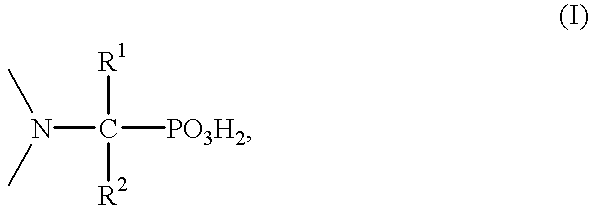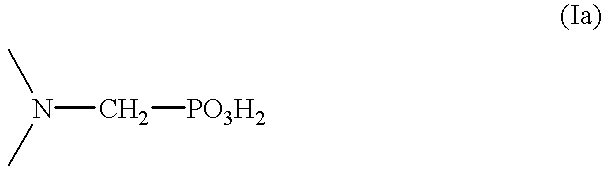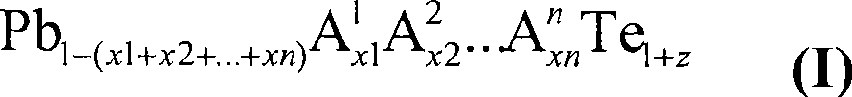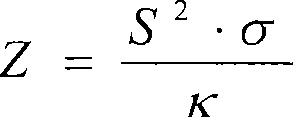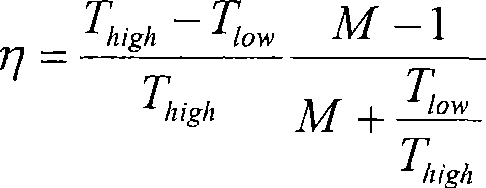Patents
Literature
425results about "Lead compounds" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Photostimulable phosphor
InactiveUS6045722AImprove clarityLow noise contentX-ray/infra-red processesRadiation applicationsPhosphorMetal
There is provided a photostimulable phosphor according to formula (I)Ba1-x-y-p-3q-zSrxMy2+M2p1+M2q3+F2-a-bBraIb:zEuwherein: M1+ is at least one alkali metal selected from the group consisting of Li, Na, K, Rb and Cs; M2+ is at least one metal selected from the group consisting of Ca Mg and Pb; M3+ is at least one metal selected from the group consisting of Al, Ga, In, Tl, Sb, Bi, Y, La, Ce, Pr, Nd, Sm, Gd, Tb, Dy, Ho, Er, Tm, Yb and Lu; 0<x< / =0.30, 0< / =y< / =0.10, 0< / =p< / =0.3, 0< / =q< / =0.1, 0.05< / =a< / =0.76, 0.20< / =b< / =0.90, a+b<1.00 and 10-6< / =z< / =0.2.
Owner:AGFA HEALTHCARE NV
Stabilization method for lead projectile impact area
Owner:FORRESTER KEITH E
Synthesis of Metal Compounds Under Carbothermal Conditions
InactiveUS20050255026A1Economical and convenient processLower capability requirementsPhosphatesLithium compoundsOxidation stateGraphite
Active materials of the invention contain at least one alkali metal and at least one other metal capable of being oxidized to a higher oxidation state. Preferred other metals are accordingly selected from the group consisting of transition metals (defined as Groups 4-11 of the periodic table), as well as certain other non-transition metals such as tin, bismuth, and lead. The active materials may be synthesized in single step reactions or in multi-step reactions. In at least one of the steps of the synthesis reaction, reducing carbon is used as a starting material. In one aspect, the reducing carbon is provided by elemental carbon, preferably in particulate form such as graphites, amorphous carbon, carbon blacks and the like. In another aspect, reducing carbon may also be provided by an organic precursor material, or by a mixture of elemental carbon and organic precursor material.
Owner:LITHIUM WERKS TECH BV +1
Synthesis of metal compounds under carbothermal conditions
InactiveUS7060206B2Economical and convenient processLower capability requirementsCell electrodesSulfur compoundsOxidation stateGraphite
Active materials of the invention contain at least one alkali metal and at least one other metal capable of being oxidized to a higher oxidation state. Preferred other metals are accordingly selected from the group consisting of transition metals (defined as Groups 4–11 of the periodic table), as well as certain other non-transition metals such as tin, bismuth, and lead. The active materials may be synthesized in single step reactions or in multi-step reactions. In at least one of the steps of the synthesis reaction, reducing carbon is used as a starting material. In one aspect, the reducing carbon is provided by elemental carbon, preferably in particulate form such as graphites, amorphous carbon, carbon blacks and the like. In another aspect, reducing carbon may also be provided by an organic precursor material, or by a mixture of elemental carbon and organic precursor material.
Owner:LITHIUM WERKS TECH BV
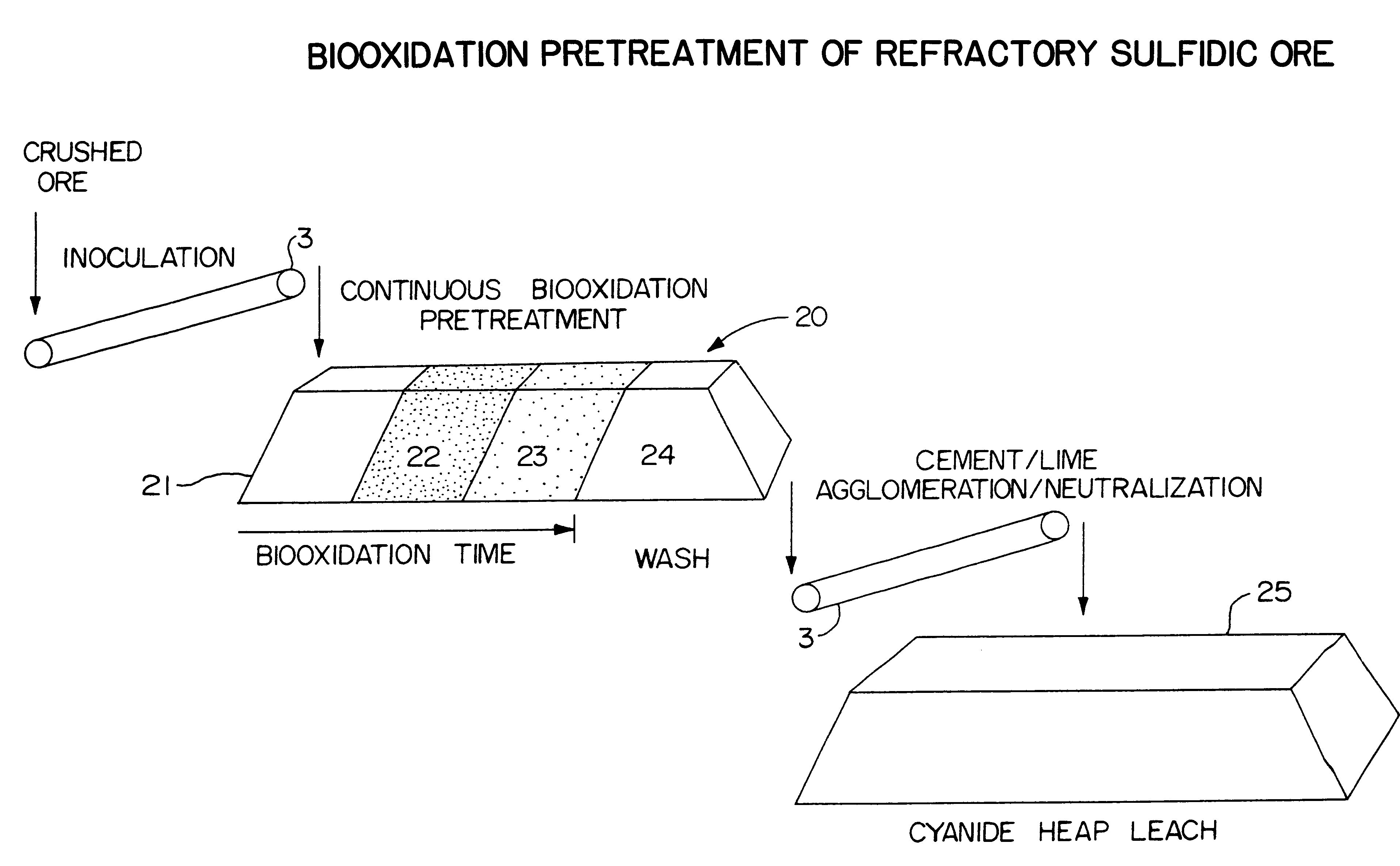
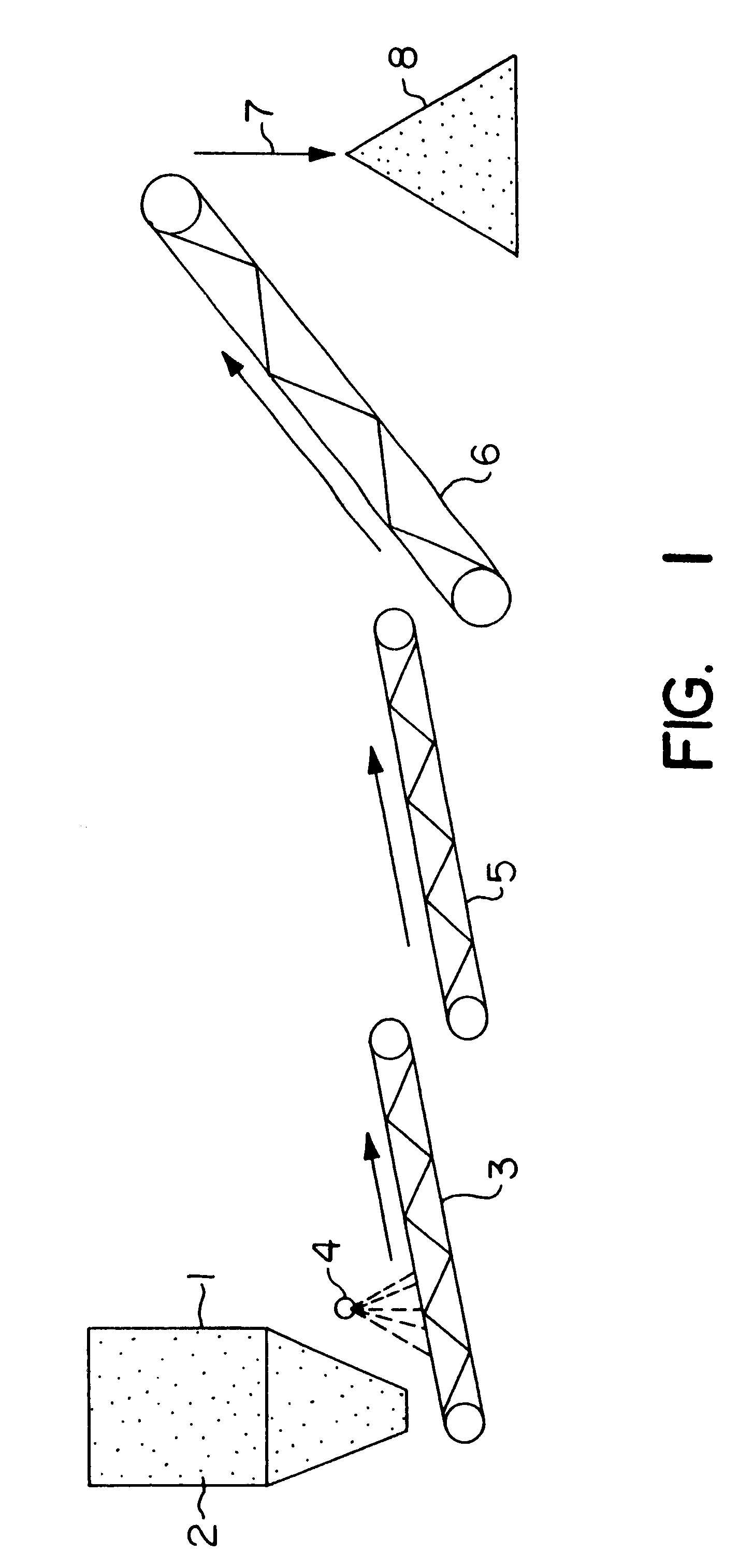
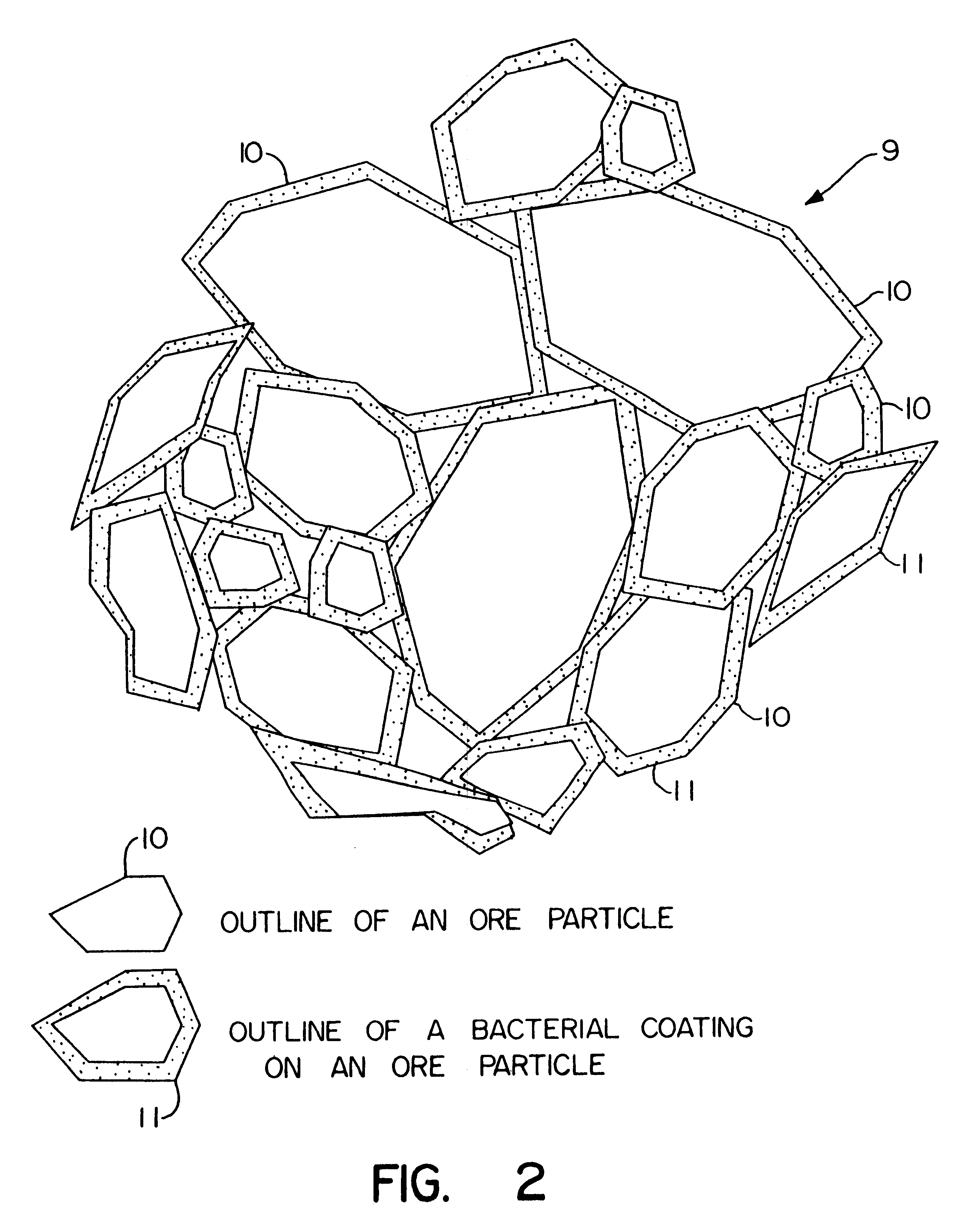
Biooxidation process for recovery of metal values from sulfur-containing ore materials
InactiveUS6383458B1Increase ratingsIncrease attractivenessTransuranic element compoundsSolvent extractionParticulatesPartial oxidation
A process for the recovery of one or more metal values from a metal ore material comprising those of one or more values and a matrix material having a sulfur content wherein the sulfur is present in an oxidation-reduction state of zero or less comprisinga. forming particulates from particles of said ore and an inoculate comprising bacteria capable of at least partially oxidizing the sulfur content;b. forming a heap of said particulates;c. biooxidizing the sulfur content andd. recovering those one or more metal values.
Owner:NEWMONT USA LTD
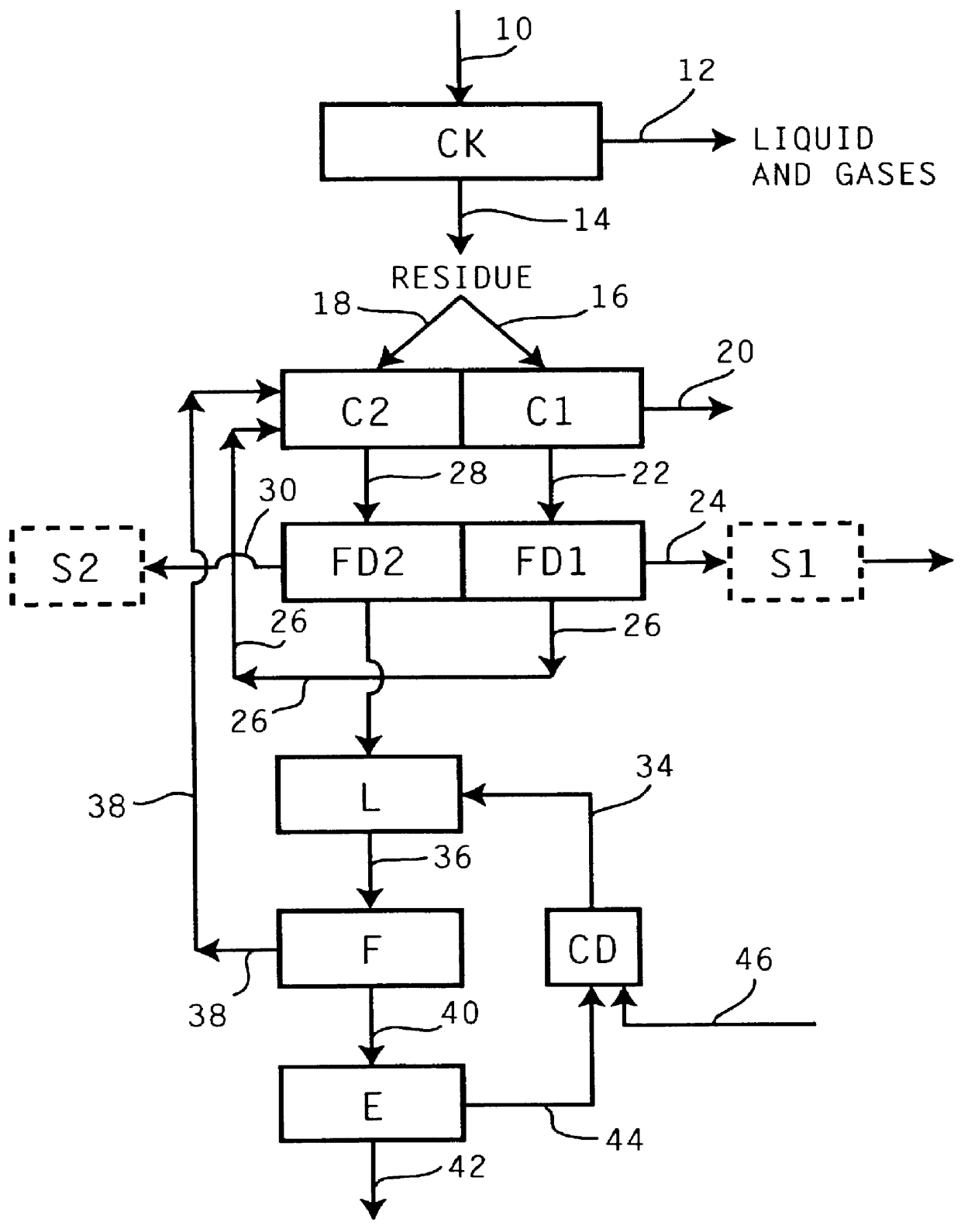
Recovery of the transition metal component of catalyst used in heavy feed hydroconversion
The invention relates to a process for recovering the transition metal component of catalysts used in the hydroconversion of heavy hydrocarbonaceous materials. In accordance with the invention, a slurry of a transition metal catalyst and hydrocarbon is catalytically desulfurized resulting in a desulfurized product and a solid residue containing the transition metal. The transition metal may be recovered by coking the residue and then dividing the coker residue into two portions are combusted with the flue dust from the first combustion zone being conducted to the second combustion zone. The flue dust from the second combustion zone is treated with ammonia and ammonium carbonate in order to obtain ammonium molybdate.
Owner:EXXON RES & ENG CO
Preparation method of perovskite quantum dots
InactiveCN107500345AGuaranteed stabilityGuaranteed fluorescence quantum efficiencyMaterial nanotechnologyLuminescent compositionsFluorescenceComputational chemistry
The invention provides a preparation method of perovskite quantum dots. The preparation method comprises steps as follows: A), Cs2CO3, oleic acid and octadecene are mixed and subjected to a heating reaction under the condition of protective atmosphere, and a cesium oleate precursor solution is obtained; B), lead halide and octadecene are mixed and subjected to heating and heat preservation under the condition of protective atmosphere, after an oleic acid and oleylamine mixed solution is added and heated to 170-190 DEG C, an obtained mixed solution is mixed and reacts with the cesium oleate precursor solution, and the perovskite quantum dots are obtained, wherein the lead halide is selected from one or more of lead chloride, lead bromide and lead iodide. According to the preparation method, the coordination effect of surface ligands of the perovskite quantum dots of different halide ions directly synthesized at a higher temperature is more remarkable, so that stability and fluorescence quantum efficiency of the synthesized perovskite quantum dots are both guaranteed.
Owner:CHANGCHUN INST OF OPTICS FINE MECHANICS & PHYSICS CHINESE ACAD OF SCI
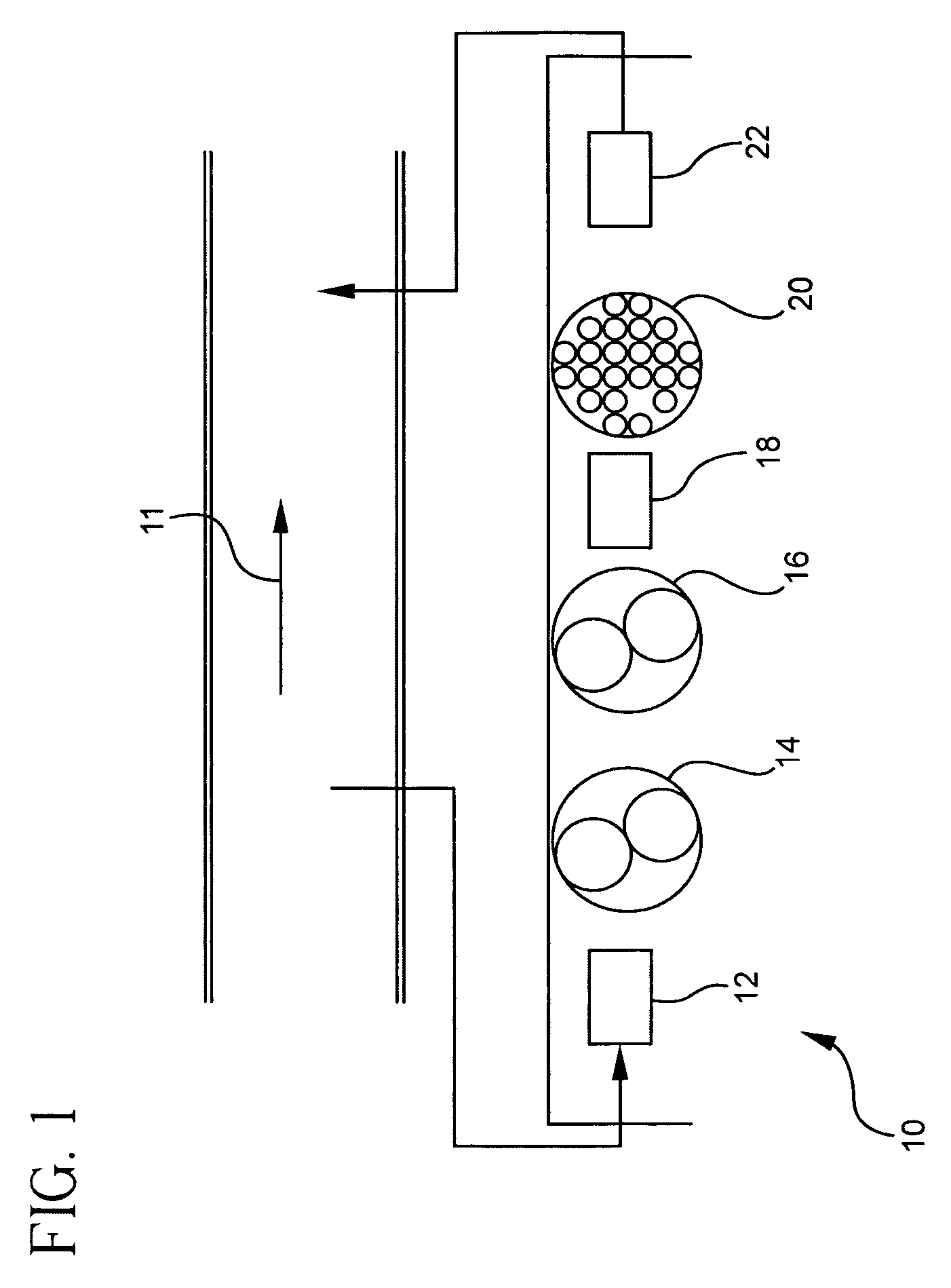
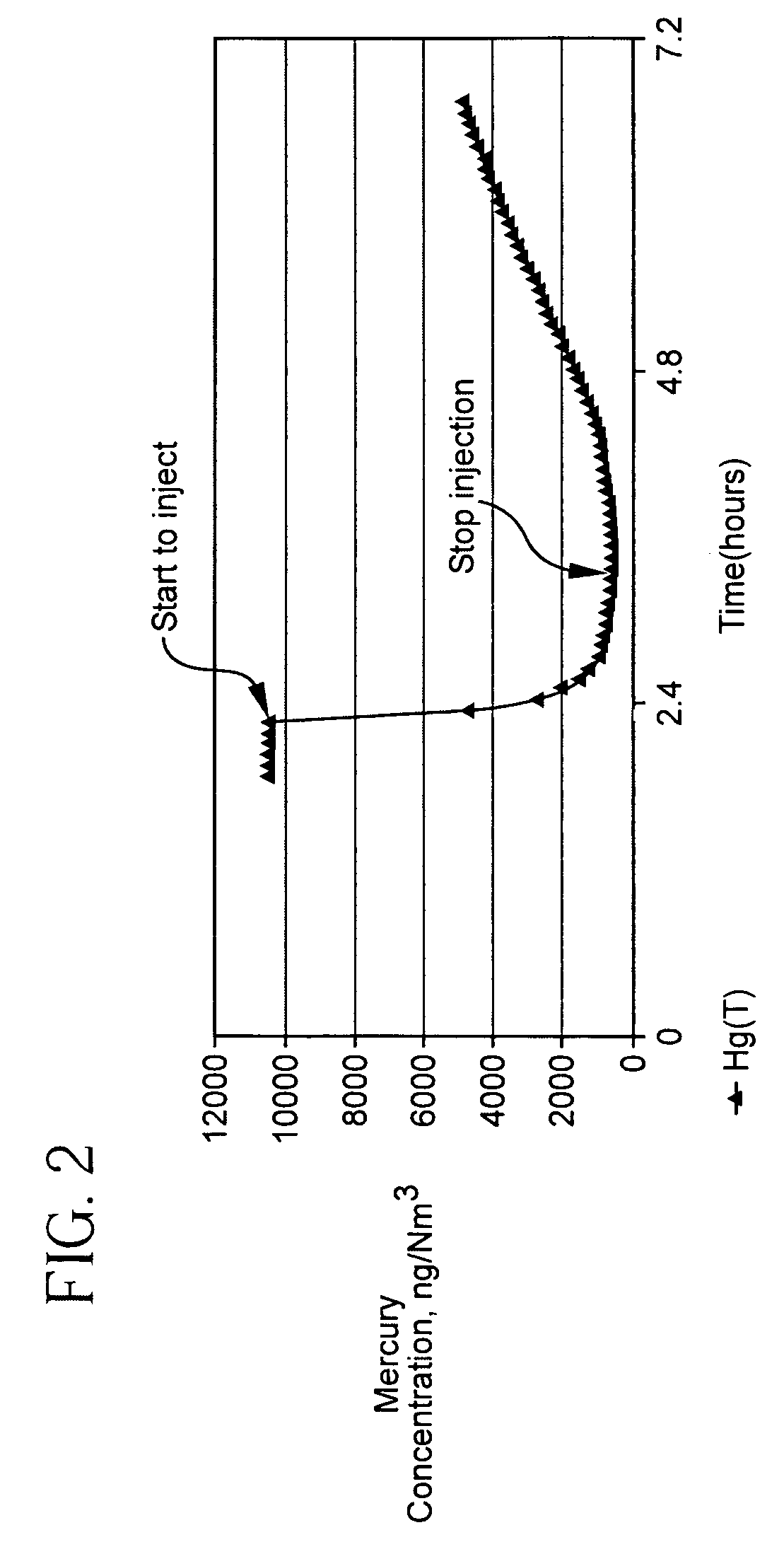
Pollutant emission control sorbents and methods of manufacture
Sorbents for removal of mercury and other pollutants from gas streams, such as a flue gas stream from coal-fired utility plants, and methods for their manufacture and use are disclosed. The methods include mixing fly ash particles with a sulfide salt and a metal salt to form a metal sulfide on the outer surface of the fly ash particles.
Owner:BASF CATALYSTS LLC
Lead recycling
ActiveUS20100040938A1Simple methodOrganic chemistryPrimary cell maintainance/servicingCITRATE ESTERLead oxide
The present invention describes a method of recycling lead from lead containing waste, the method comprising the steps of mixing the battery paste with aqueous citric acid solution so as to generate lead citrate; isolating lead citrate from the aqueous solution; and converting the lead citrate to lead and / or lead oxide.
Owner:CAMBRIDGE ENTERPRISE LTD
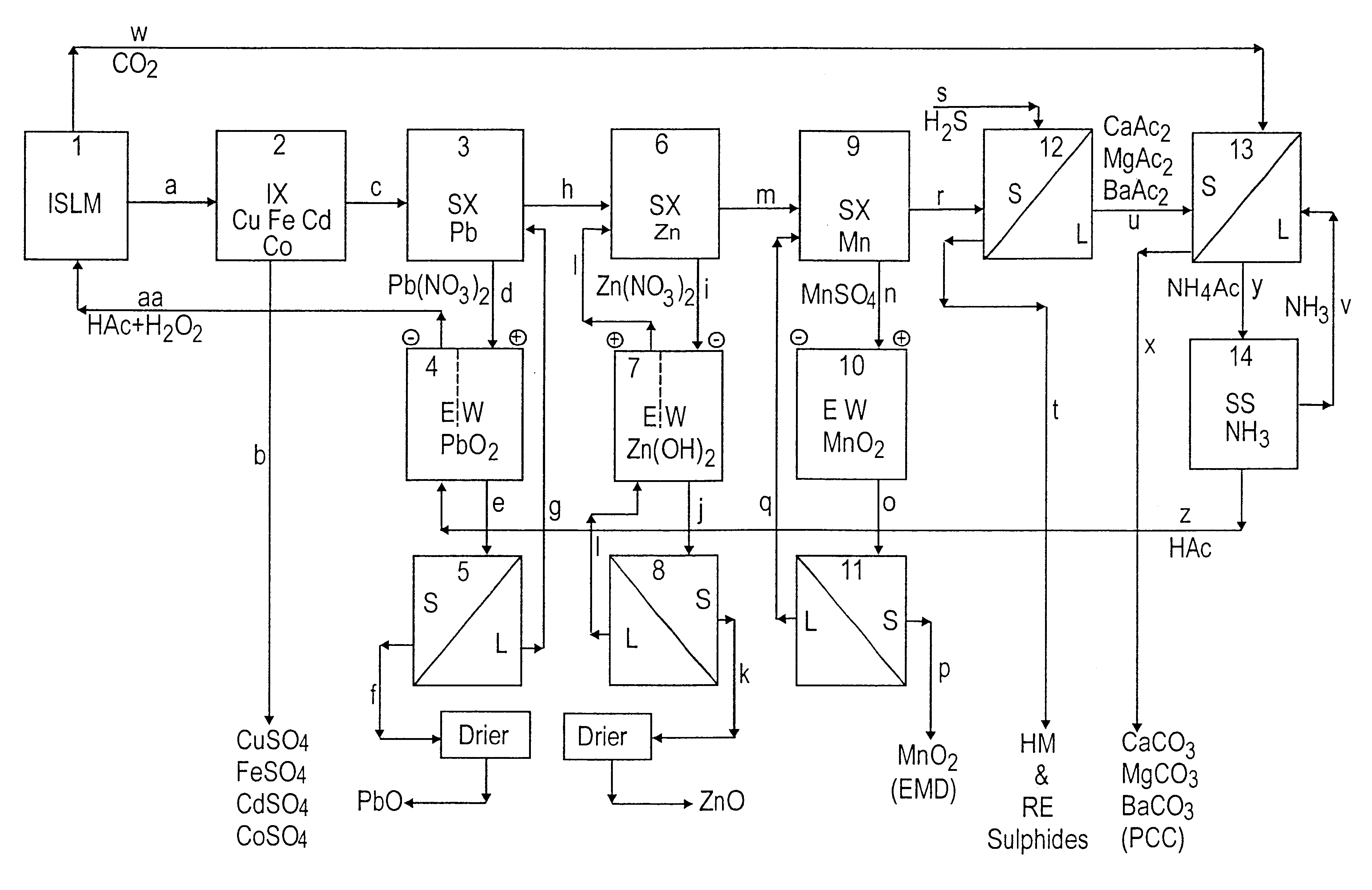
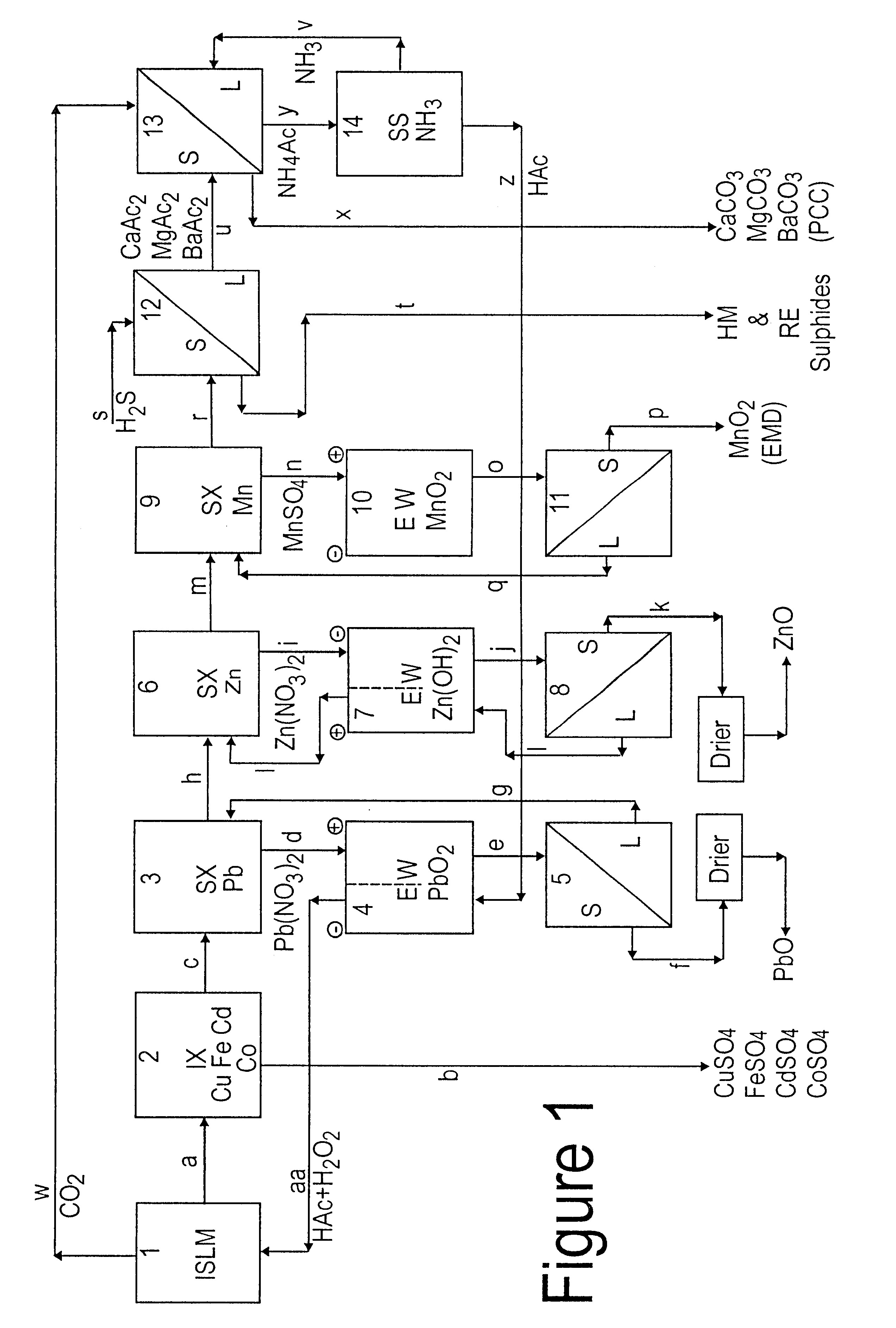
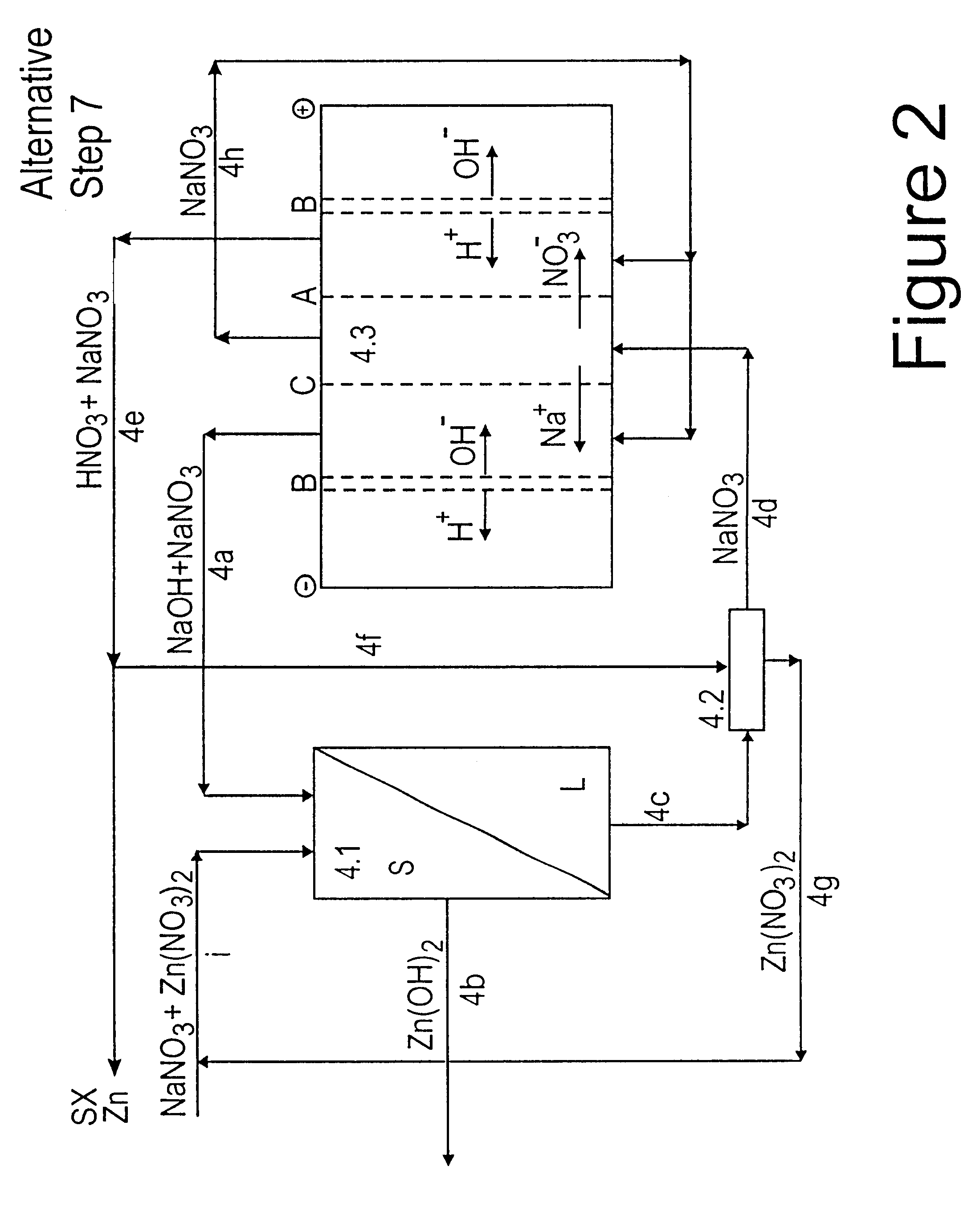
Lead, zinc and manganese recovery from aqueous solutions
InactiveUS6517701B1Low costShorten the timePhotography auxillary processesElectrolysis componentsIon exchangeManganese
Aqueous solutions containing lead, zinc and manganese are treated to recover these metals by sequential solvent extraction steps. Solvent extractants are selected to extract preferentially lead, then zinc and then manganese in that order. Any interfering metals are removed (as by ion exchange) before extraction. The loaded extractant phases are stripped with selected acids and lead, zinc and manganese each recovered from the strip solutions. Optionally calcium can be recovered when present. A preferred type of extractant (for lead especially) is substituted monothiophosphinic acids. A closed loop system is described which is advantageous with leachate from sulphide and carbonate ores.
Owner:CENTAUR MINING EXPLORATION
Method for effecting solvent extraction of metal ions using hydrocarbon soluble aminomethylene phosphonic acid compounds
Solvent extraction of one or more metal ions from an aqueous solution in the presence of hydrocarbon-soluble aminomethylenephosphonic acid derivatives.
Owner:BASF AG +1
Indium oxide-tin oxide powders and method for producing the same
InactiveUS6051166ADecrease productivityImprove sputtering efficiencyAluminium compoundsGallium/indium/thallium compoundsSaline waterIndium
A method for producing an indium oxide-tin oxide powder, which comprises supplying to react an aqueous solution of an indium salt, an aqueous solution of a tin salt and an aqueous alkaline solution into water at 40 DEG C. or more and less than 100 DEG C. so that the pH during the reaction is maintained within the range from 4 to 6, forming a precipitate, washing the formed precipitate after solid-liquid separation, and calcining the precipitate at 600 DEG C. or more and 1300 DEG C. or less.
Owner:SUMITOMO CHEM CO LTD
Synthesizing method of inorganic perovskite nanosheets
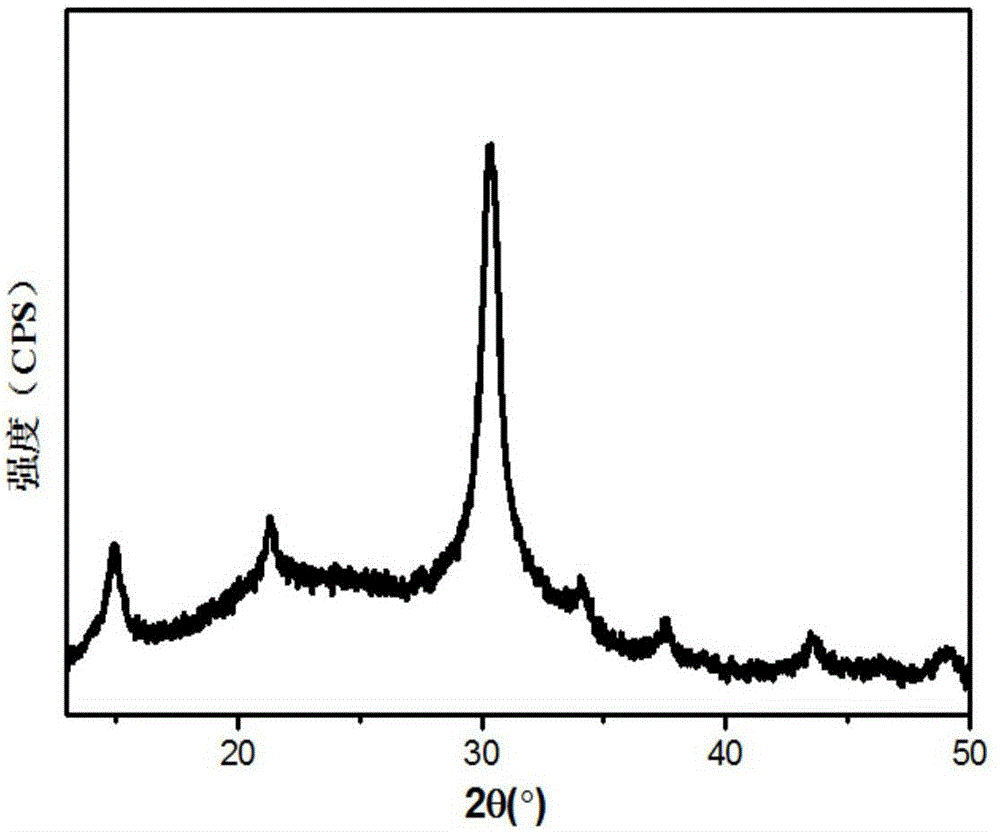
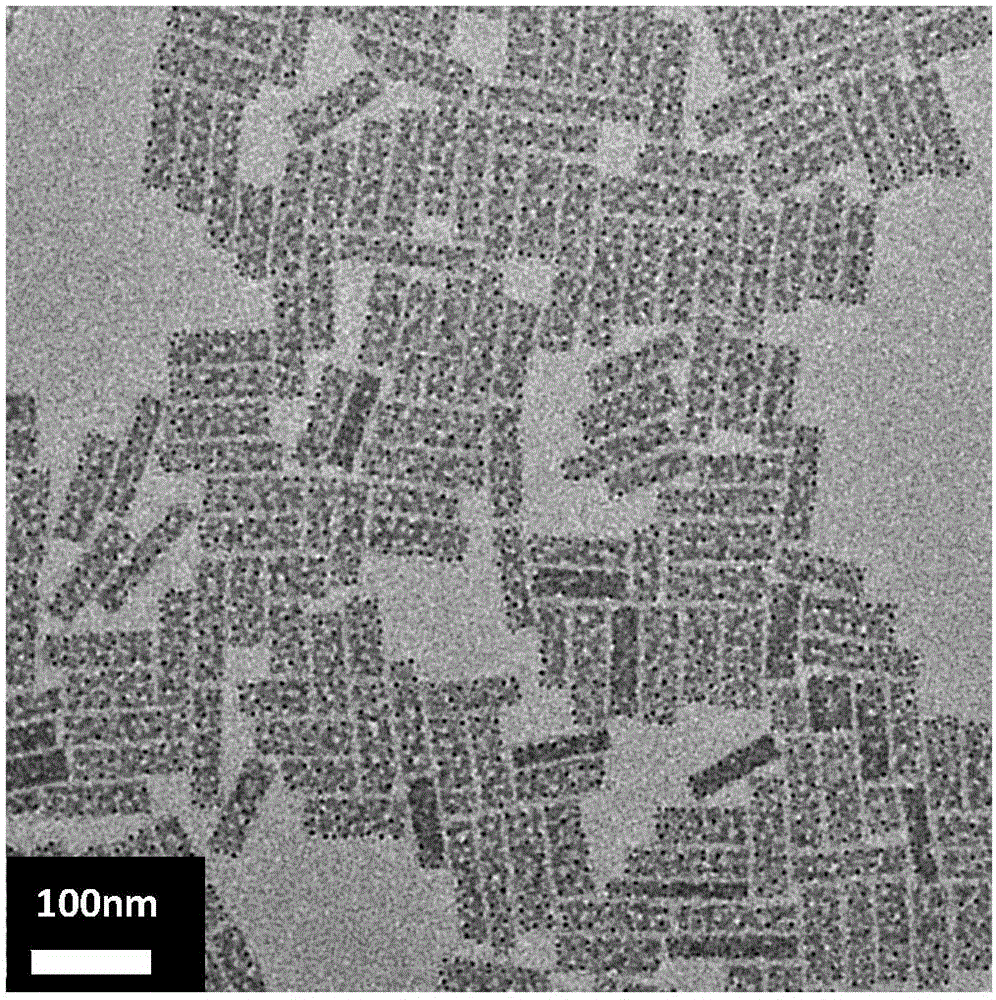
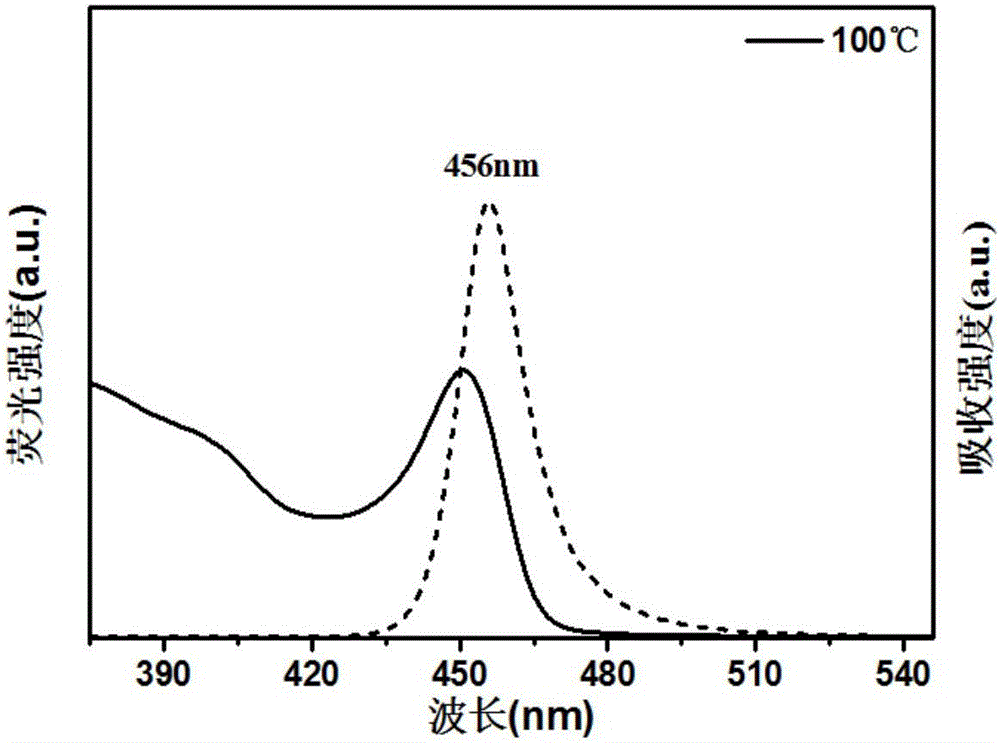
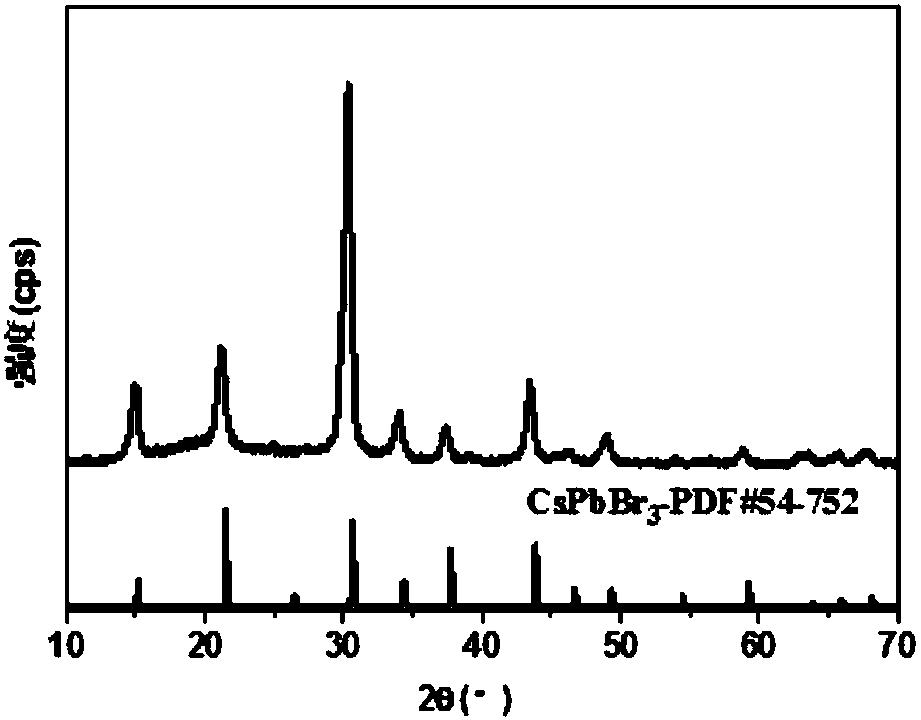
ActiveCN107522225AUniform sizeImprove controllabilityNanotechnologyLead compoundsQuantum efficiencyQuantum
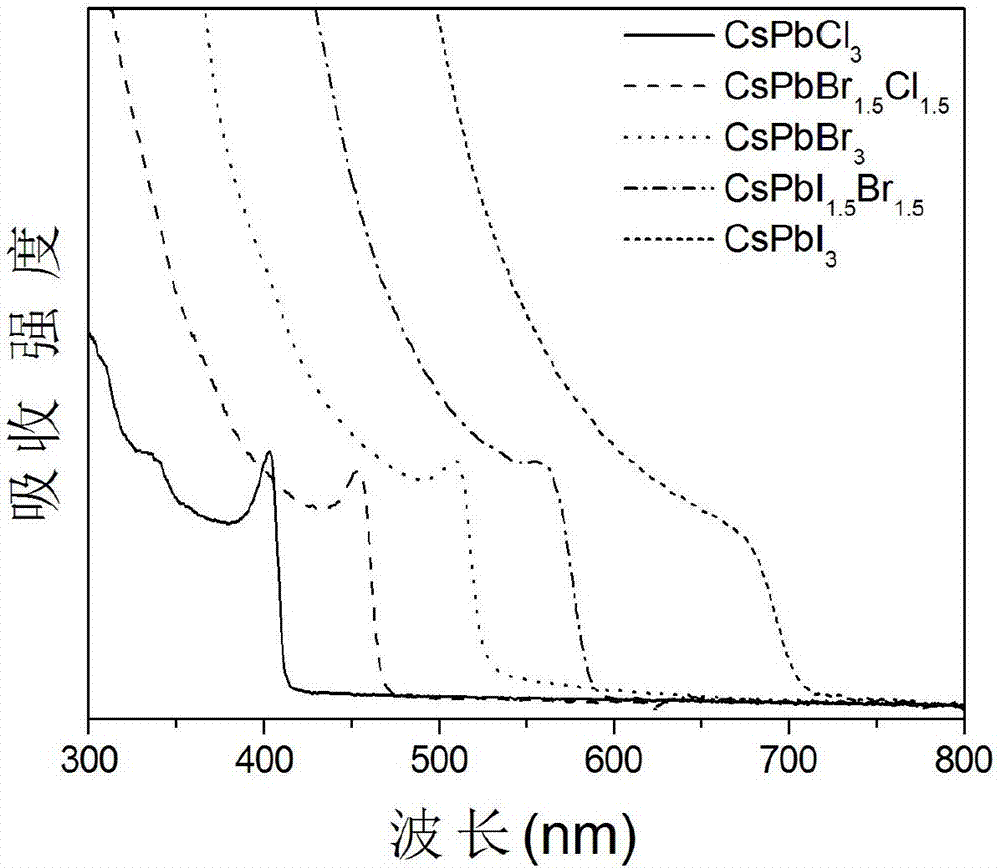
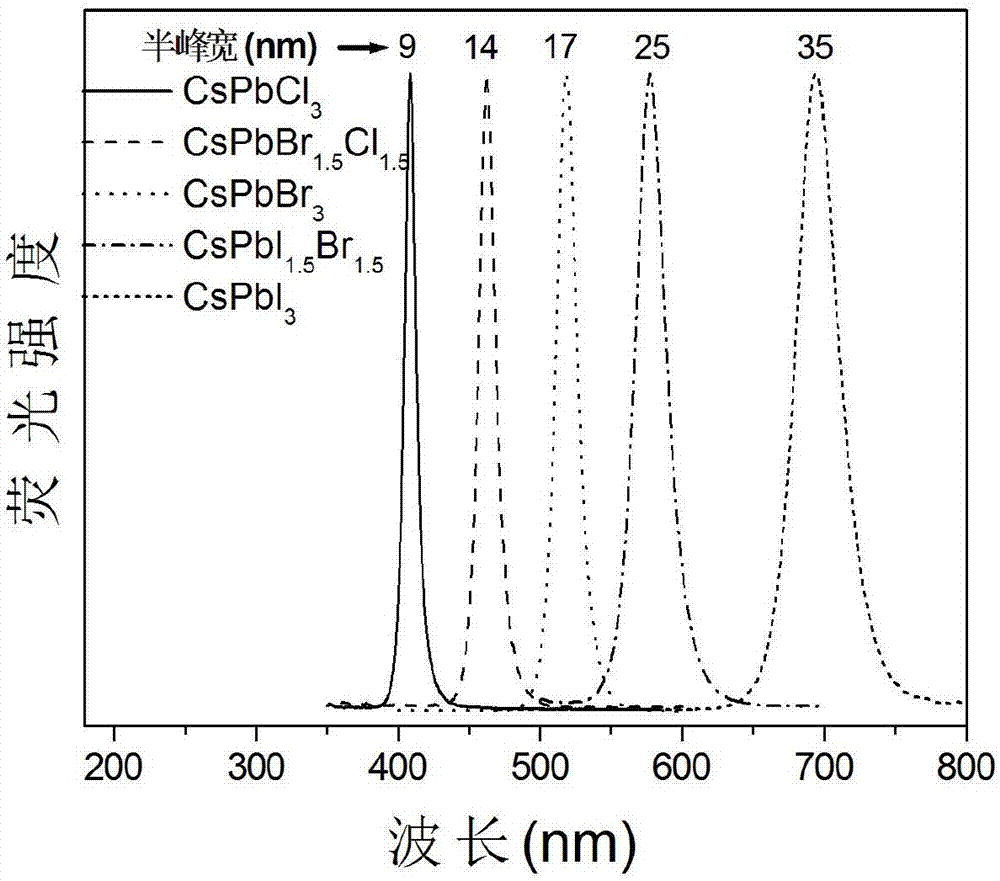
The invention relates to a synthesizing method of inorganic perovskite nanosheets. The method includes: adding the precursor solution of cesium into the precursor solution of lead halide, performing ultrasonic treatment for 10-30 minutes to obtain a mixed solution, transferring the mixed solution into a reaction kettle, performing reaction under 60-150 DEG C for 30-420 minutes, and naturally cooling to obtain reactants; performing centrifugal processing and washing to obtain the inorganic perovskite CsPbX3 nanosheets. The method has the advantages that the method is simple to operate and does not need harsh experiment conditions, and the prepared nanosheets are uniform in size, high in controllability and high in yield, and the quantum efficiency of the nanosheets reaches up to 48%.
Owner:HEBEI UNIV OF TECH
Method for synthesizing inorganic perovskite nanowires
ActiveCN108502918ALow reaction temperatureHigh yieldLuminescent compositionsLead compoundsDrawing ratioIon exchange
The invention discloses a method for synthesizing inorganic perovskite nanowires. According to the method, a CsPbBr3 nanowire which has a length of several millimeters, a diameter of only about 10 nmand an ultrahigh draw ratio is prepared at a low temperature by utilizing a solvothermal method and an anion exchange method, and nanowires CsPbI3 and CsPbCl3 are obtained by virtue of a simple anionexchange process. The method has a great effect of promoting industrial large-scale preparation of inorganic perovskite nanowires and is expected to further realize application in various aspects suchas photoelectric detection, laser and solar cells and the like. The method disclosed by the invention is simple, controllable, high in yield, uniform in morphology, low in reaction temperature and suitable for large-scale production.
Owner:HEBEI UNIV OF TECH
Method for preparing inorganic pigments, resulting inorganic pigments, and apparatus therefor
PCT No. PCT / FR96 / 01202 Sec. 371 Date May 27, 1998 Sec. 102(e) Date May 27, 1998 PCT Filed Jul. 30, 1996 PCT Pub. No. WO97 / 06215 PCT Pub. Date Feb. 20, 1997A method for preparing inorganic pigments from steel mill dust, particularly electric steel mill dust, wherein (a) the dust is separated into a magnetic fraction and a non-magnetic fraction; (b) the non-magnetic fraction is subjected to a basic leaching reaction; (c) the resulting solid batch is rinsed until neutralized and then separated; (d) the resulting batch is calcined at 450-650 DEG C.; (e) the calcined batch is treated with sulfuric acid in the presence of a catalyst; (f) the inorganic pigments are recovered; and (g) the solutions from (c) and (e) are used to precipitate other pigments.
Owner:RECUPAC
High-stability water-soluble CsPbX3 perovskite nano-crystalline preparation method
InactiveCN107381625AEasy to operate and adjustableMaterial nanotechnologyLuminescent compositionsPolymer scienceSolvent
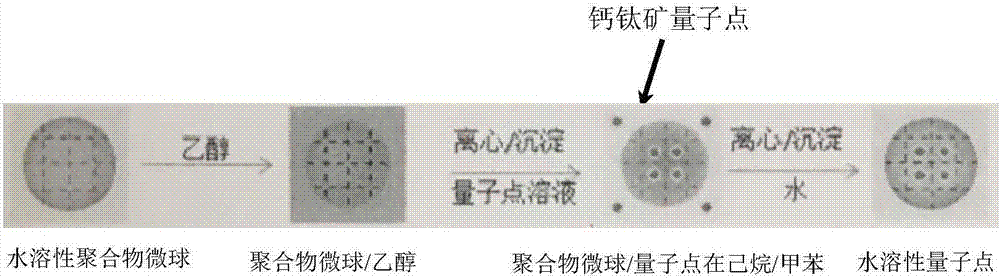
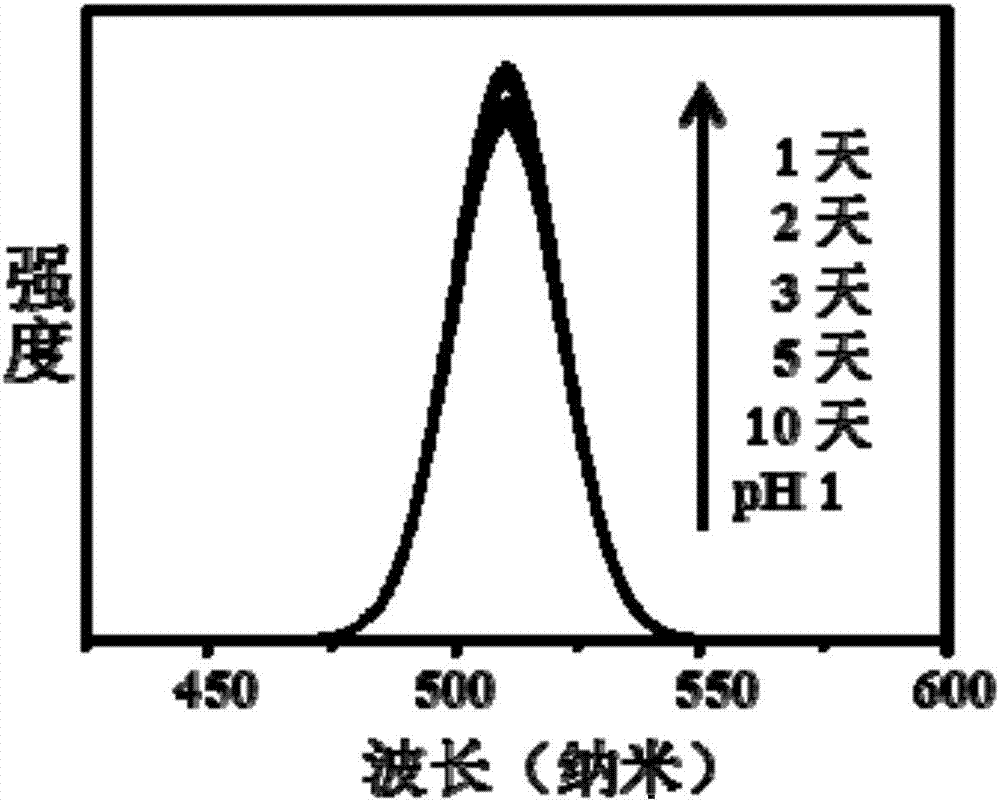
The invention discloses a high-stability water-soluble CsPbX3 perovskite nano-crystalline preparation method, and belongs to the technical field of semiconductor nano-material preparation. Water-soluble polymer particles are dispersed in hexane solvents by a solvent exchange method and then centrifuged to obtain polymer micro-sphere precipitates, CsPbX3 perovskite nano-crystalline dispersed in methylbenzene and hexane mixed solvents is mixed with the polymer micro-sphere precipitates for 24 hours, and perovskite nano-crystalline supported polymer particles are obtained by centrifugation. The polymer-supported perovskite nano-crystalline prepared by the preparation method can be dispersed in water solution, has high stability under different pH (potential of hydrogen) conditions, and the water-soluble nano-particles with fluorescence in a visible region and high fluorescence quantum efficiency are obtained by preparing different perovskite particles and have huge application values in the fields of bio-imaging, display and the like.
Owner:JILIN UNIV
Zero dimension perovskite structure luminescent material A4BX6 and preparation method thereof
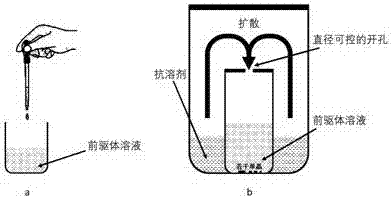
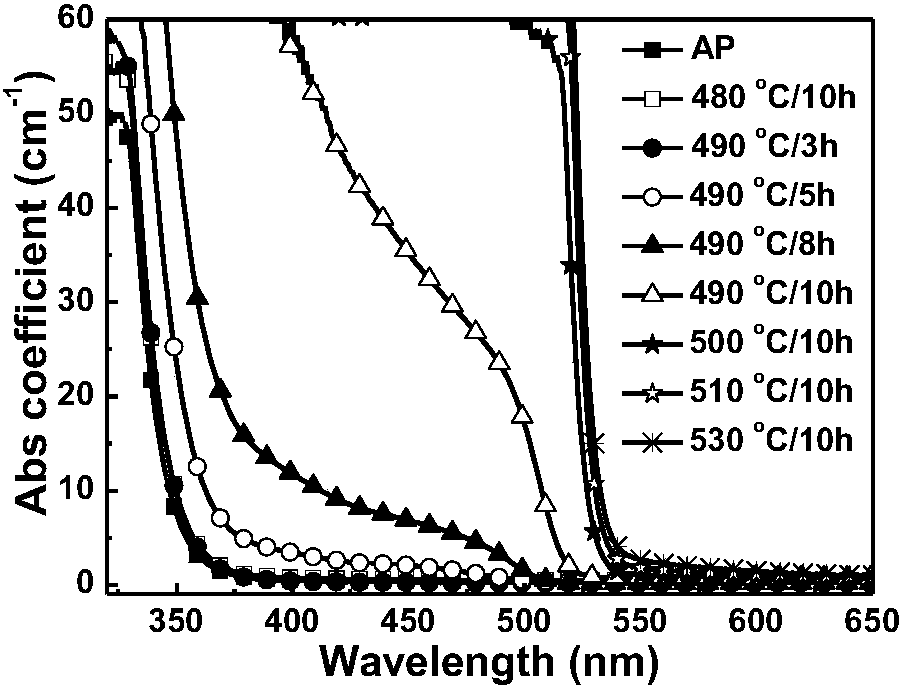
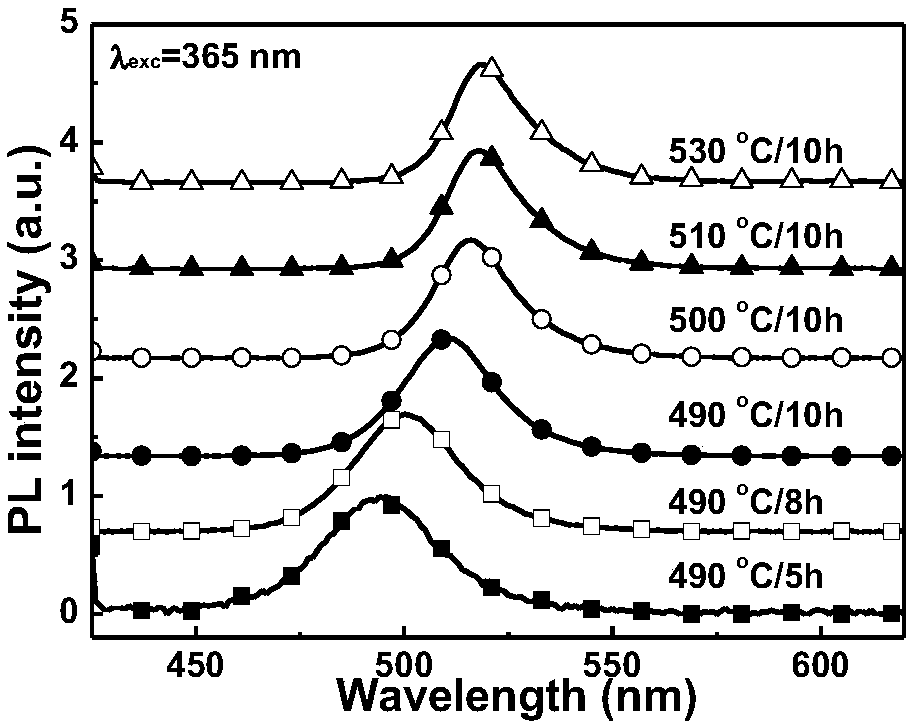
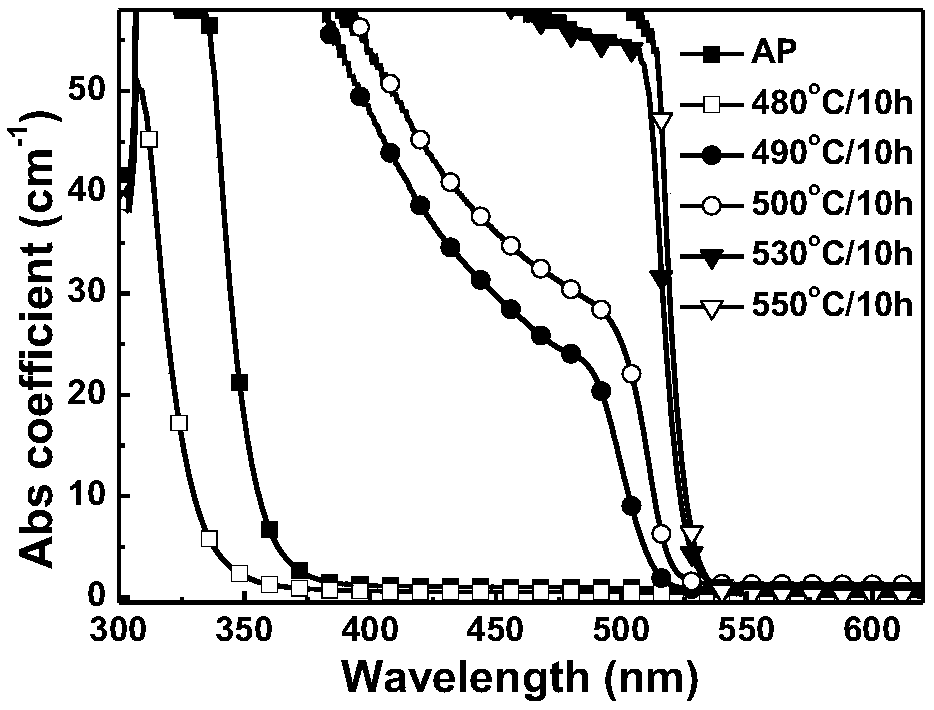
ActiveCN107267144AImprove luminous efficiencySingle crystal with few defectsPolycrystalline material growthTin compoundsSolubilityAnti solvent
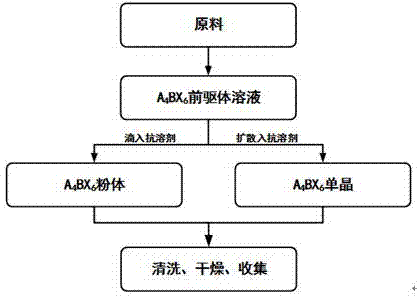
The invention discloses a zero dimension perovskite structure luminescent material A4BX6 and a preparation method thereof. The preparation method comprises the steps of utilizing a principle that the solubility of a solute in a precursor solution can be reduced through an anti-solvent, dripping or slowly diffusing the anti-solvent into the precursor solution so as to prepare the zero dimension perovskite structure efficient luminescent material A4BX6 powder or a single crystal, and concretely, dissolving an A-containing halogen compound and a B-containing halogen compound or B-containing oxide into a solvent to obtain the precursor solution; slowly dripping or diffusing the precursor solution into the anti-solvent, and separating out the zero dimension perovskite structure luminescent material A4BX6 from the precursor solution. The method has the advantages that the obtained zero dimension perovskite structure luminescent material A4BX6 has high purity, the luminous efficiency is high, the requirement on the used equipment is simple, and the like.
Owner:KUNMING UNIV OF SCI & TECH
CsPbX3 nano-crystal doped boron-containing glass and preparation method thereof
ActiveCN108424001AGood size controlEffective fluorescenceMaterial nanotechnologyLead compoundsOptical propertyBoron containing
The invention provides CsPbX3 nano-crystal doped boron-containing glass and a preparation method thereof. The atom molar percentage of elements in the CsPbX3 nano-crystal doped boron-containing glassis as follows: Si: 0 to 9.18 percent, B: 26.02 to 33.90 percent, O: 54.01 to 57.96 percent, Cs: 1.00 to 2.66 percent, Pb: 0.39 to 2.02 percent, X: 1.76 to 3.73 percent, M: 0 to 5.10 percent, N: 0 to 3.73 percent and Zn: 0 to 2.13 percent, wherein X is one or a mixture of more than two of Cl, Br or I, M is one or a mixture of more than two of Ca, Sr or Ba, and N is one or a mixture of more than twoof Li, Na or K. The process is simple and easy to operate, the price is low, the nano-crystal has controllable size, and light within a certain range of the visible light waveband can be obtained; furthermore, the CsPbX3 nano-crystal doped boron-containing glass has an excellent optical property, can obtain light within a certain range of the visible light waveband, has high transmittance and haspotential application in various fields of LEDs, solar batteries, nano-crystal lasers and the like.
Owner:WUHAN UNIV OF TECH
Mixed organic-inorganic perovskite formulations
ActiveUS20170243699A1Cost-effective performancePromote formationLight-sensitive devicesOrganic chemistryPhysical chemistryPerovskite (structure)
A formulation for use in the preferential formation of thin films of a perovskite material AMX 3 with a certain required crystalline structure, wherein said formulation comprises two or more compounds which between them comprise one or more first organic cations A; one or more metalcations M; one or more second cations A′; one or more first anions X and one or more second anions X′.
Owner:OXFORD PHOTOVOLTAICS
Method for preparing CsPbBr3 nanosheets with quantum size effects
InactiveCN106809872APreserve quantum size effectsAchieving controllable equipmentMaterial nanotechnologyLead compoundsOleic Acid TriglycerideQuantum size
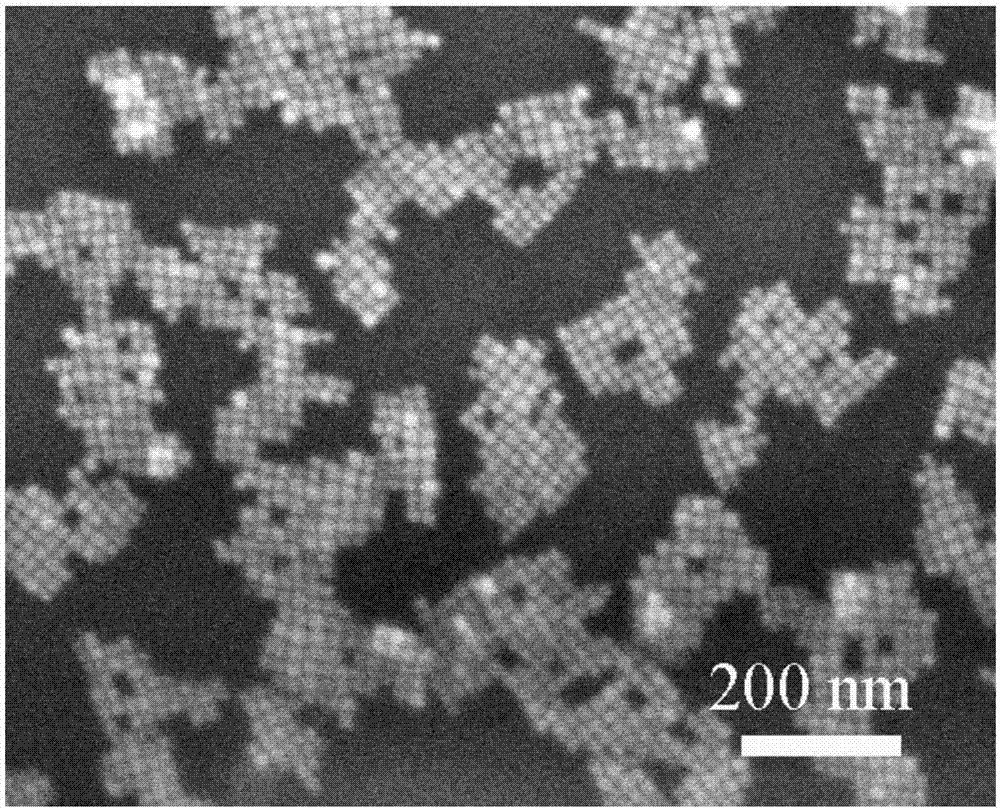
The invention discloses a method for preparing CsPbBr3 nanosheets with quantum size effects. The method includes dissolving cesium carbonate in oleic acid under argon filling conditions, and stirring the cesium carbonate and the oleic acid under heating conditions until the cesium carbonate is dissolved to obtain cesium oleate precursors; adding lead bromide, long-chain ligands and short-chain ligands into octadecene under argon filling conditions, carrying out reaction at the temperature of 100-150 DEG C until the lead bromide is dissolved, heating first reaction products until the temperature of the first reaction products reaches 120-150 DEG C, then injecting the cesium oleate precursors into the first reaction products, carrying out reaction for 5-15 s, then continuing to carry out reaction at the temperature of 100-130 DEG C for 1-5 min to obtain second reaction products and centrifuging the second reaction products. The method has the advantages that the transverse sizes of the CsPbBr3 nanosheets can be assuredly regulated and controlled in the range from 100 nm to 1 micrometer while the thicknesses which are equal to the thicknesses of a few atomic layers can be guaranteed; the thicknesses which are lower than the diameters of Bohr excitons can be kept, and accordingly the quantum size effects of the CsPbBr3 nanosheets can be reserved.
Owner:XI AN JIAOTONG UNIV
Method for Collection of Valuable Metal from ITO Scrap
ActiveUS20100084279A1Easy to collectEfficient collectionAluminium compoundsTin compoundsElectrolysisIndium
Proposed is a method for collecting valuable metal from an ITO scrap in which a mixture of indium hydroxide and tin hydroxide or metastannic acid is collected by subjecting the ITO scrap to electrolysis in pH-adjusted electrolyte, and roasting this mixture as needed to collect the result as a mixture of indium oxide and tin oxide. This method enables the efficient collection of indium hydroxide and tin hydroxide or metastannic acid, or indium oxide and tin oxide from an ITO scrap of an indium-tin oxide (ITO) sputtering target or an ITO scrap such as ITO mill ends arisen during the manufacture of such ITO sputtering target.
Owner:JX NIPPON MINING& METALS CORP
Method for producing dispersions of nanosheets
Owner:UCL BUSINESS PLC
Oxidatively regenerable adsorbents for sulfur removal
InactiveUS20090065400A1Increase capacityHigh selectivityRare earth metal oxides/hydroxidesGold compoundsSorbentSulfur
Compositions and processes are disclosed for removing sulfur and sulfur compounds from hydrocarbon fuel feedstocks. The feedstock is contacted with a regenerable sorbent such as a compound of the formula TixCeyO2 where 0<x / y≦1 and where 0<x≦1 and 0<y≦1 capable of selectively adsorbing sulfur compounds present in the hydrocarbon feedstock at about 0° C. to about 100° C. such as at about 25° C.
Owner:PENN STATE RES FOUND
Removal of lead from solid materials
InactiveUS20150050199A1Minimal costTin compoundsSolvent extractionMaterials scienceCathode Ray Tube Display
A leaching composition that substantially removes lead from solid materials and a method of using said composition. Preferably, the concentration of lead in the solid materials following processing is low enough that the solid materials can be reused and / or disposed of at minimal cost to the processor. Preferably, the solid materials comprise glass, such as cathode ray tube glass.
Owner:ENTEGRIS INC
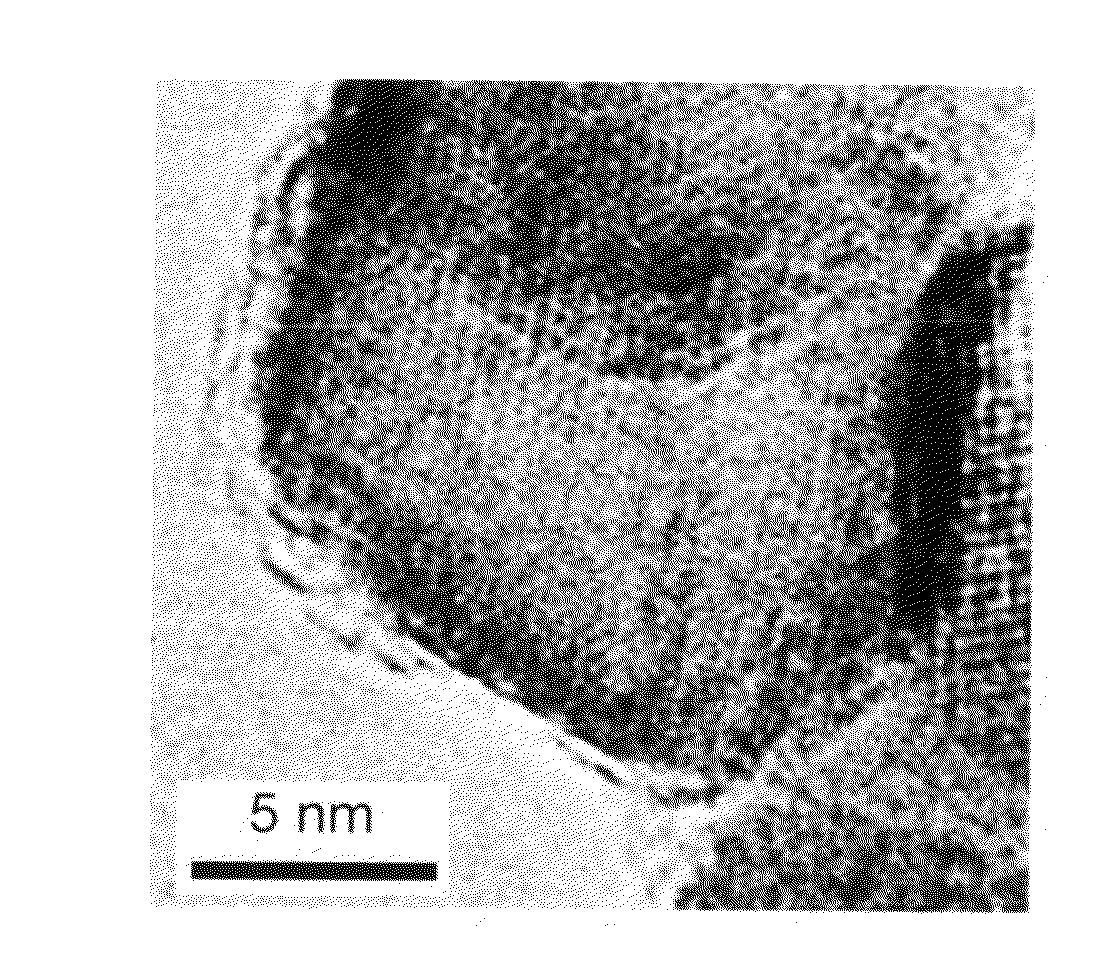
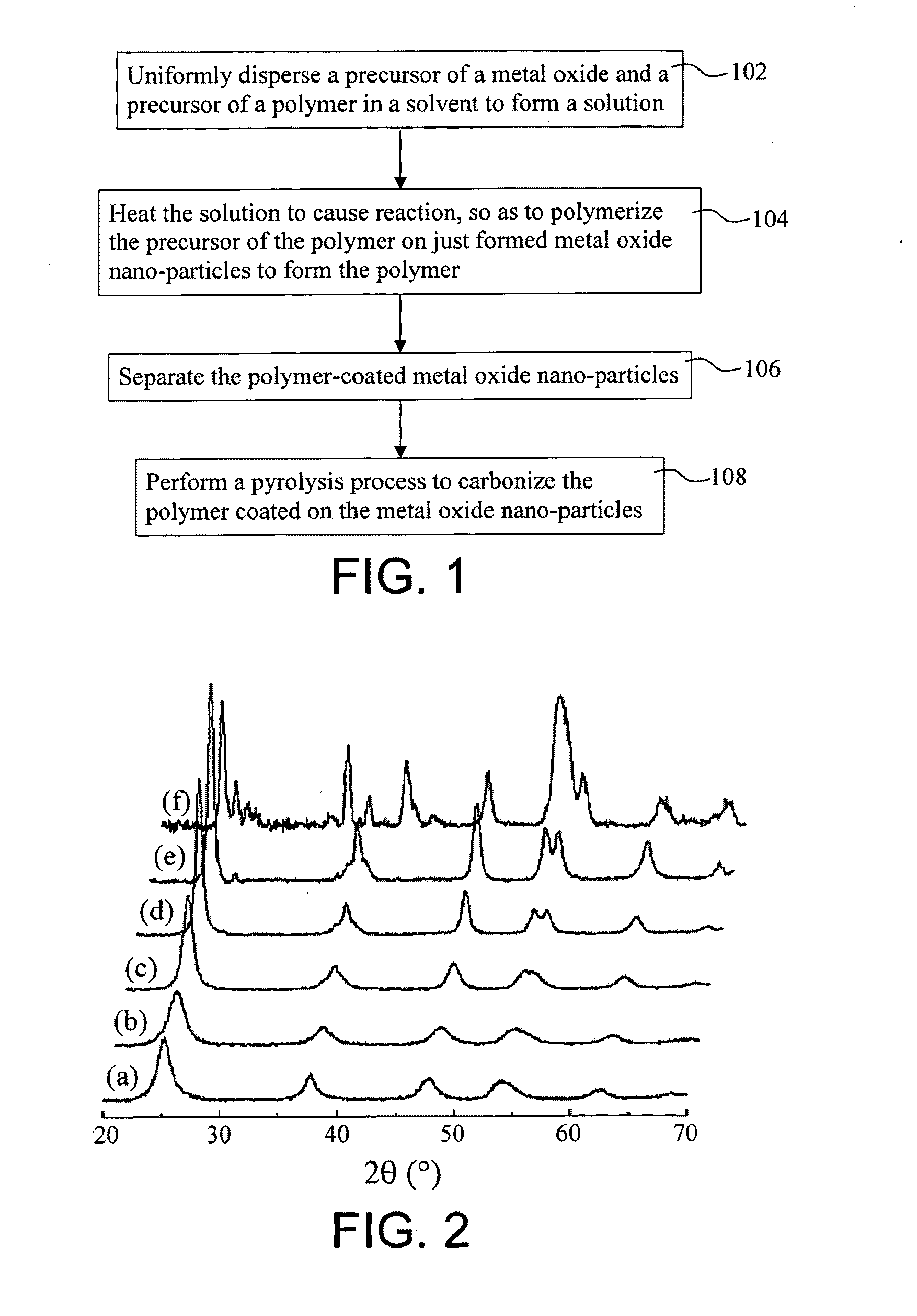
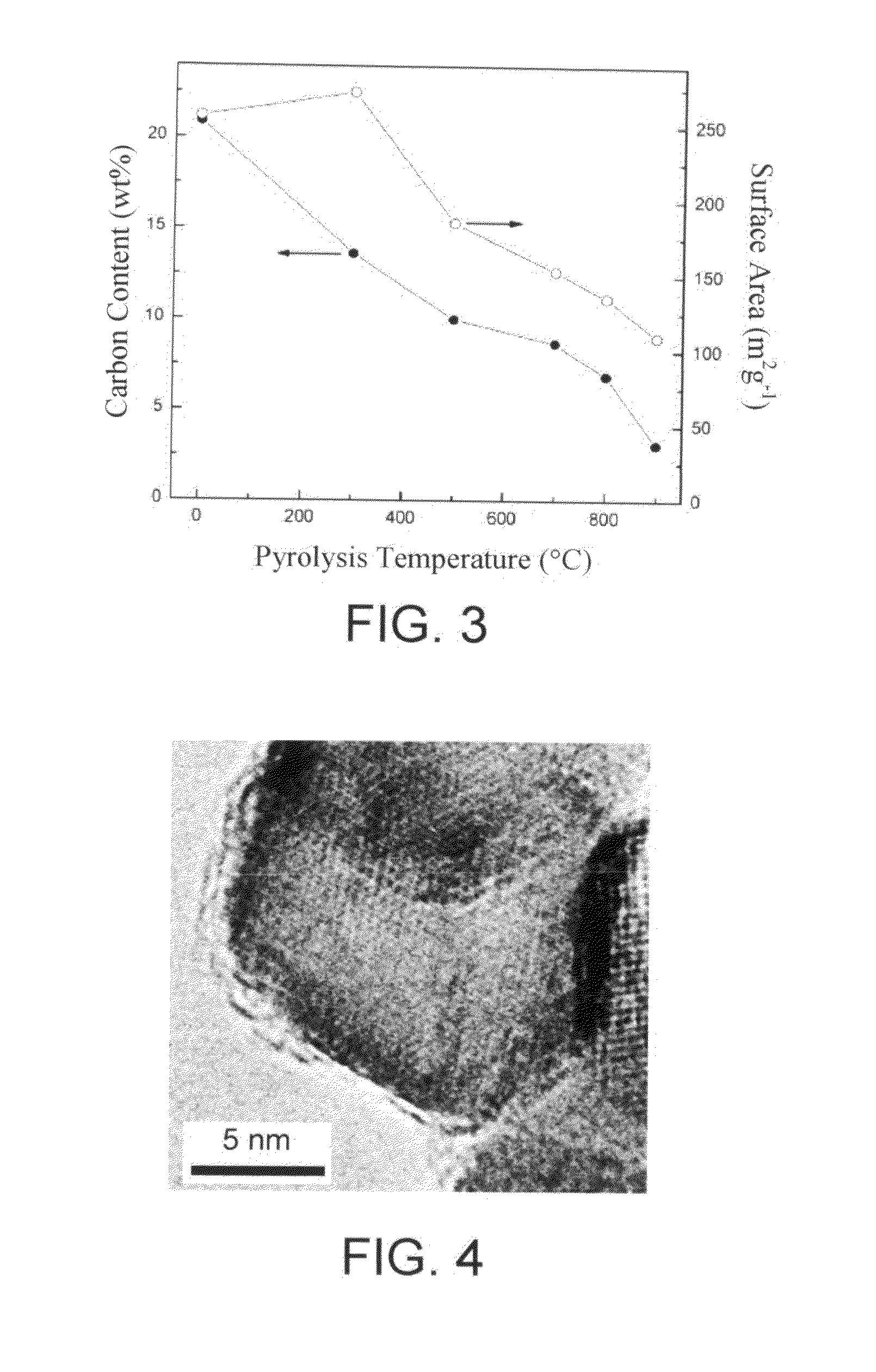
Carbon-coated metal oxide nano-particles and method of preparing the same
ActiveUS20100035750A1Easy to controlNot easy to changeMaterial nanotechnologyZirconium compoundsNanoparticleCarbon coated
A method of preparing carbon-coated metal oxide nano-particles and carbon-coated metal oxide nano-particles prepared with the same method are described. The method includes the following steps at least. A precursor of a polymer is polymerized on metal oxide nano-particles to form polymer-coated metal oxide nano-particles. Then, pyrolysis is conducted to carbonize the polymer coated on the metal oxide nano-particles, so as to form carbon-coated metal oxide nano-particles.
Owner:NATIONAL TSING HUA UNIVERSITY
Doped lead tellurides for thermoelectric applications
InactiveCN101421185AEnergy inputSelenium/tellurium compounds with other elementsSemiconductor materialsLead telluride
A p- or n-conductive semiconductor material comprises a compound of the general formula Pb1-(x1+x2+ . . . +xn)A1x1A2x2 . . . AnxnTe1+z (I) where: in each case independently n is the number of chemical elements different from Pb and Te, 1 ppm<=x1 . . . xn<=0.05, -0.05<=z<=0.05 and n>=2, A1 . . . An are different from one another and are selected from the group of the elements Li, Na, K, Rb, Cs, Be, Mg, Ca, Sr, Ba, Al, Ga, In, Tl, Si, Ge, Sn, As, Sb, Bi, S, Se, Br, I, Sc, Y, La, Ti, Zr, Hf, V, Nb, Ta, Cr, Mo, W, Mn, Re, Fe, Ru, Os, Co, Rh, Ir, Ni, Pd, Pt, Cu, Ag, Au, Zn, Cd, Hg, Ce, Pr, Nd, Sm, Eu, Gd, Tb, Dy, Ho, Er, Tm, Yb, Lu or n=1, and A1 is selected from Ti, Zr, Ag, Hf, Cu, Gr, Nb, Ta.
Owner:BASF AG
Stabilization method for lead projectile impact area
InactiveUS20030143031A1Increase surface areaLow water solubilityTin compoundsSolid waste disposalHealth riskEngineering
The invention pertains to a method for reducing the leaching of lead from lead projectile impact area acting to collect or stop such projectiles. The method includes contacting the lead projectile impact area with a seed of dry granular lead stabilizing agents. The seeding or coating of lead stabilizing agents provides a means to stabilize lead in the bullet / shot impact area while also allowing for future lead bullets, fragments, or lead shot fired into the soil to be stabilized upon contact with the lead stabilizing agent seeds or contact with rainwater leaching through the lead stabilizing agent seeds produced from the projectile coating, or excess seed from the lead shell projectiles. This method eliminates the need to remove or re-treat range soils and greatly reduces the environmental and health risks associated with the use of lead as projectiles in the open environment as well as at control trap ranges.
Owner:FORRESTER KEITH E
Green and environment-friendly synthesis method of CsPbX3 perovskite quantum dot
ActiveCN106745204AMaximize UtilizationGood optical performanceLuminescent compositionsLead compoundsSynthesis methodsEvaporation
The invention discloses a simple, green, and environment-friendly synthesis method of a CsPbX3 perovskite quantum dot, wherein the X can be Cl, Br, or I. The synthesis method is characterized in that cesium ions, lead ions, and bromine ions are dissolved in a solvent, a proper amount of carboxylic acids and amines is added and taken as the stabilizing agent; a low pressure distillation method is adopted to separate the solvent from the cesium ions, lead ions, and bromine ions; cesium ions, lead ions, and bromine ions are precipitated out due to the evaporation of the solvent, and CsPbX3 perovskite quantum dots are formed. The synthesis method has the advantages that the solvent can be repeatedly used through a low pressure distillation technology, the cost is saved, the method is green and environment-friendly, the reaction temperature is low, the operation is simple, and the synthesis method is suitable for large scale massive production.
Owner:宁波智正伟盈信息科技有限公司
Method for controlling light wavelength of full-inorganic perovskite quantum dots
InactiveCN106905960AAdjust the sizeChange sizeLuminescent compositionsLead compoundsReaction temperatureSolvent
The invention discloses a method for controlling light wavelength of full-inorganic perovskite quantum dots. PbBr2 and octadecene are mixed and a mixed solution I is obtained; oleylamine and oleic acid are added to the mixed solution I in an inert environment, and a mixed solution II is obtained; the reaction temperature is increased to 120-160 DEG C, a caesium precursor solution is quickly added to the mixed solution II, the temperature is kept for 5 s, quantum dots are dispersed in a solvent, and a CsPbBr3 quantum dot solution is obtained; the CsPbBr3 quantum dot solution is dropped and applied to a silicon substrate cleaned in advance, and a film sample is formed; the film sample is placed in a vacuum device to be heated, and the light wavelength of full-inorganic perovskite quantum dots is controlled. The method is simple to operate, the size of the CsPbBr3 quantum dots can be changed after heat treatment, and the light wavelength is adjustable.
Owner:JILIN NORMAL UNIV
Tellurium inorganic reaction systems for conductive thick film paste for solar cell contacts
This disclosure relates to electroconductive paste formulations useful in solar panel technology. In one aspect, the disclosure relates to an inorganic reaction system for use in electroconductive paste compositions, wherein the inorganic reaction system comprises a lead containing matrix composition and a tellurium containing matrix composition. In another aspect, the disclosure relates to an electroconductive paste composition comprising a conductive metal component, an inorganic reaction system and an organic vehicle. Another aspect of the disclosure relates to a solar cell produced by applying an electroconductive paste composition of the invention to a silicon wafer. Yet another aspect relates to a solar cell module assembled using solar cells produced by applying an electroconductive paste composition to a silicon wafer, wherein the electroconductive paste composition comprises an conductive metal component, an inorganic reaction system and an organic vehicle.
Owner:HERAEUS PRECIOUS METALS NORTH AMERICA CONSHOHOCKEN
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com