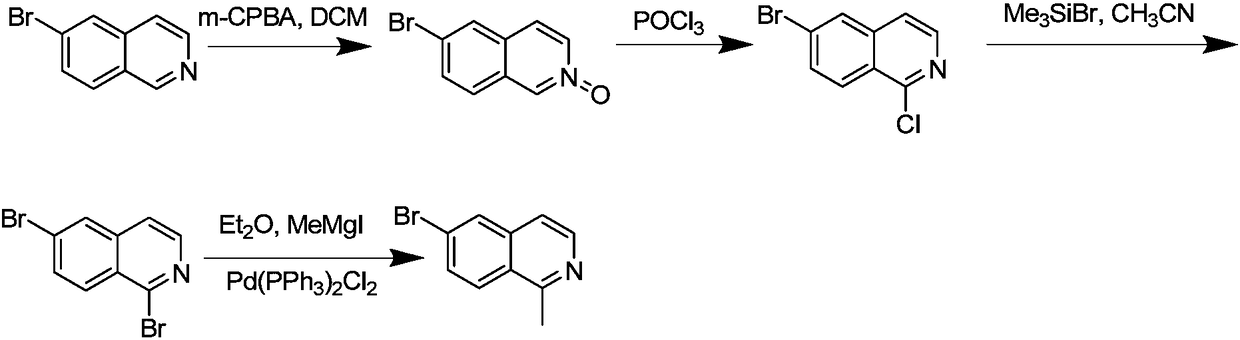
The invention relates to a method for synthesizing a pharmaceutical intermediate nitrogen heterocyclic bromo-compound. The method comprises that 6-bromoisoquinoline, dichloromethane and m-chloroperoxybenzoic acid as raw materials undergo a reaction to produce a mixture 6-bromoisoquine oxynitride, phosphorus oxychloride is added into the mixture so that solid and water phase products are obtained,the solid products are dried, the water phase products are repeatedly washed and are extracted multiple times, the organic phase is spin-dried, the solid and water phases are treated so that 6-bromo-1-chloroisoquinoline is obtained, the solid crude product and the water crude product are mixed, the mixture is treated through a silica gel column of 100-200 meshes, the mixture is eluted through a petroleum ether PE: ethyl acetate EA eluent A to form a pure product 6-bromo-1-chloroisoquinoline, trimethylbromosilane, acetonitrile and 6-bromo-1-chloroisoquinoline undergo a reaction, pH of the reaction product is adjusted to 7 so that 1, 6-dibromoisoquinoline is obtained, and the 1, 6-dibromoisoquinoline is added into dichloroditriphenyl phosphine and a homemade Grignard reagent so that a finalproduct 6-bromo-1-methylisoquinoline is obtained. The method has the advantages of clear processes, less waste, high yield, raw material saving and operation easiness.
Owner:北京六合宁远医药科技股份有限公司
Preparation method of stable silicon-coated pure-phase CsPb2Br5 inorganic nanocrystal
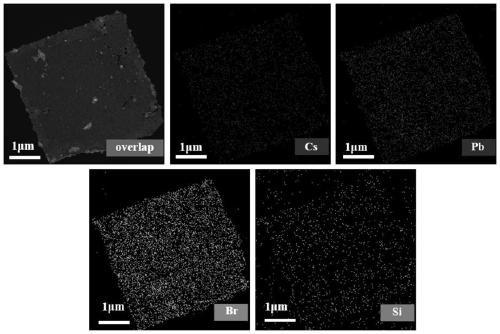
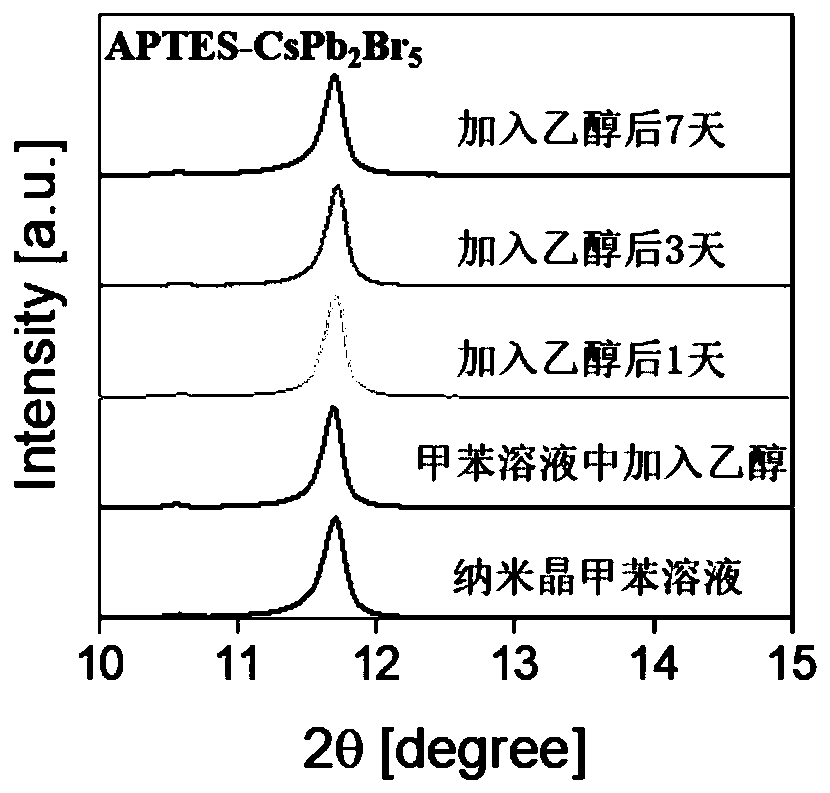
ActiveCN111517364APrecise control ratioReduce defect statesMaterial nanotechnologyLead compoundsPhysical chemistryLead acetate
The invention provides a preparation method of a stable silicon coated pure-phase CsPb2Br5 inorganic nanocrystal. The preparation method comprises the following steps: adding octadecene, oleic acid and APTES into cesium carbonate and anhydrous lead acetate to prepare a reaction precursor solution, performing heating and stirring, performing heating to 60 DEG C under vacuum, and introducing nitrogen to remove reaction gas generated in the heating process; cutting back to vacuum, heating to 90 DEG C, and introducing nitrogen; heating to 120 DEG C in vacuum, keeping the temperature until cesium carbonate and anhydrous lead acetate are completely dissolved, introducing nitrogen, heating to 140-150 DEG C, injecting trimethylbromosilane, reacting for 1 hour, and cooling to room temperature; andperforming mixing with methyl acetate, and purifying the mixture for seven times to obtain the stable silicon-coated pure-phase CsPb2Br5 nanocrystal. According to the method, a silicon shell layer isprepared by adopting APTES, the stability of the nanocrystal is improved, the structure of the silicon shell layer is not damaged after multiple times of purification, and the high stability is achieved while it is guaranteed that the pure-phase CsPb2Br5 nanocrystal is synthesized.
Owner:UNIV OF ELECTRONIC SCI & TECH OF CHINA
Method for synthesizing bromotrimethylsilane
ActiveCN102153582AAvoid it happening againHigh purityGroup 4/14 element organic compoundsPhosphorus tribromideChemical products
The invention discloses a method for synthesizing bromotrimethylsilane, and relates to a synthetic production technology for a chemical product. The method comprises the following steps of: under oxygen-free conditions, stirring in a reaction kettle in which phosphorus tribromide and silyl ether are sequentially added, raising the temperature to 1005 DEG C, keeping the temperature, performing reflux reaction for 60.5 hours, rectifying under normal pressure to obtain the bromotrimethylsilane, and stopping to receive a finished product of the bromotrimethylsilane when the temperature in the kettle is raised to 135 DEG C. By the method, a silyl ether-phosphorus tribromide method is improved and innovated, side reactions are avoided, the obtained product has purity, and the finished product of the bromotrimethylsilane which has the content of about 99 percent can be obtained; and the stirring at normal temperature is changed into high-temperature reflux, so that the reaction time is greatly shortened.
Owner:扬州三友合成化工有限公司
High-regioselectivity bromination method for phenol compounds
ActiveCN109761759AHigh regional selectivityAchieve recyclingOrganic compound preparationCarboxylic acid esters preparationSolventNitrogen gas
The invention discloses a high-regioselectivity bromination method for phenol compounds. According to the high-regioselectivity bromination method, bromotrimethylsilane serving as a bromination reagent, aryl sulfoxide serving as an activating agent and the phenol compounds are stirred and react for 1-12 hours at the temperature of 0-50 DEG C in a solvent and under the nitrogen atmosphere, thus high-regioselectivity bromination of the phenol compounds is achieved, and bromophenol compounds are obtained by separating and purifying through filtering, extraction or column chromatography methods. The high-regioselectivity bromination method adopts the aryl sulfoxide as the activating agent, the sulfoxide substituent group is large, on the one hand, regioselectivity of a phenol compound bromination reaction is high, when the hydroxyl para-position of the phenol compounds has no substituent group, a product of para-bromination is obtained in a regioselectivity mode, however when the hydroxylpara-position of the phenol compounds has the substituent group, a product of ortho-bromination is obtained in a regioselectivity mode, on the other hand, by-products can be simultaneously recycled byseparating and purifying through the filtering and extraction methods, and the separating and purifying costs are lowered compared with the column chromatography.
Owner:XINYANG NORMAL UNIVERSITY
Preparation method of high-stability adefovir dipivoxil
PendingCN110143983AExtended shelf lifeSmall particle sizeGroup 5/15 element organic compoundsOrganic chemistry methodsPurineActive ingredient
The invention belongs to the field of pharmaceutical synthesis. A preparation method of high-stability adefovir dipivoxil is characterized by comprising the following steps: 1) a compound 1 undergoestrimethylbromosilane substitution, followed by hydrolysis with a NaOH aqueous solution to obtain a compound 2; 2) the compound 2 and a compound 3 are catalyzed by triethylamine in N-methylpyrrolidoneto obtain a compound 4; 3) the compound 4 is refined to obtain a compound 5, namely [[2-(6-amino-9H-purine-9-yl)ethoxy]methyl]phosphodi(pivaloyloxymethyl)ester. The preparation method of high-stability adefovir dipivoxil has advantages of good stability of bulk drugs and simple production process, and is suitable for large-scale commercial production.
Owner:BEIJING JIMEITANG MEDICINE RES CO LTD
Synthetic method of cefpirome sulfate
ActiveCN101284840BLow costHigh yieldAntibacterial agentsOrganic chemistryTrimethylsilyl chlorideCefotaxime
The invention relates to a synthesis method for making cefpirome sulfate. The method comprises the following steps that: cefotaxime and 2, 3-cyclopenopyridine, with the molar ratio of between 1:7 and 1:12, are mixed with trimethyl bromosilicane used as protecting agent inside organic solvent to carry out substitution reaction, thereby generating cefpirome dihydrobromide; and the salt-forming reaction of the cefpirome dihydrobromide is carried out to make cefpirome sulfate. According to the synthesis method of cefpirome sulfate provided by the technical proposal of the invention, cefotaxime and trimethyl bromosilicane are respectively used as raw material and protecting agent to make cefpirome sulfate. Because enough supply and technical maturity of cefotaxime are realized in China and trimethyl bromosilicane is more active than trimethylchlorosilane and is cheaper than trimethyliodosiliane, the synthesis method has the advantages of low cost of raw material, high yield, simple synthetic route and convenient operation.
Owner:GUANGDONG JIUMING PHARMA
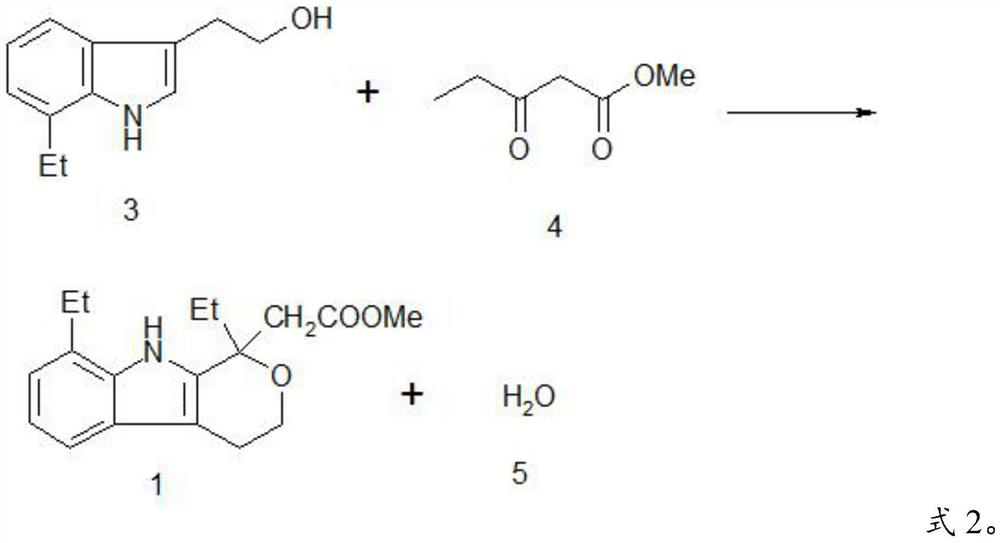
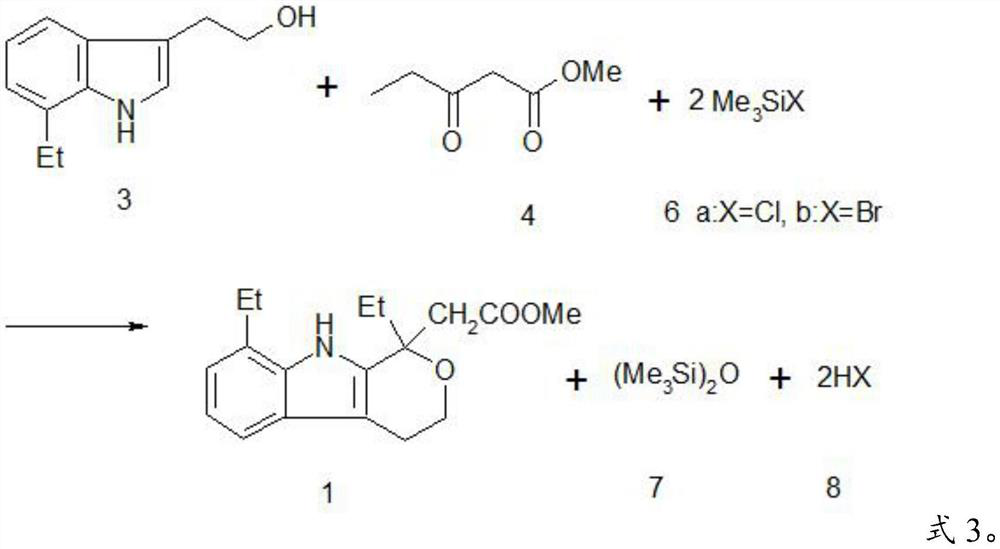
A kind of method preparing etodolac methyl ester
ActiveCN111303172BReduce workloadHigh yieldGroup 4/14 element organic compoundsOrganic synthesisEthyl group
The invention provides a method for preparing etodolac methyl ester, which relates to the technical field of organic synthesis. The method provided by the invention comprises the following steps: (1) after the reaction raw materials are mixed, a ring closure reaction is carried out at 20-25° C. to obtain a reaction liquid; the reaction raw materials include 7-ethyltryptol, 3-oxopentanoic acid Methyl ester, trimethylhalosilane and methanol, excluding concentrated sulfuric acid; the trimethylhalosilane is trimethylchlorosilane or trimethylbromosilane; (2) cooling the reaction solution to 10-15°C , and filtered to obtain etodolac methyl ester and mother liquor. The method provided by the invention has high yield and does not use concentrated sulfuric acid which is extremely harmful and easily causes product oxidation. Further, the present invention further improves the product yield through the continuous application of the mother liquor, can obtain almost quantitative products, reduces the workload of solvent treatment, and is easy to operate; and the trimethylhalosilane is easy to recover and recycle.
Owner:ZHEJIANG INT STUDIES UNIV
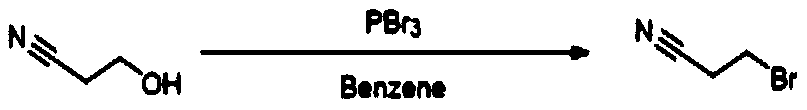
Preparation method of 3-bromopropionitrile
ActiveCN110790679AHigh purityHigh yieldOrganic compound preparationCarboxylic acid nitrile purification/separationMeth-Biochemical engineering
The invention discloses a preparation method of 3-bromopropionitrile, wherein the preparation method comprises the following steps: taking acrylonitrile as a raw material, carrying out trimethylbromosilane intermolecular addition reaction, and distilling and purifying to obtain high-purity 3-bromopropionitrile. According to the method, cheap acrylonitrile is used as the raw material, and the high-purity 3-bromopropionitrile is obtained through the trimethylbromosilane intermolecular addition reaction and finally distillation and purification; the raw material is cheap and easy to obtain, and the method is suitable for commercial production, environmentally friendly, small in amount of generated three wastes and high in yield.
Owner:ASTATECH CHENGDU BIOPHARM CORP
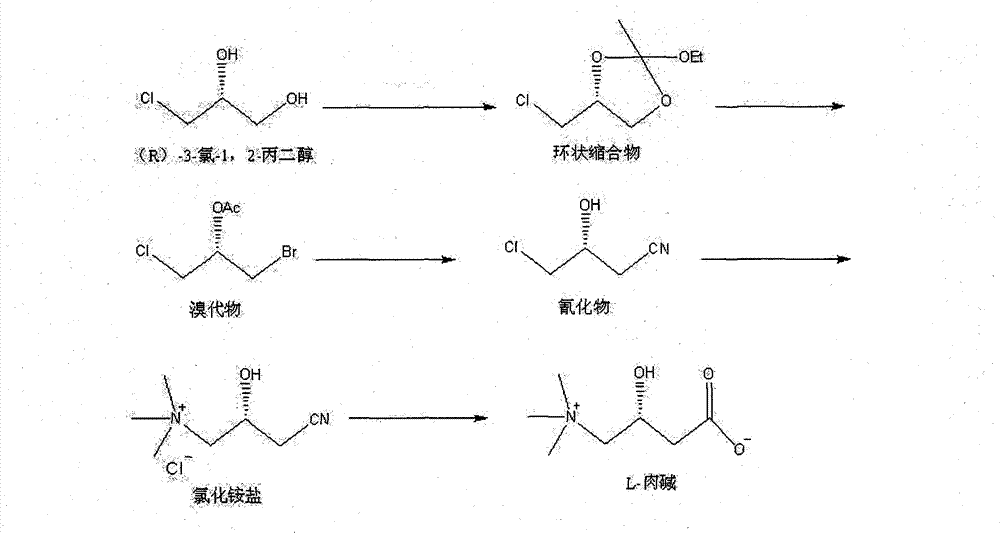
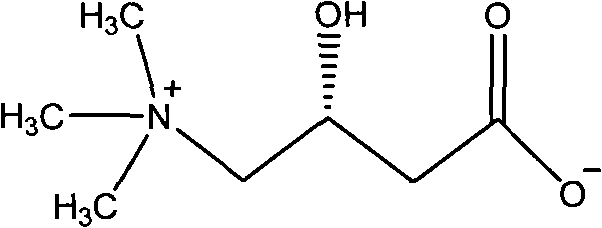
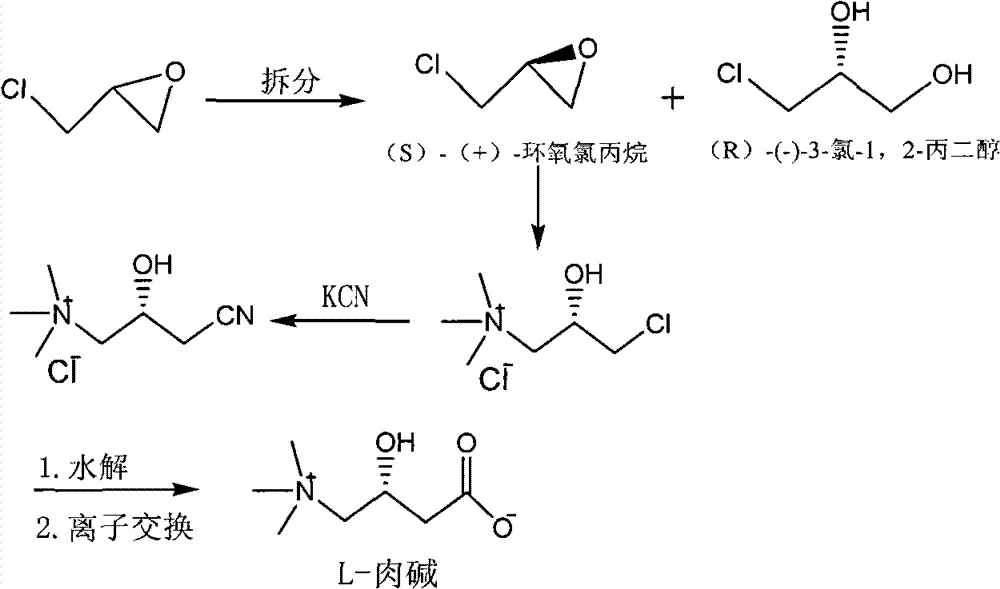
Method for synthesizing L-carnitine by using (R)-(-)-3-chlorine-1,2-propylene glycol as chiral initiative raw material
InactiveCN101838212BOrganic compound preparationAmino-carboxyl compound preparationTrimethylsilyl bromideTriethyl orthoacetate
The invention provides a method for synthesizing L-carnitine by using (R)-(-)-3-chlorine-1,2-propylene glycol as a chiral initiative raw material, which belongs to the field of medicament chemistry. The method comprises the following steps of: mixing a side product, namely (R)-(-)-3-chlorine-1,2-propylene glycol used as the initiative raw material with triethyl orthoacetate for reacting to produce an annular condensation compound, wherein the side product is generated when the L-carnitine is prepared by chirally splitting racemic epoxy chloropropane; reacting the annular condensation compound with trimethylsilyl bromide to obtain brominated substance; reacting the brominated substance with sodium cyanide to produce cyanide; reacting the cyanide with trimethylamine to produce ammonium chloride salt; and performing hydrolysis and ion exchange on the ammonium salt under the acidic condition to produce the L-carnitine finally. The method has the advantage that: the L-carnitine is prepared by using the side product, namely (R)-(-)-3-chlorine-1,2-propylene glycol which is generated when the L-carnitine is prepared by chirally splitting racemic epoxy chloropropane as the chiral initiative raw material and by adopting low-cost and readily available chemical raw materials, the reaction condition is simple and mild and the conversion efficiency is high.
Owner:UNIV OF SCI & TECH BEIJING
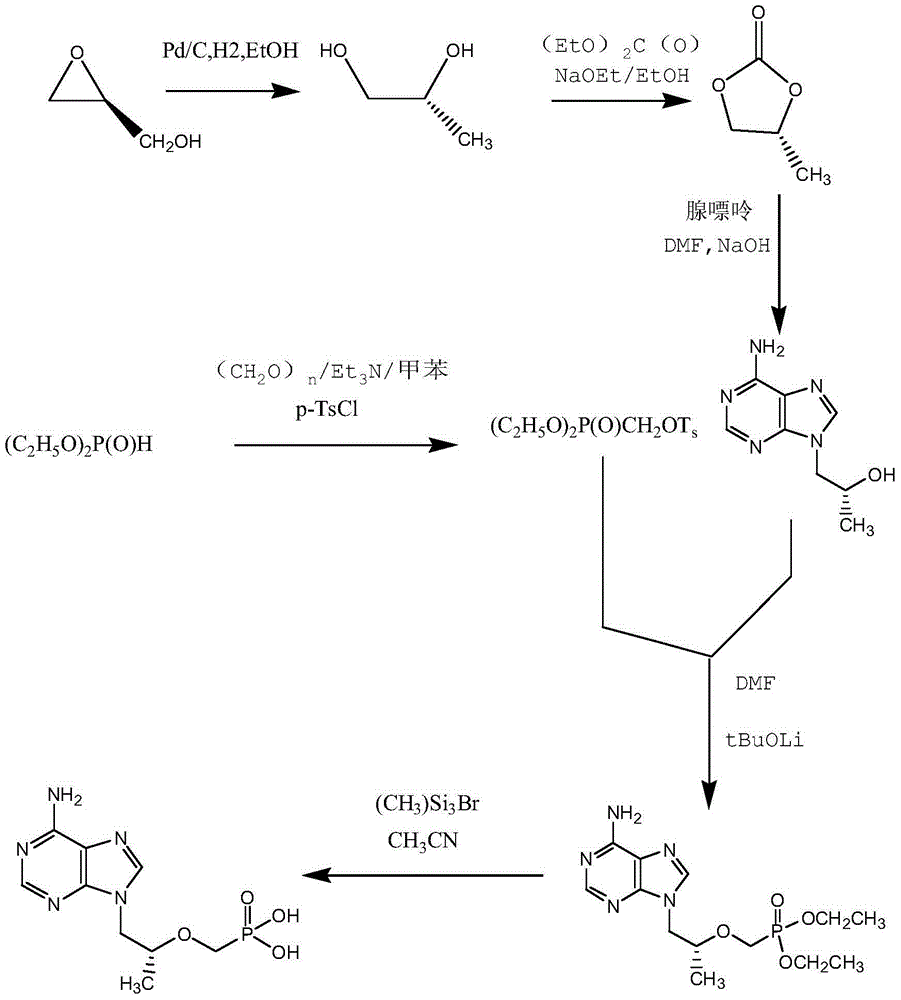
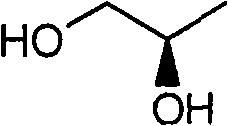
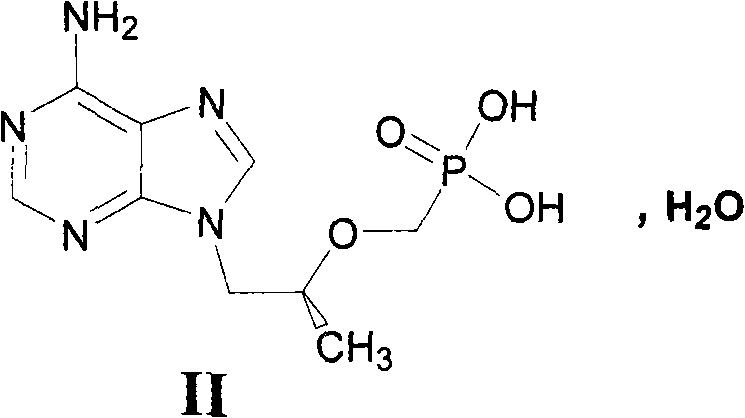
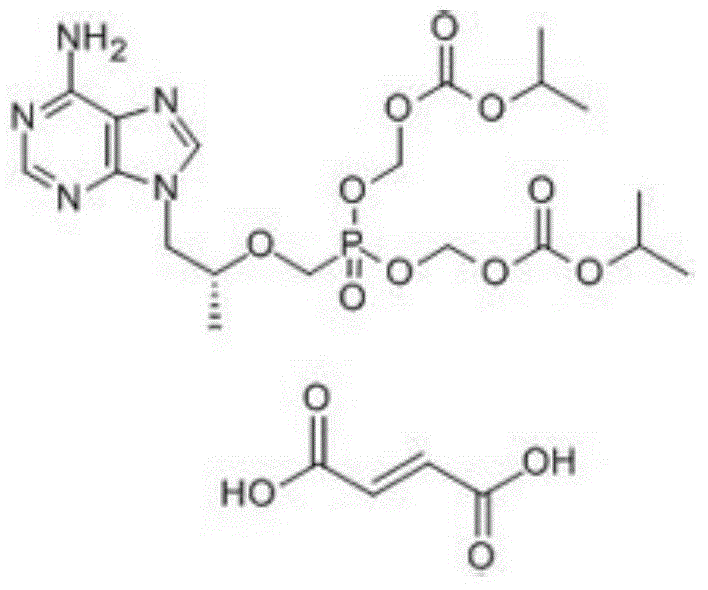
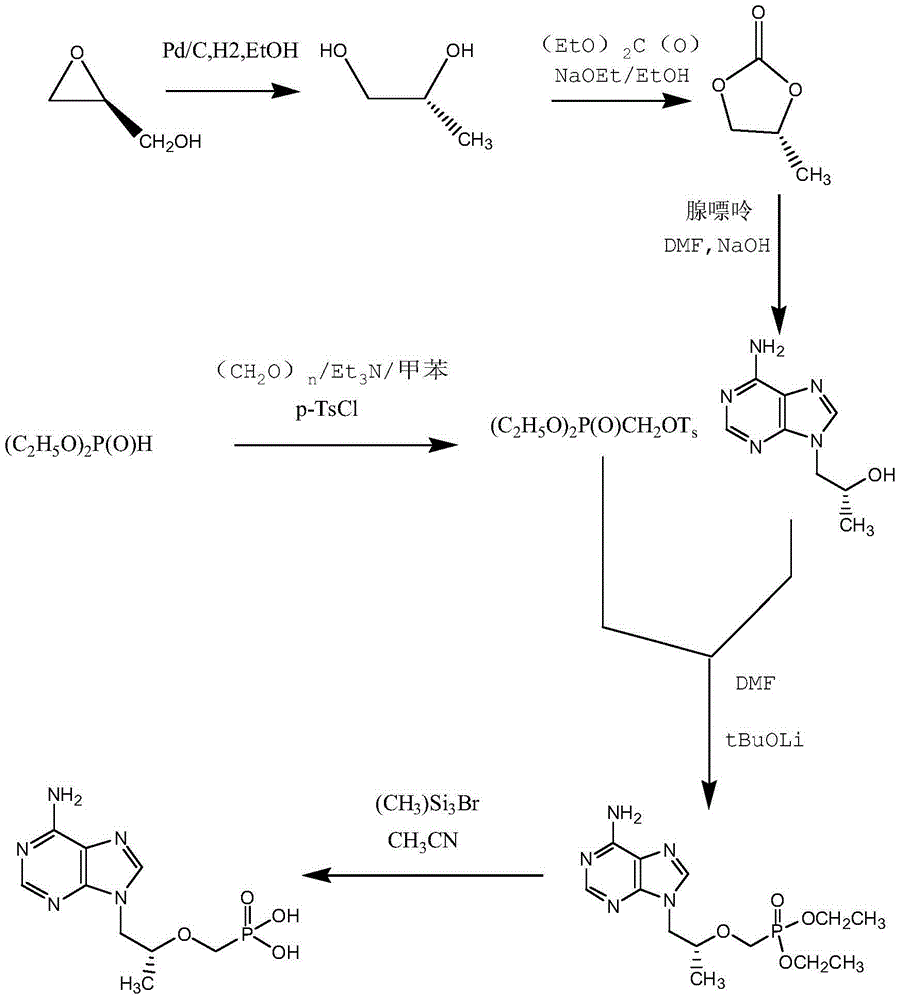
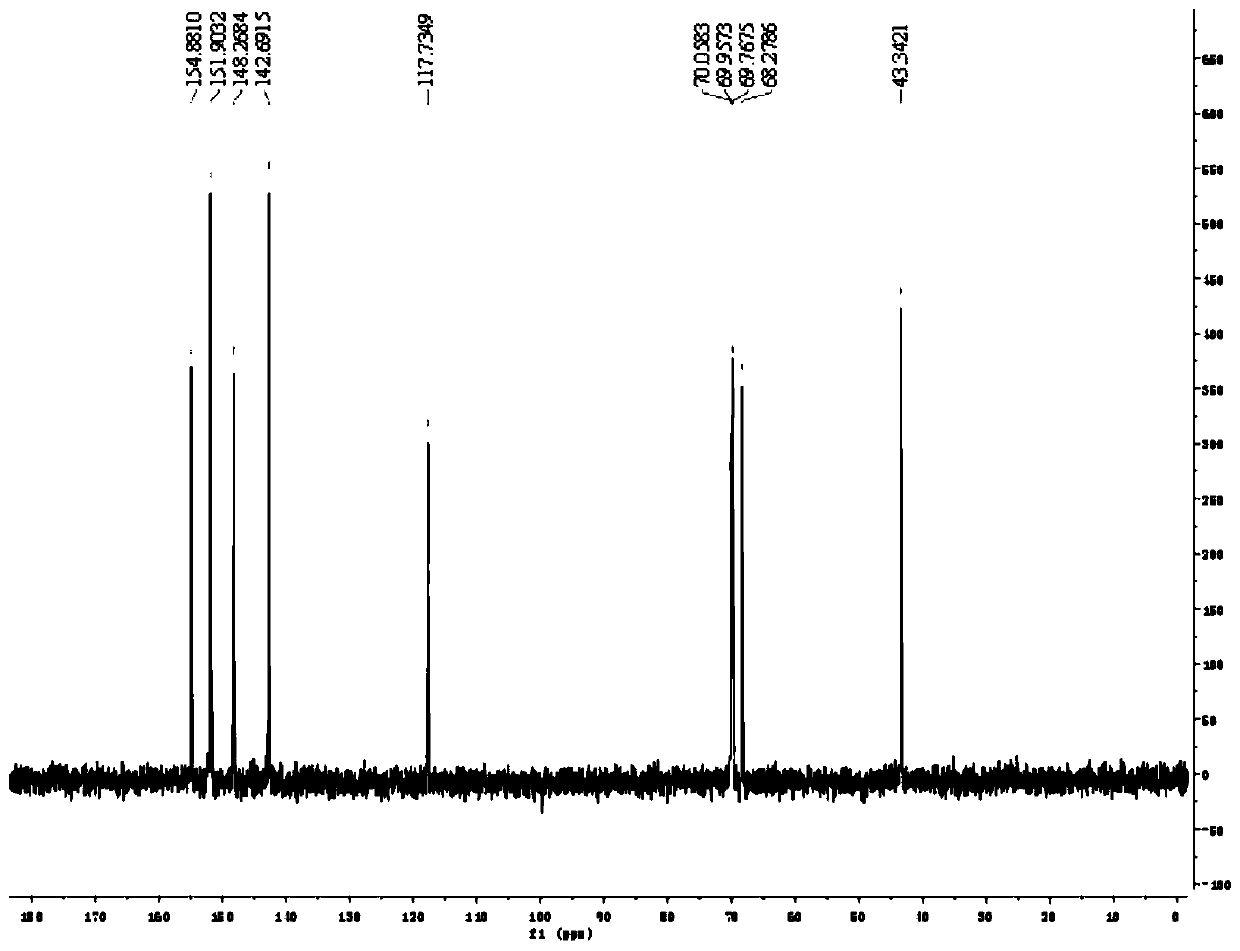
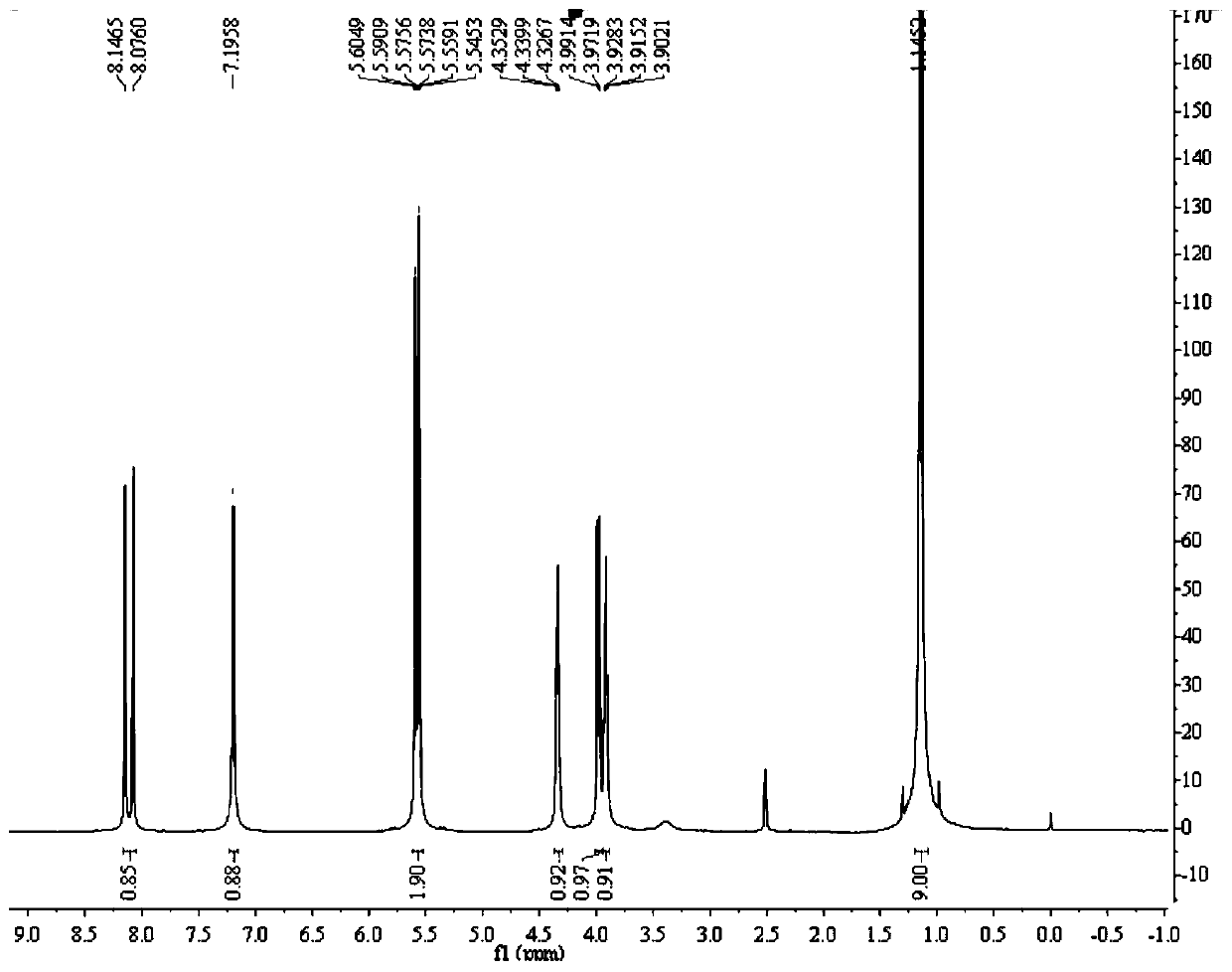
Preparation method of (R)-9-[2-(phosphoryl methoxyl)propyl]-adenine
ActiveCN105646585AHigh yieldReduce generationGroup 5/15 element organic compoundsAntiviral drugDiethyl methylphosphonate
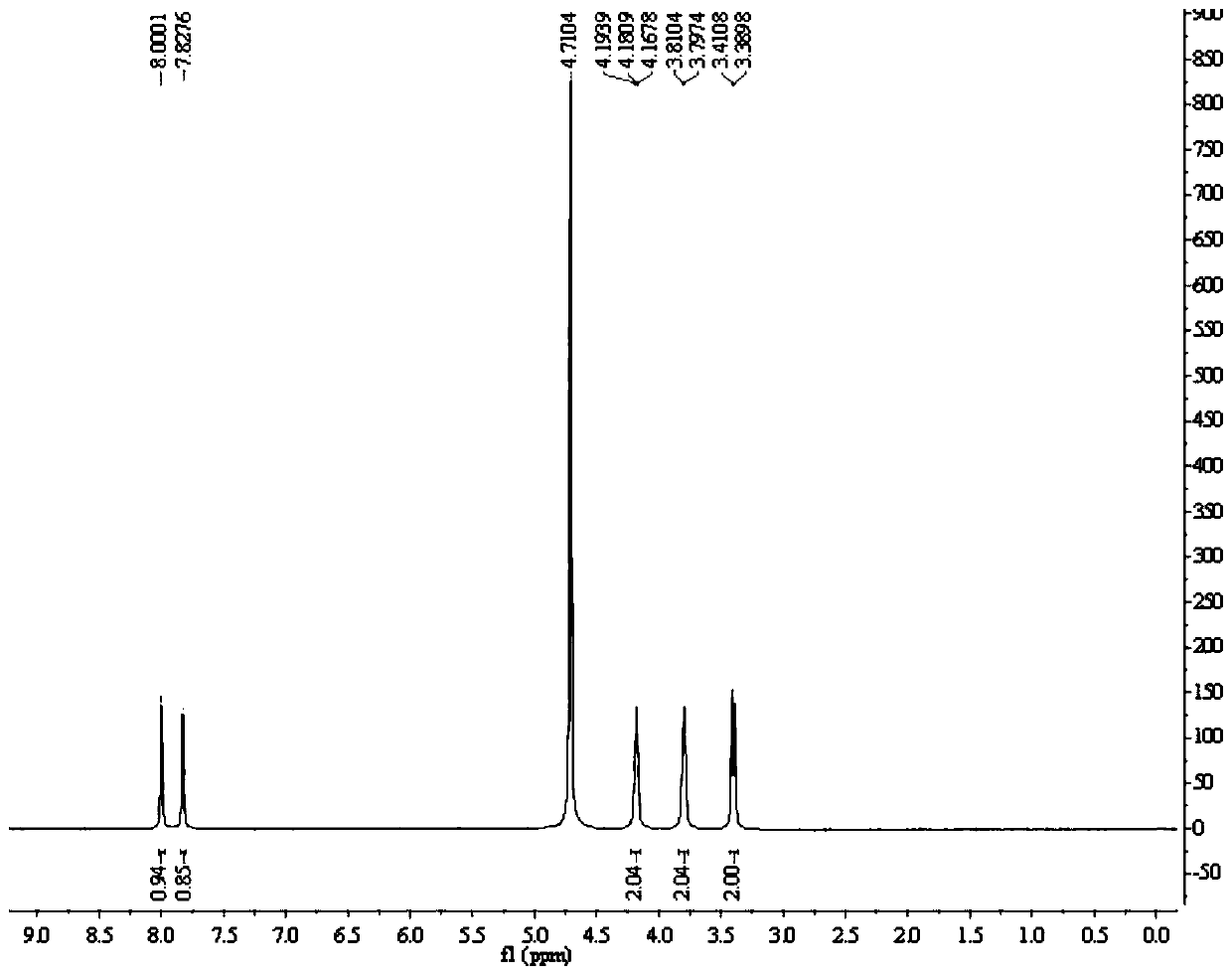
The invention discloses a preparation method of (R)-9-[2-(phosphoryl methoxyl)propyl]-adenine, and relates to a preparation method of an antiviral drug for an acquired immune deficiency syndrome. The method comprises the steps that when (R)-9-[2-(diethyl phosphoryl methoxyl)propyl]-adenine is prepared, (R)-hydroxypropyl adenine and diethyl p-toluenesulfonyloxymethylphosphonate (DESMP) are subjected to a condensation reaction under the action of magnesium tert-butoxide to generate the (R)-9-[2-(diethyl phosphoryl methoxyl)propyl]-adenine; PMPA diethyl ester is subjected to deethylation through trimethylbromosilane by taking water as solvent to obtain the (R)-9-[2-(phosphoryl methoxyl)propyl]-adenine (PMPA). According to the technology, the product yield is increased, the technology is cleaner, the production cost is lowered, the product yield is increased, the raw materials are easy to obtain, and industrialization is easy to achieve.
Owner:扬州三友合成化工有限公司
Method for synthesizing bromotrimethylsilane
ActiveCN102153582BReduce usageHigh purityGroup 4/14 element organic compoundsRefluxPhosphorus tribromide
Owner:扬州三友合成化工有限公司
A method for highly regioselective bromination of phenolic compounds
ActiveCN109761759BHigh regional selectivityAchieve recyclingOrganic compound preparationCarboxylic acid esters preparationMeth-Ortho position
Owner:XINYANG NORMAL UNIVERSITY
Novel method for preparing tenofovir
InactiveCN101906119BImprove protectionReduced responseOrganic active ingredientsGroup 5/15 element organic compoundsTrimethylsilyl bromideHuman immunodeficiency
The invention relates to a novel method for preparing tenofovir, in particular to a method for preparing a tenofovir compound, in particular the monohydrate of the tenofovir for resisting hepatitis B viruses and human immunodeficiency viruses. The method comprises the following steps of: using (substituted) triphenylchloromethane XIII to protect hydroxy in the end position of (R)-1,2-propanediol, condensing the obtained product with dialkyl sulfonyloxy methyl phosphonate V to obtain XV, hydrolyzing the XV in the aqueous solution of an organic acid to remove the protective group of the hydroxyl at the end position of XV to obtain an intermediate XI, condensing sulfonate XII of the intermediate XI with adenine to obtain an intermediate VII, transforming the intermediate VII into the corresponding trimethylsilyl phosphonate VIII by trimethylsilyl bromide (TMSBr), and hydrolyzing the VIII to obtain the target product. The method has the advantages of short process route, convenient operation, low cost and easy access of raw materials and favorability for large scale production.
Owner:INST OF PHARMACOLOGY & TOXICOLOGY ACAD OF MILITARY MEDICAL SCI P L A
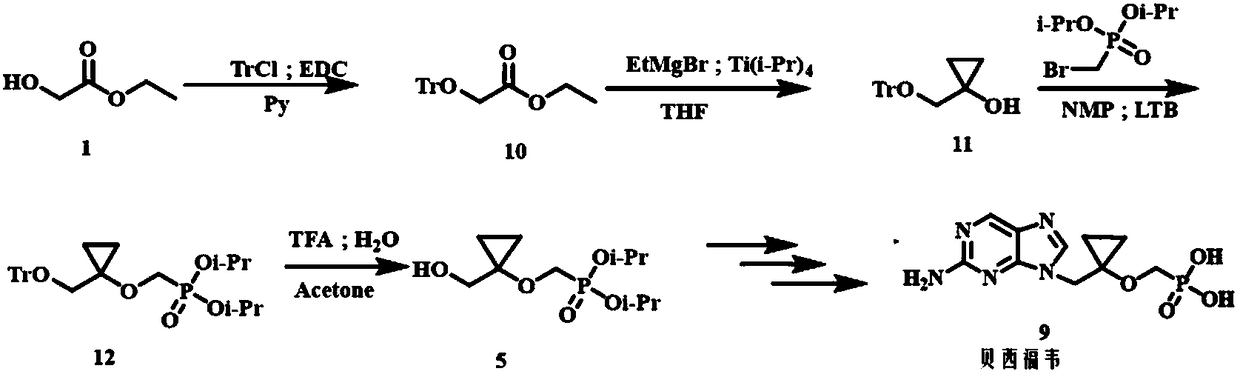
Method for preparing Besifovir
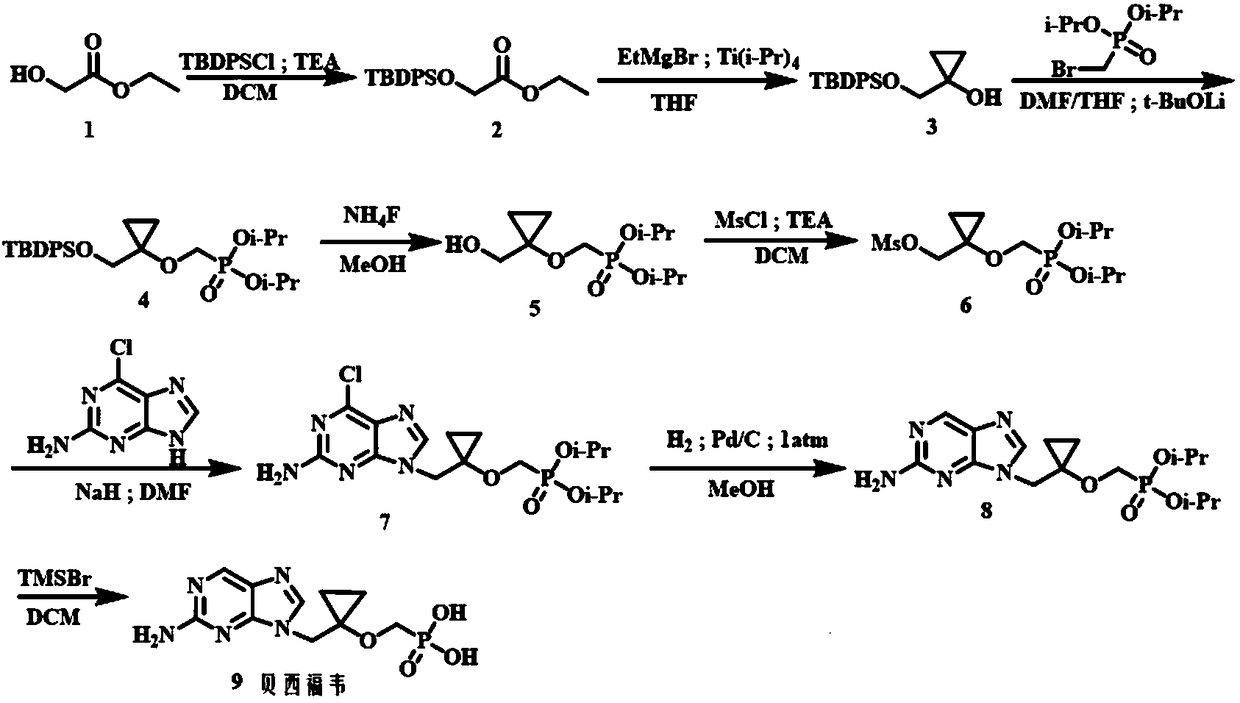
ActiveCN108997429AReduce the difficulty of purificationLow costGroup 5/15 element organic compoundsDiethyl methylphosphonateSilanes
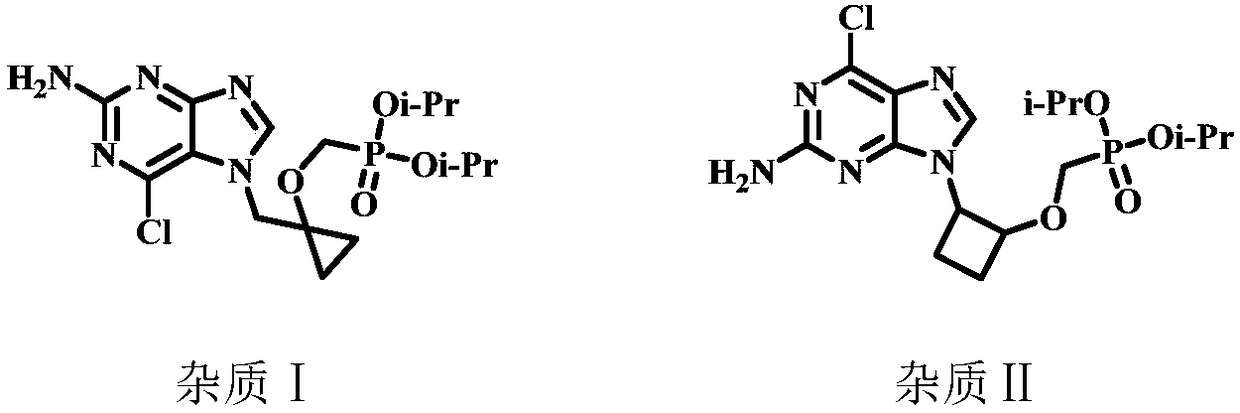
The invention relates to a method for preparing P-[[[1-[(2-amino-9H-purine-9-yl) methyl] cyclopropyl] oxy] methyl]-phosphoric acid (Besifovir). The method comprises the following steps: 1) enabling acompound of formula (1) as shown in the specification to react with tertiary butyl diphenylchlorosilane so as to prepare a compound of formula (2) as shown in the specification; 2) enabling the compound of formula (2) to react with ethyl magnesium bromide so as to prepare a compound of formula (3) as shown in the specification; 3) enabling the compound of the formula (3) to react with p-toluenesulfonyl oxymethyldiethoxyphosphine under a condition that tertiary butanol lithium is adopted as an alkali so as to prepare a compound of formula (23) as shown in the specification; 4) carrying out ammonium fluoride hydrolysis on the compound of formula (23) so as to prepare a compound of formula (24) as shown in the specification; 5) enabling the compound of formula (24) to react with a compound offormula (28) as shown in the specification so as to prepare a compound as a solid of formula (22) as shown in the specification; 6) carrying out reduction dechloridation on the compound of formula (22) as shown in the specification under a condition of a catalyst and a hydrogen supplier so as to prepare a compound as a solid of formula (25) as shown in the specification; 7) carrying out trimethylbromide silane hydrolysis on the compound of formula (25), so as to obtain Besifovir as shown in the specification. The method is cheap in raw material and intermediate material, easy in raw materialand intermediate material obtaining, low in cost, high in yield, gentle in condition and good in security.
Owner:广州粤美医药科技有限公司
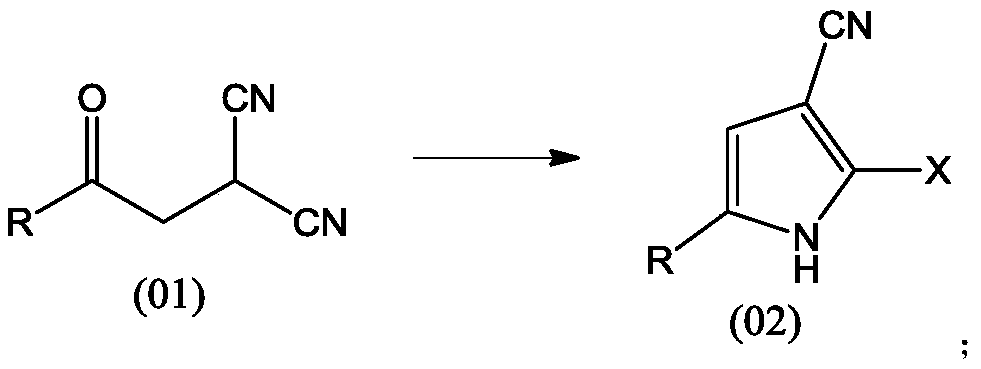
A kind of preparation method of pyrrole compound
The invention provides a preparation method of a pyrrole compound and belongs to the technical field of pharmacy. The preparation method comprises that a raw material compound and a halogenation reagent undergo a reaction in an organic solvent to produce a target compound. The halogenation reagent is trimethylchlorosilane, trimethylbromosilane, triethylchlorosilane, tert-butyl dimethylchlorosilane, phenyl dimethylchlorosilane, oxalyl chloride, acetyl chloride, phosphorus oxychloride, phosphorus pentachloride, phosphorus pentabromide, thionyl chloride, sulfuryl chloride or their composition. The preparation method can prevent harsh conditions such as use of hydrogen chloride gas, is carried out under simple reaction conditions, is easy to operate and control and is suitable for industrial production.
Owner:SUNSHINE LAKE PHARM CO LTD
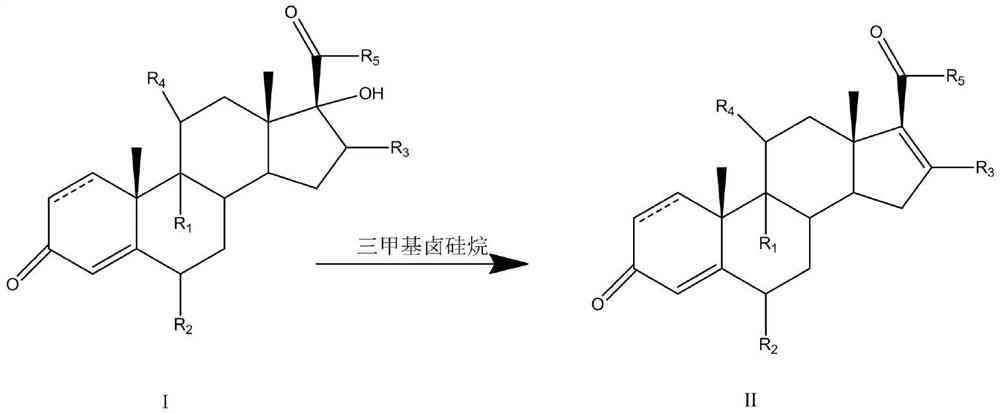
A kind of preparation method of 16-ene steroid compound
ActiveCN110294783BHigh molar yieldHigh puritySteroidsBulk chemical productionTrimethylsilyl chlorideSide reaction
The present invention provides a method for preparing a 16-ene steroid compound. The compound of formula I is used as a substrate, and trimethylhalosilane is used as a dehydrating agent. The methyl halosilane is selected from one or more of trimethylchlorosilane, trimethylbromosilane and trimethyliodosilane. The beneficial effect of the present invention is to provide a method for preparing a 16-ene steroid compound with mild reaction conditions, friendly environment, easy operation, low cost and high molar yield, using trimethylhalosilane as a dehydrating agent, which is cheap and easy to obtain, The new process of the invention has more industrial value, can effectively control side reactions, and improve the reaction yield and quality.
Owner:TIANJIN PHARMA GROUP CORP
Preparation method of adefovir
InactiveCN106317115AShort reaction timeSave man hoursGroup 5/15 element organic compoundsHuman bodySilanes
The invention discloses a preparation method of adefovir. The preparation method comprises the following steps: adding 9-[2-(diethoxylphosphorylmethoxyl)ethyl]adenine into a water solution of HBr, heating to carry out reactions, cooling, and adjusting the pH to 2-3.5 to precipitate white solids namely white adefovir. The preparation method has the advantages that during the hydrolysis of 9-[2-(diethoxylphosphorylmethoxyl)ethyl]adenine, the step of evaporating organic solvents is eliminated, the reaction time is reduced, thus the preparation time is shortened; trimethyl iodo-silane, trimethyl bromo-silane, or trimethyl chloro-silane is replaced by a water solution of HBr, and the cost is reduced by nearly 100 to 400 yuan. Secondary solvent acetonitrile is not used, influence of pollution and solvent residue on drug quality is reduced; trimethyl bromo-silane is easy to decompose and is harmful to human body, and after HBr substitution, the harm to workers is largely reduced.
Owner:天津市亨必达化学合成物有限公司
A kind of phosphonic acid grafted polyimide high temperature proton exchange membrane and preparation method thereof
The invention belongs to the technical field of a fuel battery and particularly relates to a phosphoric acid grafted polyimide high-temperature proton exchange membrane and a preparation method thereof. The proton exchange membrane is prepared by a method comprising the following steps: firstly performing a bromination reaction on a benzene ring of a common diamine monomer to obtain bromized diamine; replacing bromine on the bromized diamine with phosphonate by using n-butyllithium and chlorophosphate so as to obtain phosphate grafted diamine; performing polycondensation reaction by using the phosphate grafted diamine, the common diamine and dianhydride to prepare the polyimide; and finally hydrolyzing the phosphonate on the polyimide by using trimethyl bromo-silicane and hydrochloric acid to prepare the phosphoric acid grafted polyimide high-temperature proton exchange membrane. The proton exchange membrane prepared by the method disclosed by the invention is excellent in proton conduction capability at a high temperature; the phosphoric acid is chemically boned in a polymer, hard to leak and good in hydrolysis resistance and stability; the phosphoric acid can also cooperate with N in the polyimide to form a continuous hydrogen bond network so as to realize water-free proton conduction.
Owner:WUHAN UNIV OF TECH
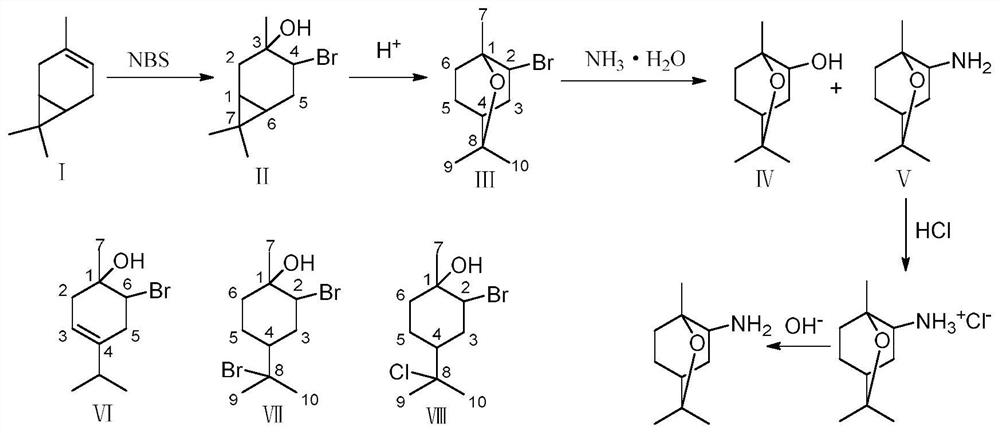
Method for preparing 1,8-cineole derivative from 3-carene
ActiveCN113603705AHigh reactivityAbundant natural reservesOrganic compound preparationPreparation by halogen introductionMethyl palmoxirateBromosuccinimide
The invention discloses a method for preparing a 1,8-cineole derivative from 3-carene, which comprises the following steps: reacting 3-carene with N-bromosuccinimide at room temperature to generate a product A with 3-hydroxy-4-bromo-carane as a main component; reacting the product A under the action of trimethylbromosilane and the like to generate a product B, and conducting separating and purifying to obtain 2-bromo-1,8-cineole; then conducting reacting with ammonia water to generate a mixture of 2-hydroxy-1,8-cineole and 2-amino-1,8-cineole; adding HCl to convert the 2-amino-1,8-cineole into ammonia salt, separating out the ammonia salt in an organic solvent, and separating the ammonia salt from the 2-hydroxy-1,8-cineole; and dissolving the solid ammonia salt in water, adding alkali to convert the ammonia salt into amine again, and extracting and recycling the amine through an organic solvent. The conversion rate of all reaction raw materials is close to 100%, and the total selectivity of 2-hydroxy-1,8-cineole and 2-amino-1,8-cineole is greater than 98%.
Owner:INST OF CHEM IND OF FOREST PROD CHINESE ACAD OF FORESTRY
Preparation method of organic-inorganic hybrid halide micro-nano tubes
InactiveCN110819344ASmall particle sizeVery high confinement effectLuminescent compositionsMicro nanoReaction temperature
The invention discloses a preparation method of organic-inorganic hybrid halide micro-nano tubes, and belongs to the technical field of preparation of semiconductor micro-nano tube materials. The preparation method comprises following steps: introducing 4-aminopyridine, anhydrous zinc acetate, octadecene, oleic acid and oleylamine into a three-necked bottle, magnetically stirring, heating to 110 DEG C under the protection of nitrogen gas, keeping the temperature for 1 hour, naturally cooling to 80-60 DEG C, keeping the temperature for 30 minutes, injecting trimethylbromosilane, and convertingan obtained solution into a white turbid solution, thereby obtaining the (C5H7N2)2ZnBr4 micro-nano tubes. By changing the volume ratio or the reaction temperature of oleic acid to oleylamine, the sizeand the morphology of the (C5H7N2)2ZnBr4 micro-nano tubes can be controlled. Synthesis of the micro-nano tubes in organic-inorganic hybrid halides is realized for the first time, the vacancy of the halide synthesis technology is filled, and conditions are provided for research in the field of luminescent materials.
Owner:JILIN UNIV
Tenofovir microcapsule and preparation method thereof
InactiveCN110179767AImprove securityImprove toleranceOrganic active ingredientsGroup 5/15 element organic compoundsDiethyl methylphosphonateOil phase
The present invention discloses a tenofovir microcapsule and a preparation method thereof, and relates to the technical field of tenofovir. The tenofovir comprises the following components according to parts by weight: 56-60 parts of R-propylene oxide, 32-35 parts of diethyl p-toluenesulfonyloxymethylphosphonate, 2-4 parts of adenine, 12-15 parts of trimethyl bromo-silicane and 7-9 parts of dimethoxyethoxy aluminum hydride; and the microcapsule comprises the following components in parts by weight: 22-25 parts of chitin, 15-17 parts of microcrystalline cellulose, 14-16 parts of sodium alginate, 3-6 parts of hydrolase, 4-6 parts of thioglycolic acid, 6-8 parts of glucose, 4-5 parts of epichlorohydrin, 3-9 parts of an oil phase, 1-3 parts of a curing agent and 2-3 parts of a dispersing agent. The tenofovir capsule is improved in absorption rate and also relatively small in smell.
Owner:南京望知星医药科技有限公司
A kind of tenofovir preparation method suitable for industrialized production
ActiveCN104098605BMild conditions for crystallizationMild reaction conditionsGroup 5/15 element organic compoundsLithiumFiltration
The invention provides a tenofovir preparation method suitable for industrialized production. According to the method, tenofovir is obtained through catalyzing and hydrolyzing a raw material intermediate (R)-9-[2-(diethylphosphonomethoxy)propyl]adenine via trimethylbromosilane, wherein the raw material intermediate (R)-9-[2-(diethylphosphonomethoxy)propyl]adenine is obtained through the following steps: firstly, obtaining (R)-9-(2-hydroxypropyl)adenine via the ring-opening condensation of adenine and (R)-epoxypropane under an alkaline condition; secondly, catalyzing the (R)-9-(2-hydroxypropyl)adenine through lithium t-butoxide; thirdly, conducting etherification on the catalyzed (R)-9-(2-hydroxypropyl)adenine and p-benzenesulfonyloxymethyl phosphoric acid diethylester, so as to obtain the (R)-9-[2-(diethylphosphonomethoxy)propyl]adenine. The method is characterized in that purified water of appropriate proportion is added in a mixed product obtained through catalyzing and hydrolyzing the (R)-9-[2-(diethylphosphonomethoxy)propyl] adenine via trimethylbromosilane; the tenofovir is obtained through spontaneous crystallization at an appropriate temperature and cooling rate, as well as at an appropriate stirring speed. According to the invention, the obtained tenofovir product has the characteristics of high yield, strong indissolubility in operation under room temperature, simplicity in filtration and collection due to large particles, short production time, low energy consumption, and the like.
Owner:FUJIAN COSUNTER PHARMA CO LTD
Phosphoric acid grafted polyimide high-temperature proton exchange membrane and preparation method thereof
InactiveCN104497310APrevent leakageImprove hydrolytic stabilityFuel cell detailsPhosphatePhosphoric acid
The invention belongs to the technical field of a fuel battery and particularly relates to a phosphoric acid grafted polyimide high-temperature proton exchange membrane and a preparation method thereof. The proton exchange membrane is prepared by a method comprising the following steps: firstly performing a bromination reaction on a benzene ring of a common diamine monomer to obtain bromized diamine; replacing bromine on the bromized diamine with phosphonate by using n-butyllithium and chlorophosphate so as to obtain phosphate grafted diamine; performing polycondensation reaction by using the phosphate grafted diamine, the common diamine and dianhydride to prepare the polyimide; and finally hydrolyzing the phosphonate on the polyimide by using trimethyl bromo-silicane and hydrochloric acid to prepare the phosphoric acid grafted polyimide high-temperature proton exchange membrane. The proton exchange membrane prepared by the method disclosed by the invention is excellent in proton conduction capability at a high temperature; the phosphoric acid is chemically boned in a polymer, hard to leak and good in hydrolysis resistance and stability; the phosphoric acid can also cooperate with N in the polyimide to form a continuous hydrogen bond network so as to realize water-free proton conduction.
Owner:WUHAN UNIV OF TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com

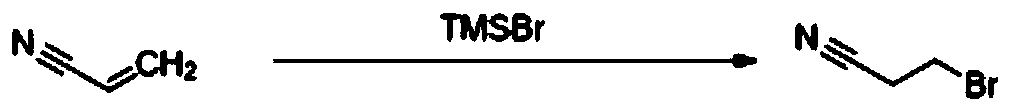
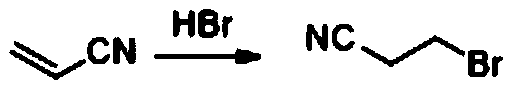
![Preparation method of (R)-9-[2-(phosphoryl methoxyl)propyl]-adenine Preparation method of (R)-9-[2-(phosphoryl methoxyl)propyl]-adenine](https://images-eureka.patsnap.com/patent_img/099b9d3f-eb26-47a2-924c-b8c1414daf4b/160204153919.PNG)
![Preparation method of (R)-9-[2-(phosphoryl methoxyl)propyl]-adenine Preparation method of (R)-9-[2-(phosphoryl methoxyl)propyl]-adenine](https://images-eureka.patsnap.com/patent_img/099b9d3f-eb26-47a2-924c-b8c1414daf4b/489278DEST_PATH_IMAGE003.PNG)
![Preparation method of (R)-9-[2-(phosphoryl methoxyl)propyl]-adenine Preparation method of (R)-9-[2-(phosphoryl methoxyl)propyl]-adenine](https://images-eureka.patsnap.com/patent_img/099b9d3f-eb26-47a2-924c-b8c1414daf4b/697537DEST_PATH_IMAGE004.PNG)