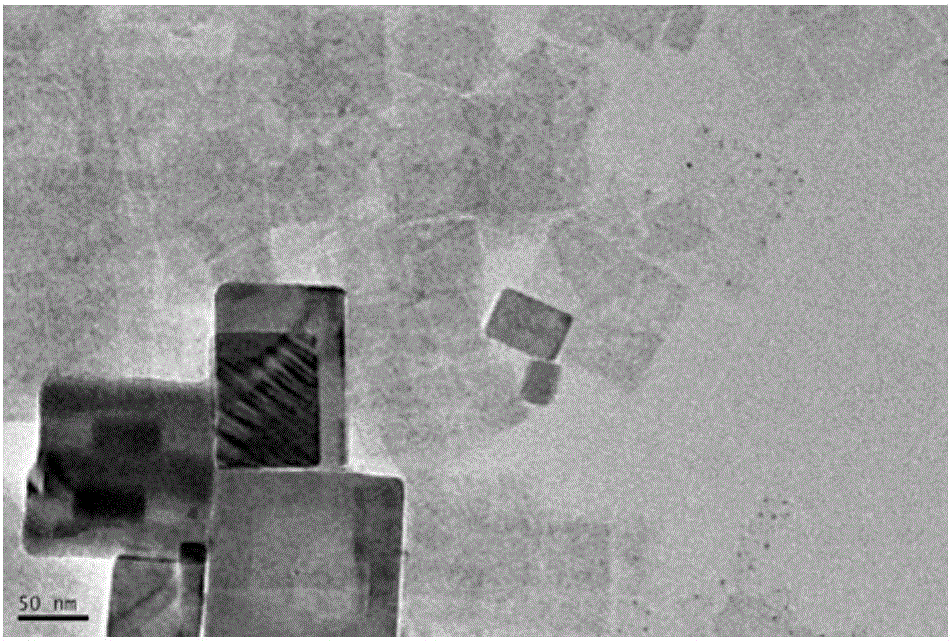
Method for preparing CsPbBr3 nanosheets with quantum size effects
A technology of size effect and nanosheets, which is applied in the field of preparation of CsPbBr3 nanosheets, can solve problems such as the inability to control the lateral size of nanosheets
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] 1) Add 0.05g of cesium carbonate powder to a 50mL three-necked flask containing 5mL of oleic acid, stir and react for 0.5h at 120°C, the cesium carbonate powder is completely dissolved, and the cesium oleate precursor is obtained; in the process, pass in Argon removes the water produced by the reaction;
[0030] 2) Add 0.04g of lead bromide, 0.25mL oleic acid, 0.25mL oleylamine, 0.15mL octanoic acid and 0.15mL octylamine to 10mL octadecene in sequence, and stir the reaction at 120℃ for 0.5h to lead bromide powder Completely dissolve to obtain a mixed solution; In this process, argon gas is introduced, and the reaction system is always kept in an inert atmosphere;
[0031] 3) After the temperature of the mixed solution in step 2) rises to 120°C, quickly inject 1 mL of the cesium oleate precursor cooled to room temperature, and after reacting for 15 seconds, transfer to a 100°C oil bath to continue the reaction. The total reaction time is 5min (total reaction time includes 15...
Embodiment 2
[0035] 1) Add 0.05g of cesium carbonate powder to a 50mL three-necked flask containing 5mL of oleic acid, stir and react for 0.5h at 120°C, the cesium carbonate powder is completely dissolved, and the cesium oleate precursor is obtained; in the process, pass in Argon removes the water produced by the reaction;
[0036] 2) Add 0.04g of lead bromide, 0.25mL oleic acid, 0.25mL oleylamine, 0.25mL octanoic acid and 0.25mL octylamine to 10mL octadecene solvent, and stir the reaction at 120℃ for 0.5h to lead bromide The powder is completely dissolved to obtain a mixed solution; argon gas is introduced during this process, and the reaction system is always kept in an inert atmosphere;
[0037] 3) After the temperature of the mixed solution in step 2) rises to 130°C, quickly inject 1 mL of the cesium oleate precursor cooled to room temperature, and transfer to an oil bath at 110°C for 15 seconds to continue the reaction. The total reaction time is 5min (total reaction time includes 15s aft...
Embodiment 3
[0041] 1) Add 0.05g of cesium carbonate powder to a 50mL three-necked flask containing 5mL of oleic acid, stir and react for 0.5h at 120°C, the cesium carbonate powder is completely dissolved, and the cesium oleate precursor is obtained; in the process, pass in Argon removes the water produced by the reaction;
[0042] 2) Add 0.04g of lead bromide, 0.25mL of oleic acid, 0.25mL of oleylamine, 0.35mL of octanoic acid and 0.35mL of octylamine to 10mL of octadecene solvent, and stir the reaction at 120℃ for 0.5h to lead bromide The powder is completely dissolved and a mixed solution is obtained; argon gas is introduced during this process, and the reaction system is always kept in an inert atmosphere;
[0043] 3) After the temperature of the mixed solution in step 2) is increased to 140°C, quickly inject 1 mL of the cesium oleate precursor cooled to room temperature, and after reacting for 10 seconds, transfer to an oil bath at 120°C to continue the reaction. The total reaction time is...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com