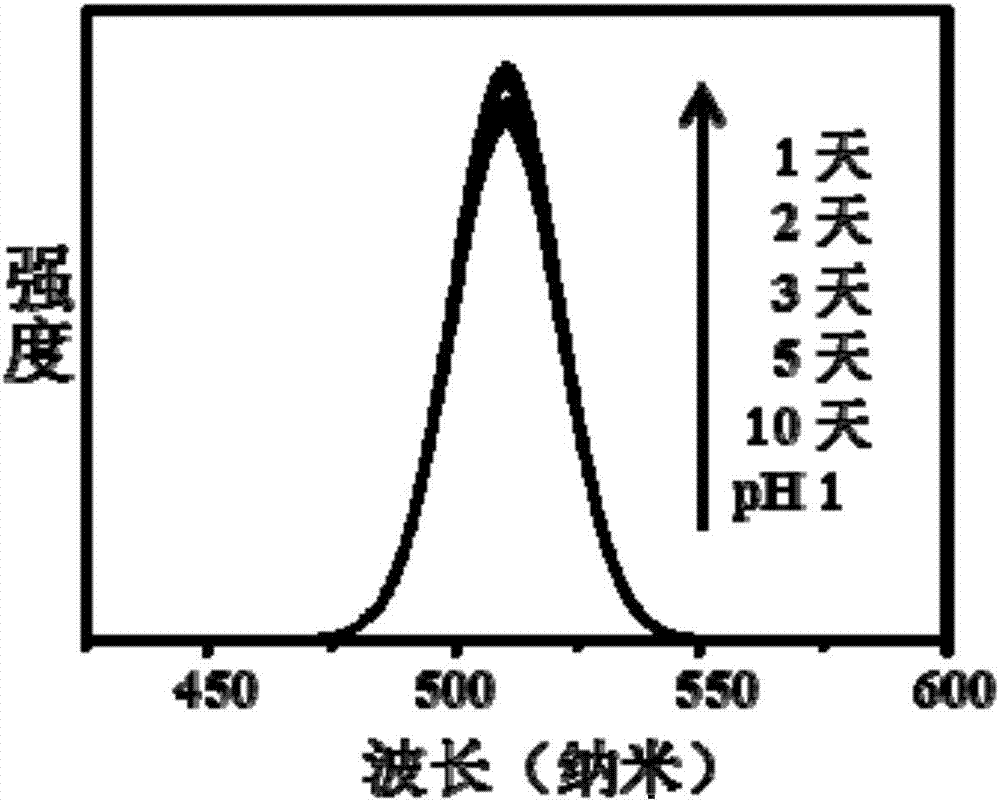
High-stability water-soluble CsPbX3 perovskite nano-crystalline preparation method
A high-stability, perovskite technology, applied in nanotechnology, nanotechnology, nanotechnology, etc. for materials and surface science, can solve problems such as nanocrystal instability, and achieve the effect of simple and adjustable operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
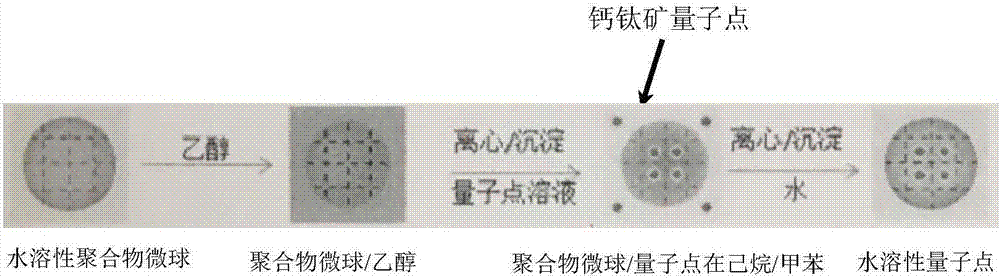
[0025] First, add 0.01 g (1 mL) of water-soluble polystyrene microspheres (0.1 to 10 microns in size) into 10 mL of ethanol, and disperse them further by ultrasonication for 30 minutes to obtain a homogeneous solution, followed by centrifugation to redisperse the precipitate in hexane After sonicating for 2 hours, the solution was centrifuged again to obtain a precipitate of polystyrene microspheres.
[0026] Then the prepared 5mL CsPbBr 3 Perovskite nanocrystal solution (containing 0.001mmol perovskite nanocrystal, diameter 4-12nm, solvent is toluene and ethane with a volume ratio of 1:10) was added to the polystyrene precipitate, ultrasonicated for 1 hour, and then stirred for 24 Hour. The solution is centrifuged to obtain composite particles of perovskite and polystyrene. The obtained particles were redispersed into a hexane solution, and the perovskite-supported polystyrene particles were obtained by precipitation and redispersed into hexane. Finally, the as-prepared pr...
Embodiment 2
[0028] First, add 0.01 g (1 mL) of water-soluble polystyrene microspheres (0.1 to 10 microns in size) into 10 mL of ethanol, and disperse them further by ultrasonication for 30 minutes to obtain a homogeneous solution, followed by centrifugation to redisperse the precipitate in hexane Post-sonic for 2 hours. The solution was centrifuged again to obtain a pellet of polystyrene microspheres.
[0029] Then the prepared 5mL CsPbBr 3 The perovskite nanocrystal solution (containing 0.002mmol perovskite nanocrystal, diameter 4-12nm, solvent is toluene and ethane with a volume ratio of 1:4) was added to the polystyrene precipitate, ultrasonicated for 1 hour, and then stirred for 24 Hour. Finally, the solution was centrifuged to obtain composite particles of perovskite and polystyrene. The obtained particles were redispersed into a hexane solution, and the perovskite-supported polystyrene particles were obtained by precipitation and redispersed into hexane. Finally, the prepared pr...
Embodiment 3
[0031] First, add 0.02g (1mL) of water-soluble polymethylmethacrylate microspheres (0.1-10 microns in size) into 10mL of ethanol, and disperse further by ultrasonication for 30 minutes to obtain a homogeneous solution, followed by centrifugation to redisperse the precipitate into Post-sonicate in hexane for 2 hours. The solution was centrifuged again to obtain precipitates of polymethyl methacrylate microspheres.
[0032] Then the prepared 5mL CsPb(Br / Cl) 3 Perovskite nanocrystal solution (containing 0.001mmol perovskite nanocrystal, with a diameter of 4-12nm, and the solvent is toluene and ethane with a volume ratio of 1:4) was added to the polymethyl methacrylate precipitation, ultrasonicated for 1 hour, and then 24 hours under stirring. The solution is centrifuged to obtain composite particles of perovskite and polymethyl methacrylate. The obtained particles were redispersed in hexane solution, and then dispersed in hexane to obtain perovskite-supported polymethyl methac...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com