Nitrogen-doped carbon material supporting cobalt catalyst and method therewith of catalytic hydrogenation reductive amination to produce amine compounds
A nitrogen-doped carbon and cobalt catalyst technology, which is applied in the direction of catalyst activation/preparation, reductive alkylation preparation, organic compound preparation, etc., can solve the problems of high reaction pressure, equipment corrosion, poor product quality, etc., and achieve the goal of reaction Mild conditions, reduced reaction pressure, and reduced production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] A nitrogen-doped carbon material supported cobalt catalyst, prepared by the following method:
[0030] 1) Dissolve 5.5g 2-methylimidazole in 20mL water to obtain 2-methylimidazole solution, dissolve 0.45g cobalt nitrate hexahydrate in 3mL water to obtain cobalt nitrate solution, add cobalt nitrate solution to 2-methylimidazole In the imidazole solution, a mixed solution was obtained, and the mixed solution was stirred and reacted at room temperature (25° C.) for 6 hours. After the reaction was completed, the obtained mixed system was centrifuged and filtered, and the filter cake was washed with water and methanol in sequence, and the obtained solid was vacuum Dry to obtain MOF material ZIF-67;
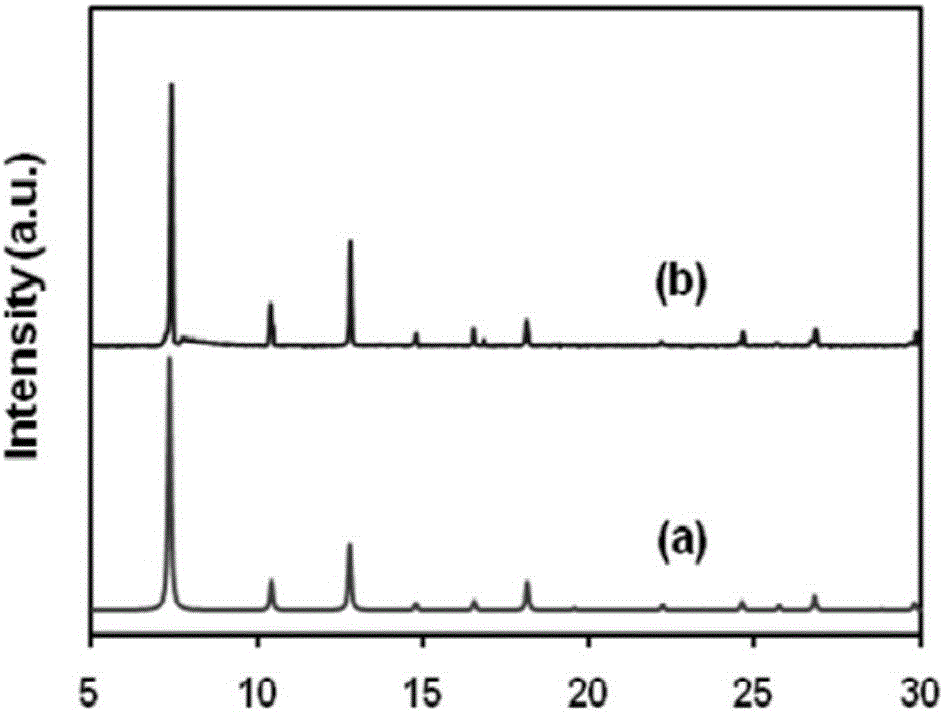
[0031] Carry out X-ray diffraction analysis to the nitrogen-doped carbon material supported cobalt catalyst prepared in Example 1, the X-ray diffraction pattern of gained is as follows figure 1 As shown, it shows that we have successfully prepared ZIF-67.
[0032] 2) Under the...
Embodiment 2
[0035] The operation and steps are the same as in Example 1, only the calcination temperature is changed, and the calcination temperature is changed to 800°C, and the obtained nitrogen-doped carbon material supports the cobalt catalyst Co / N-C, which is marked as catalyst B.
Embodiment 3
[0037] The operation and steps were the same as in Example 1, only the calcination temperature was changed, and the calcination temperature was changed to 900° C., and the obtained nitrogen-doped carbon material supported cobalt catalyst Co / N-C, which was marked as catalyst C.
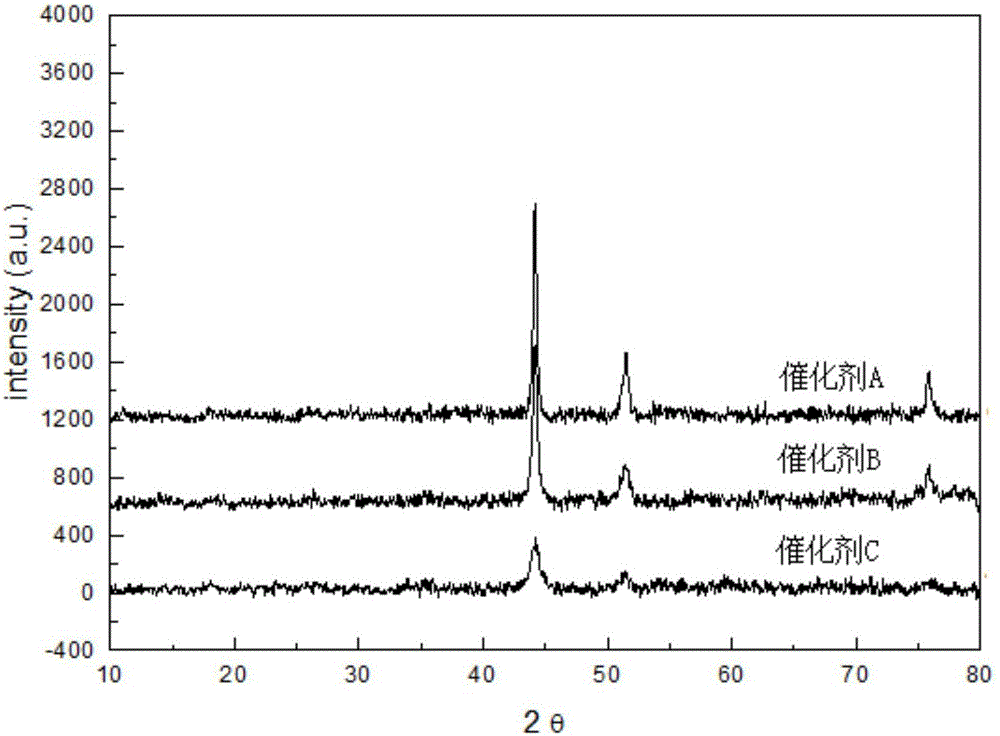
[0038] Carry out X-ray diffraction analysis to the nitrogen-doped carbon material supported cobalt catalyst prepared in embodiment 1-3, the X-ray diffraction pattern of gained is as follows image 3 shown, from image 3 It can be seen that the three peaks at 44.2°, 51.6°, and 76.0° correspond to the three crystal planes of Co(111), Co(200), and Co(200) respectively (JCPDS No.15-0806), further indicating that the cobalt nanoparticles exist. Compared image 3 , it can be found that there are more characteristic peaks of metallic cobalt, and the characteristic peaks of ZIF-67 disappear.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com