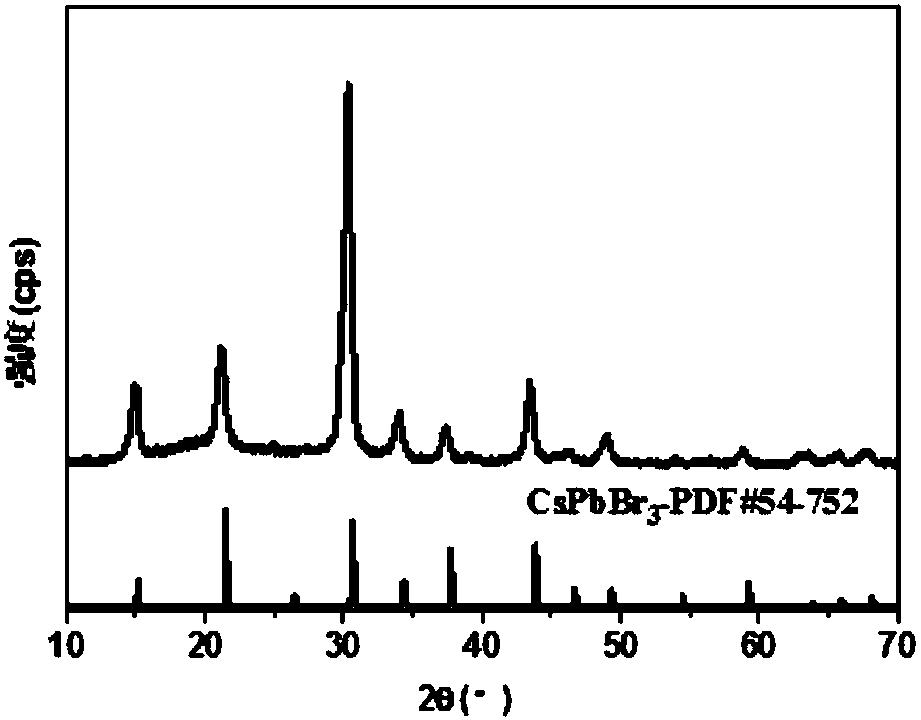
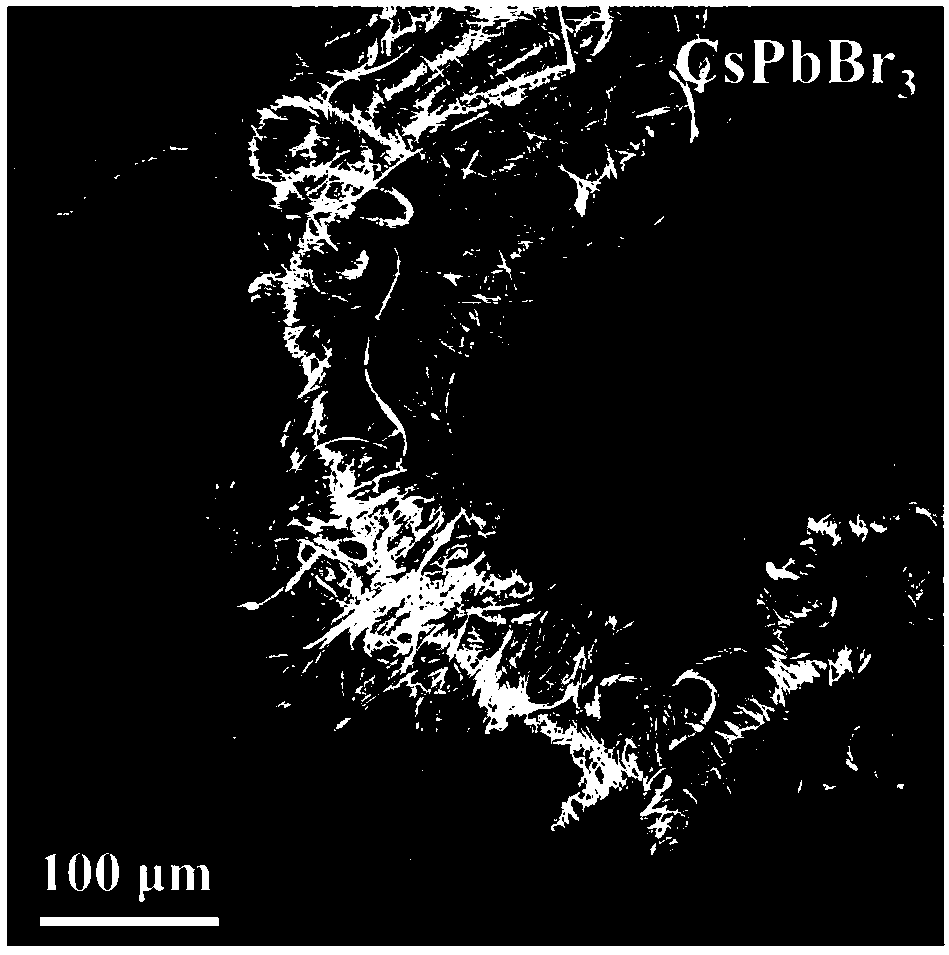
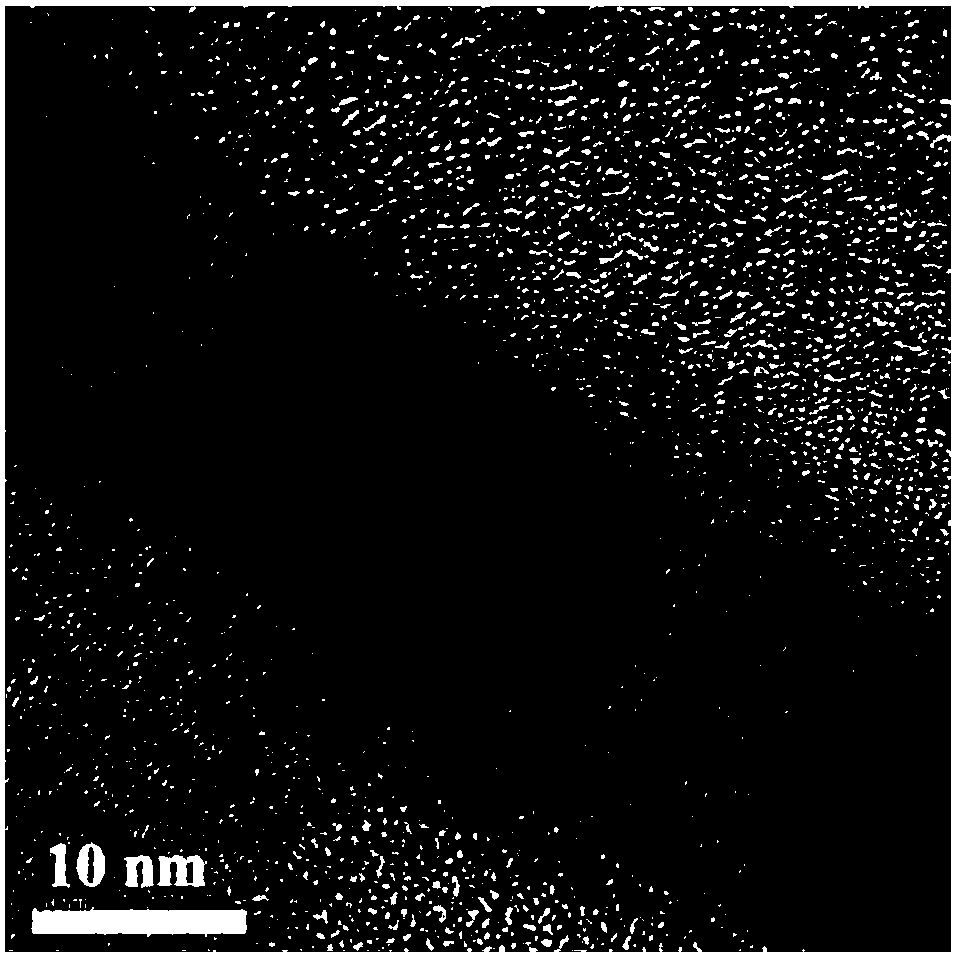
Method for synthesizing inorganic perovskite nanowires
A synthesis method and nanowire technology, applied in inorganic chemistry, chemical instruments and methods, lead compounds, etc., can solve the problems of low yield of nanowire method, high cost of high temperature treatment process, complexity of experimental process, etc., and achieve uniform morphology. , the effect of low reaction temperature and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Step 1, take by weighing 0.7mmol cesium carbonate (Cs 2 CO 3 ) into a flask containing 1.0 mL of oleic acid and 8.0 mL of octadecene, stirred at 120° C. for 25 min to completely dissolve the cesium carbonate powder, and then naturally cooled to room temperature to form a cesium precursor solution.
[0046] Step 2. Weigh 2.0mmol of lead bromide into a flask containing 2.0mL of oleic acid, 2.0mL of oleylamine and 16mL of octadecene, stir at 110°C for 25min to completely dissolve the lead bromide powder, and then cool in an ice-water bath to room temperature to form a lead bromide precursor solution.
[0047] Step 3. Heat the cesium oleate precursor solution obtained in step 1 to 80°C, measure 2mL and add it to the reaction kettle, cool to room temperature, then add 20mL lead bromide precursor obtained in step 2 at room temperature, and sonicate 15min.
[0048] Step 4. React the mixed solution obtained in Step 3 at a heating temperature of 120° C. for 70 h, and naturall...
Embodiment 2
[0053] Step 1. Weigh 0.7 mmol of cesium carbonate and add it to a flask containing 1.0 mL of oleic acid and 8.0 mL of octadecene, stir at 120°C for 25 minutes to completely dissolve the cesium carbonate powder, then cool naturally to room temperature to form a cesium precursor body solution.
[0054] Step 2. Weigh 2.0mmol of lead bromide into a flask containing 2.0mL of oleic acid, 2.0mL of oleylamine and 16mL of octadecene, stir at 110°C for 25min to completely dissolve the lead bromide powder, and then cool in an ice-water bath to room temperature to form a lead bromide precursor solution.
[0055] Step 3. Weigh 2.0mmol lead iodide and add it to a flask containing 2.0mL oleic acid, 2.0mL oleylamine and 16mL octadecene, stir at 110°C for 25min to completely dissolve the lead iodide powder, and then use an ice-water bath to cool to room temperature to form a lead iodide precursor solution.
[0056] Step 4. Heat the cesium oleate precursor solution obtained in step 1 to 80°C ...
Embodiment 3
[0063] Step 1. Weigh 0.7 mmol of cesium carbonate and add it to a flask containing 1.0 mL of oleic acid and 8.0 mL of octadecene, stir at 120°C for 25 minutes to completely dissolve the cesium carbonate powder, then cool naturally to room temperature to form a cesium precursor body solution.
[0064] Step 2. Weigh 2.0mmol of lead bromide into a flask containing 2.0mL of oleic acid, 2.0mL of oleylamine and 16mL of octadecene, stir at 110°C for 25min to completely dissolve the lead bromide powder, and then cool in an ice-water bath to room temperature to form a lead bromide precursor solution.
[0065] Step 3. Weigh 2.0mmol lead chloride and add it to a flask containing 2.0mL oleic acid, 2.0mL oleylamine, 2.0mL trioctylphosphine and 16mL octadecene, stir at 110°C for 25min to make the lead chloride powder completely dissolved, and then cooled to room temperature using an ice-water bath to form a lead chloride precursor solution.
[0066] Step 4. Heat the cesium oleate precurso...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com