Preparation method of stable silicon-coated pure-phase CsPb2Br5 inorganic nanocrystal
An inorganic nano- and silicon-coated technology, which is applied in the field of preparation of pure-phase CsPb2Br5 inorganic nanocrystals, can solve the problems of loss of stability, surface detachment of surface ligand oleylamine, etc. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] This example provides a stable silicon-coated pure-phase CsPb 2 Br 5 The preparation method of inorganic nanocrystal specifically comprises the following steps:
[0021] Step 1, in the round bottom flask of 50mL, add 0.1mmol cesium carbonate (Cs 2 CO 3 ) with 0.3mmol anhydrous lead acetate (Pb(Ac) 2 ), then add 5mL octadecene (ODE), 2.8mmol oleic acid (OA) and 2.9mmol 3-aminopropyltriethoxysilane (APTES) to prepare a reaction precursor solution;
[0022] Step 2. Place the flask containing the reaction precursor solution in a magnetic heating stirrer with a rotation speed of 1000rpm, and connect the double-row tubes that can switch nitrogen and vacuum environments. First, the reaction precursor solution is raised to 60°C, switch nitrogen three times; then switch back to vacuum conditions, raise the temperature to 90°C, switch nitrogen three times again; then continue to heat up to 120°C under vacuum conditions and keep it warm for 20 minutes. 2 CO 3 and Pb(Ac) 2 A...
Embodiment 2
[0026] Prepare stable silicon-coated pure-phase CsPb according to the steps of Example 1 of the present invention 2 Br 5 Inorganic nanocrystals, the difference is that only the amount of anhydrous lead acetate in step 1 is adjusted to 0.4 mmol, and the other steps remain unchanged.
Embodiment 3
[0028]Prepare stable silicon-coated pure-phase CsPb according to the steps of Example 2 of the present invention 2 Br 5 For inorganic nanocrystals, the difference is that only the last heating temperature in step 2 is adjusted to 150° C., and other steps remain unchanged.
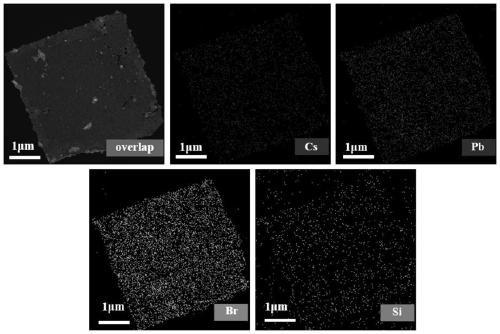
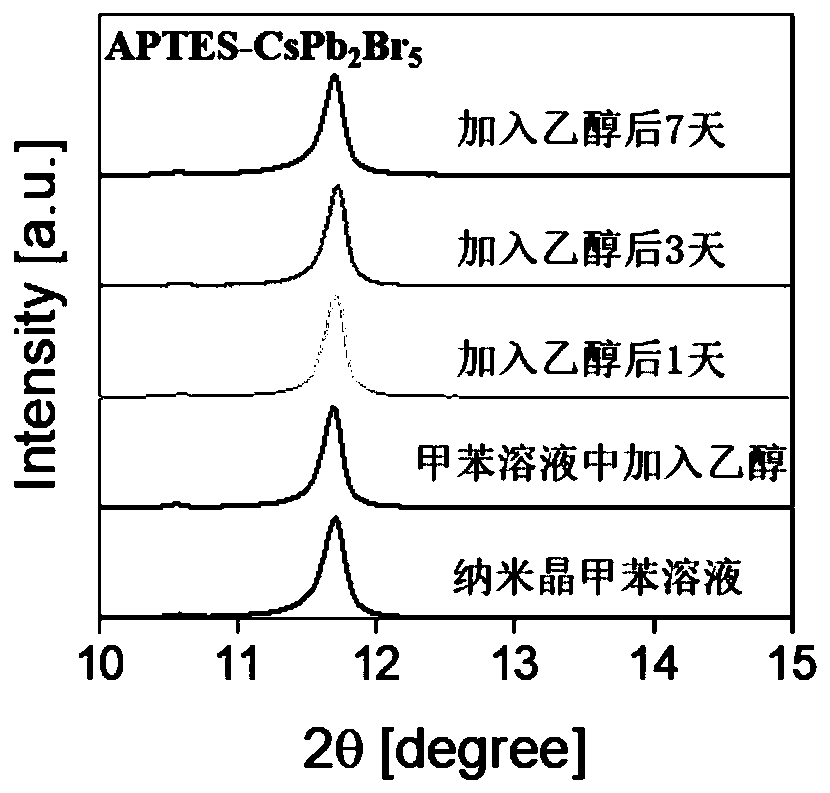
[0029] To illustrate the effect of different purification times on silicon-coated CsPb 2 Br 5 Influence of the purity of nanocrystals, Example 1 of the present invention prepared silicon-coated CsPb purified 1, 3, 5 and 7 times respectively 2 Br 5 nanocrystals, made of figure 1 The TEM image shown shows that after purification once, CsPb 2 Br 5 There are still a lot of CsPbBr mixed in the nanocrystals (square nanosheets) 3 Nanocrystalline (small particles at the edge), with the increase of purification times, CsPbBr 3 The nanocrystals gradually decreased, and after 7 times of purification, the CsPbBr 3 disappear, resulting in pure phase CsPb coated with Si 2 Br 5 Nanocrystalline.
[0030] Depend...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com