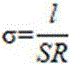Preparation method for amorphous state sulfide solid electrolyte
A solid electrolyte and sulfide technology, applied in the directions of electrolytes, electrolyte immobilization/gelation, circuits, etc., can solve the problems of low ionic conductivity, easy moisture absorption and hydrolysis, and increase energy consumption, etc. Simple, solve the effect of lower product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Lithium metal, elemental sulfur, and phosphorus pentasulfide are weighed in a glove box filled with nitrogen in a molar ratio of 6:3:1, and 0.2333g of lithium metal, 0.5333g of elemental sulfur, and 1.2334g of phosphorus pentasulfide are weighed, and the above raw materials and 36g of zirconia balls Put it into a 100ml zirconia ball mill jar, take it out from the glove box after completely sealing it. Then use a planetary ball mill to mill at 200 rpm for 24 hours to obtain an amorphous sulfide solid electrolyte Li 3 P.S. 4 .
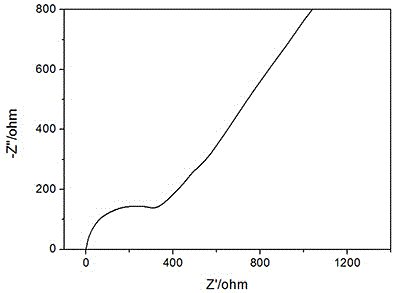
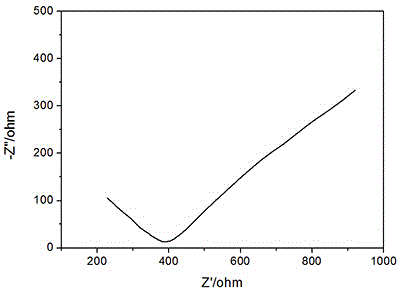
[0025] Press the sample into a disc with a diameter of 15mm and a thickness of about 0.5mm, clamp the disc between the stainless steel discs, apply epoxy glue on the exposed solid electrolyte, and let it stand for 10 minutes until it is cured. Connect the stainless steel sheets at both ends to the positive and negative electrodes respectively, measure the AC impedance diagram on the electrochemical workstation, and calculate the conductivity of ...
Embodiment 2
[0034] Lithium metal, elemental sulfur, and phosphorus pentasulfide are in a molar ratio of 8:4:1. In a glove box filled with argon, weigh 0.2759g lithium metal, 0.6305g elemental sulfur and 1.0936g phosphorus pentasulfide, and oxidize the above raw materials and 36g Put the zirconium balls into a 100ml zirconia ball mill jar, and take it out from the glove box after completely sealing. Then use a planetary ball mill to mill at 200 rpm for 24 hours to obtain an amorphous sulfide solid electrolyte Li 8 P 2 S 9 .
[0035] Press the sample into a disc with a diameter of 15mm and a thickness of about 0.5mm, clamp the disc between the stainless steel discs, apply epoxy glue on the exposed solid electrolyte, and let it stand for 10 minutes until it is cured. Connect the stainless steel sheets at both ends to the positive and negative electrodes respectively, measure the AC impedance diagram on the electrochemical workstation, and calculate the conductivity of the sulfide solid el...
Embodiment 3
[0038] Lithium metal, elemental sulfur, and phosphorus pentasulfide are in a molar ratio of 8:4:1. In a glove box filled with nitrogen, weigh 0.2759g lithium metal, 0.6305g elemental sulfur, and 1.0936g phosphorus pentasulfide. The above raw materials and 36g zirconia The balls were put into a 100ml zirconia ball milling jar, completely sealed and taken out from the glove box. Then use a planetary ball mill at 250 rpm for 48 hours to obtain an amorphous sulfide solid electrolyte Li 8 P 2 S 9 .
[0039] Press the sample into a disc with a diameter of 15mm and a thickness of about 0.5mm, clamp the disc between the stainless steel discs, apply epoxy glue on the exposed solid electrolyte, and let it stand for 10 minutes until it is cured. Connect the stainless steel sheets at both ends to the positive and negative electrodes respectively, measure the AC impedance diagram on the electrochemical workstation, and calculate the conductivity of the sulfide solid electrolyte accordin...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Conductivity | aaaaa | aaaaa |
| Conductivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com