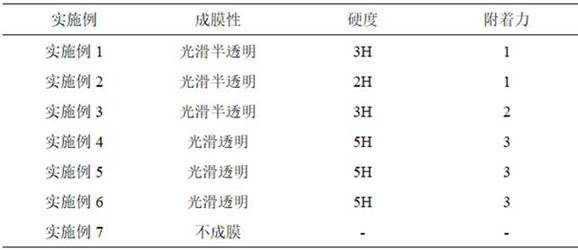
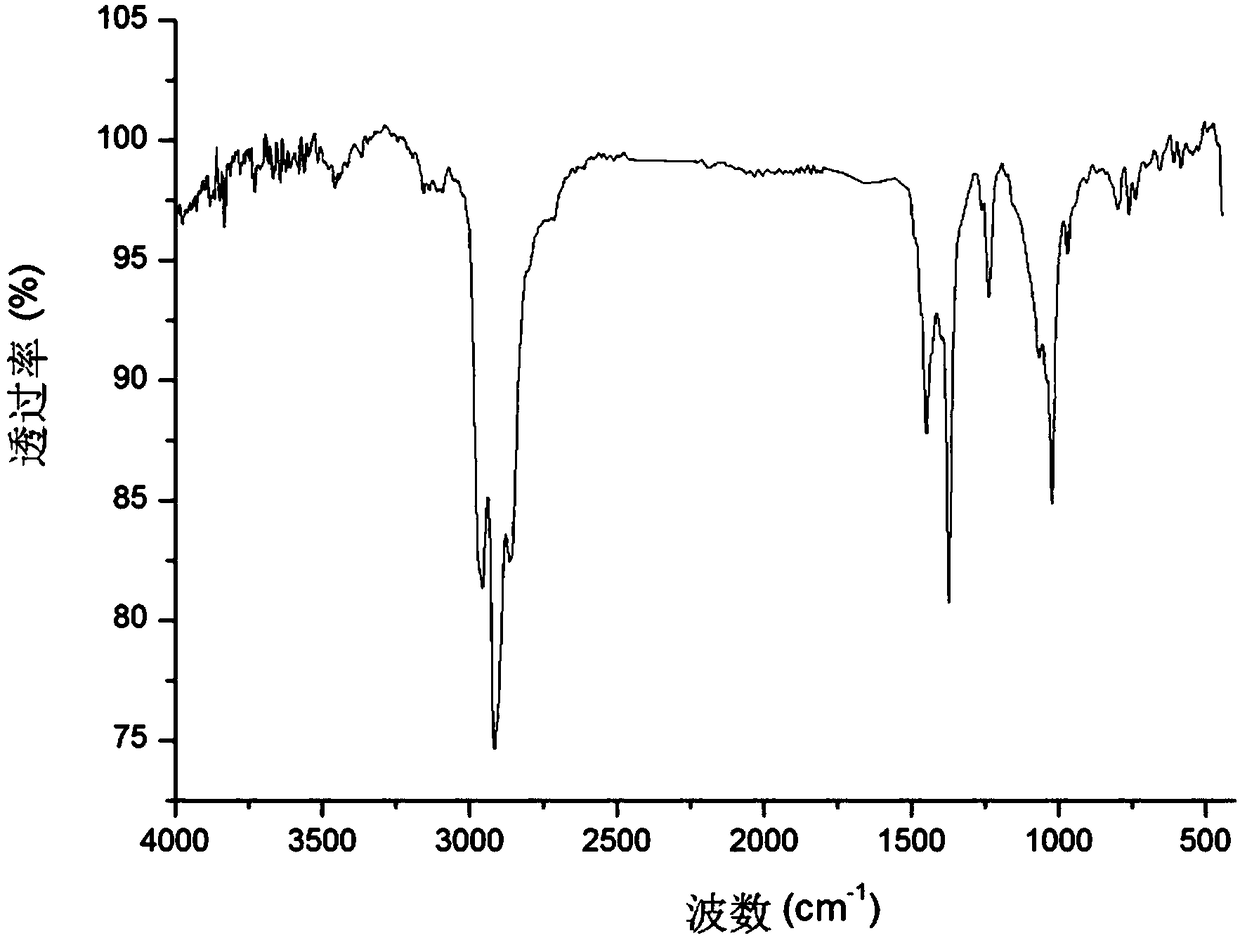
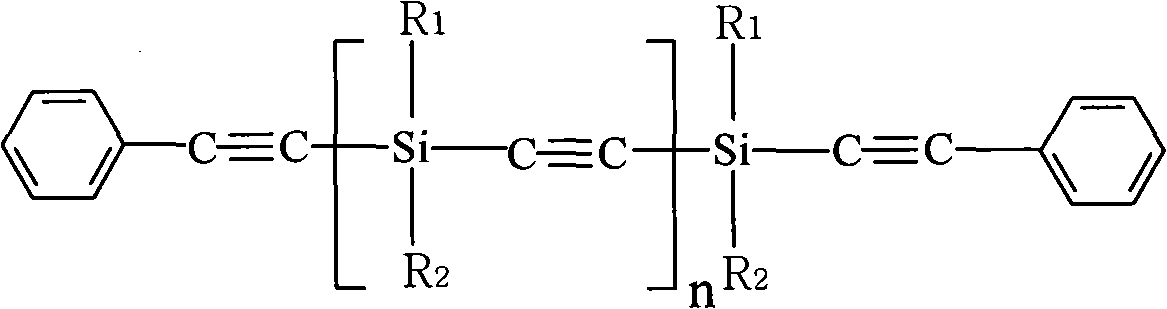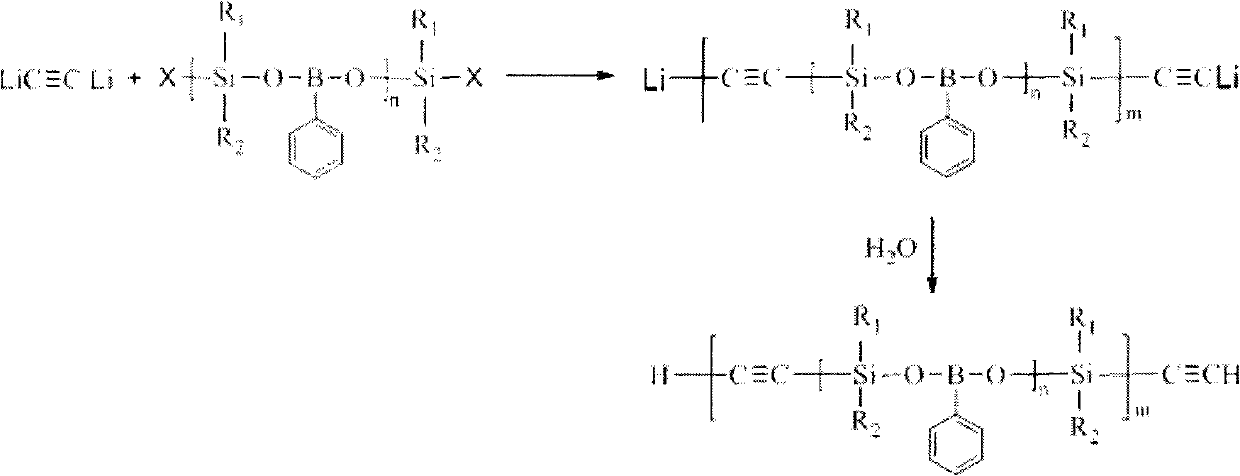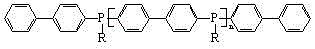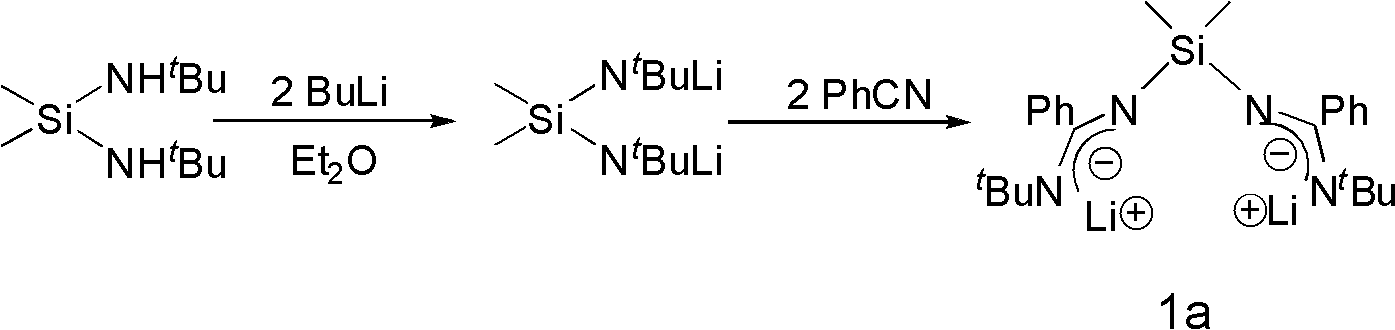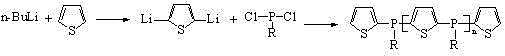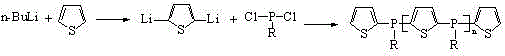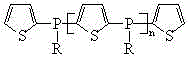Patents
Literature
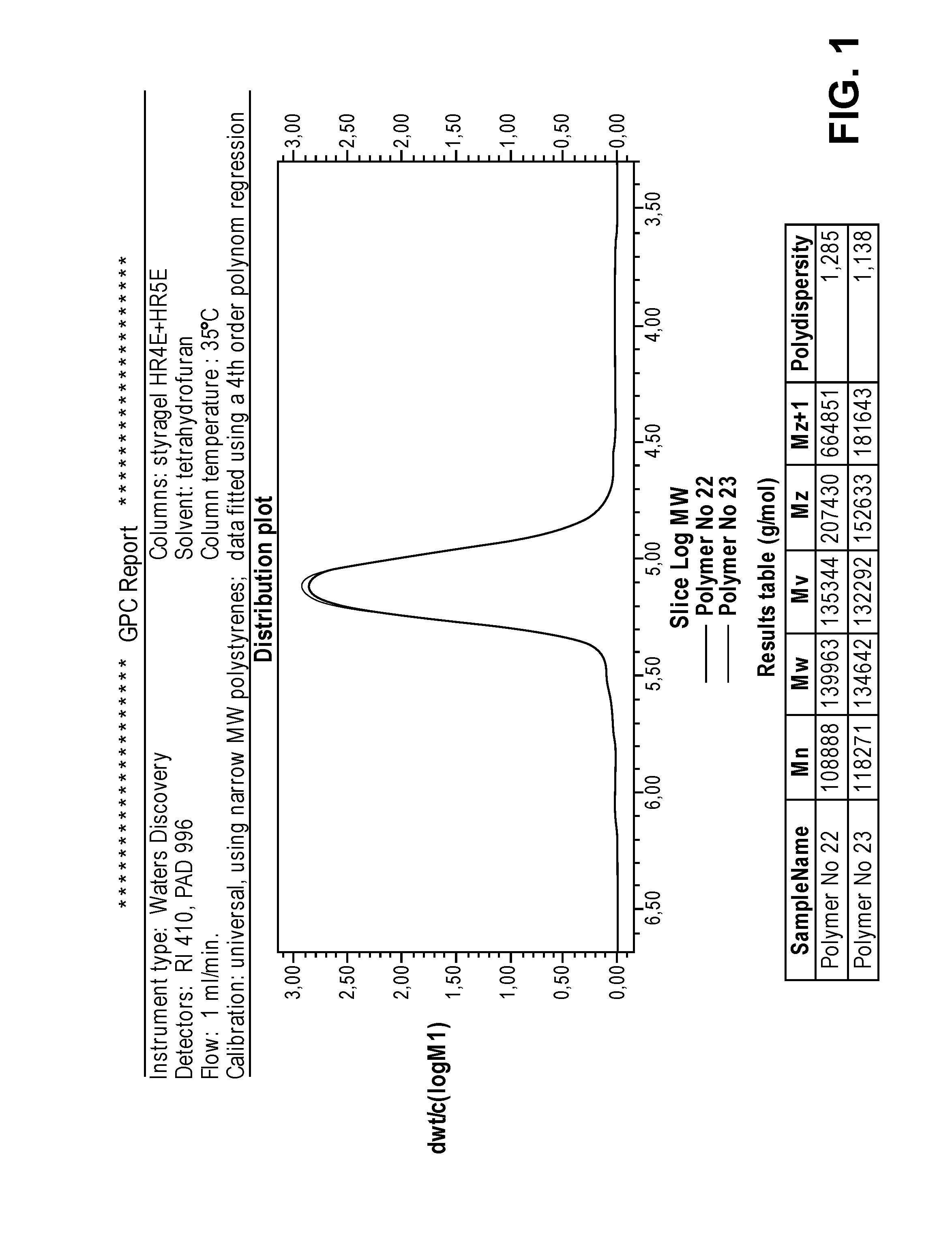
32 results about "Dilithium" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
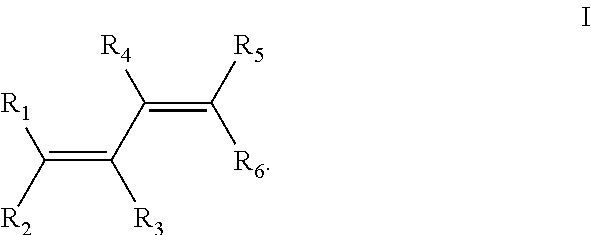
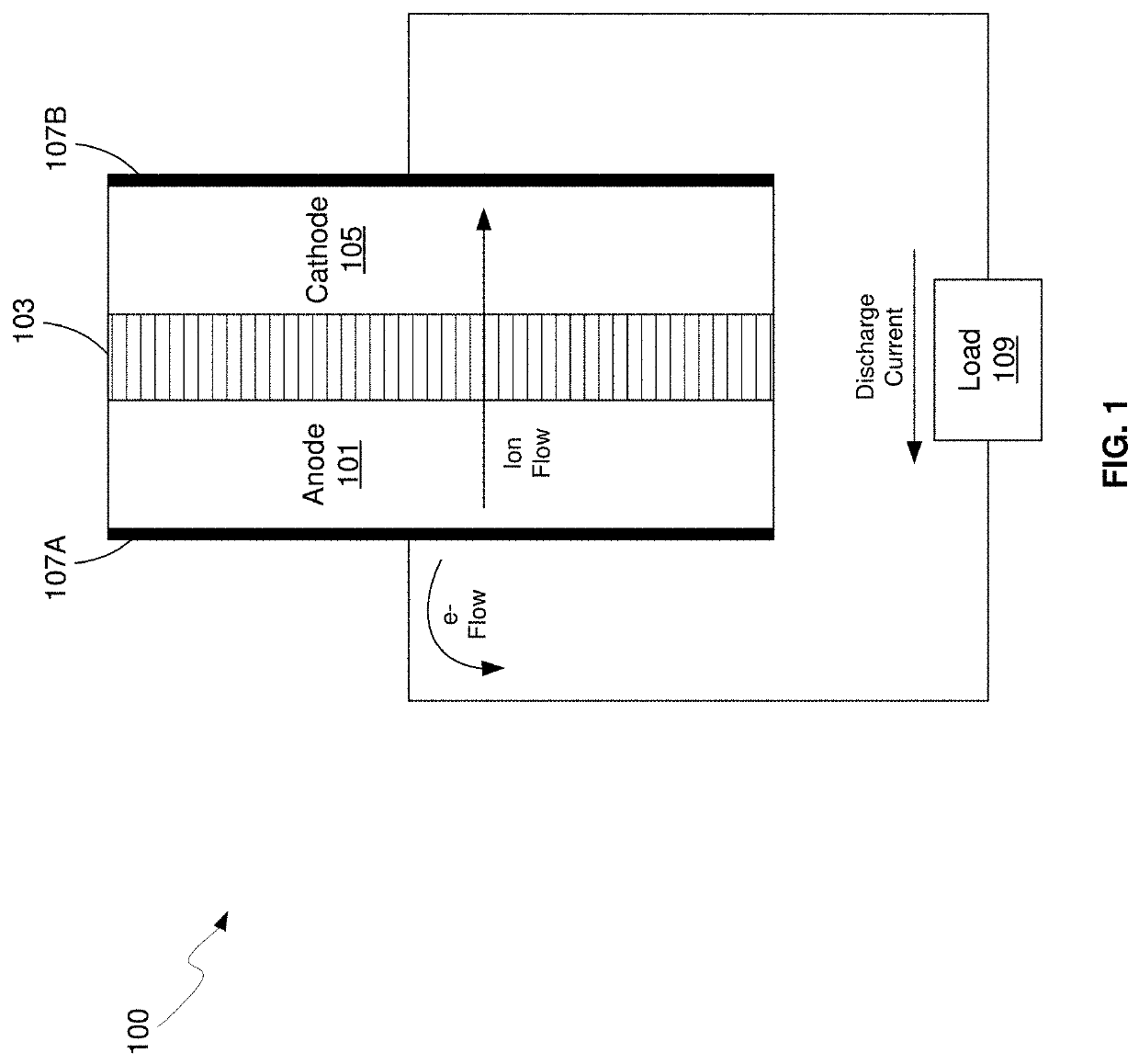
Dilithium, Li₂, is a strongly electrophilic, diatomic molecule comprising two lithium atoms covalently bonded together. Li₂ is known in the gas phase. It has a bond order of 1, an internuclear separation of 267.3 pm and a bond energy of 102 kJ mol⁻¹ or 1.06eV in each bond. The electron configuration of Li₂ may be written as σ².
Cathode active material and lithium ion rechargeable battery using the material
InactiveUS20120308890A1High activityGood chemical stabilityPrimary cellsPositive electrodesDilithiumCathode material
Provided is a lithium ion rechargeable battery less suffering from swelling even when stored at high temperatures. Disclosed are a cathode active material, a cathode for a lithium ion rechargeable battery using the cathode active material, and a lithium ion rechargeable battery using the cathode. The cathode active material includes particles, each of the particles including a cathode material capable of intercalating and deintercalating lithium ions, and a film formed on at least part of surfaces of the particles. The film includes a compound represented by Chemical Formula (1). Examples of the compound represented by Chemical Formula (1) include lithium squarate and dilithium squarate. Preferably, the lithium ion rechargeable battery is a prismatic battery.
Owner:HITACHT MAXELL LTD
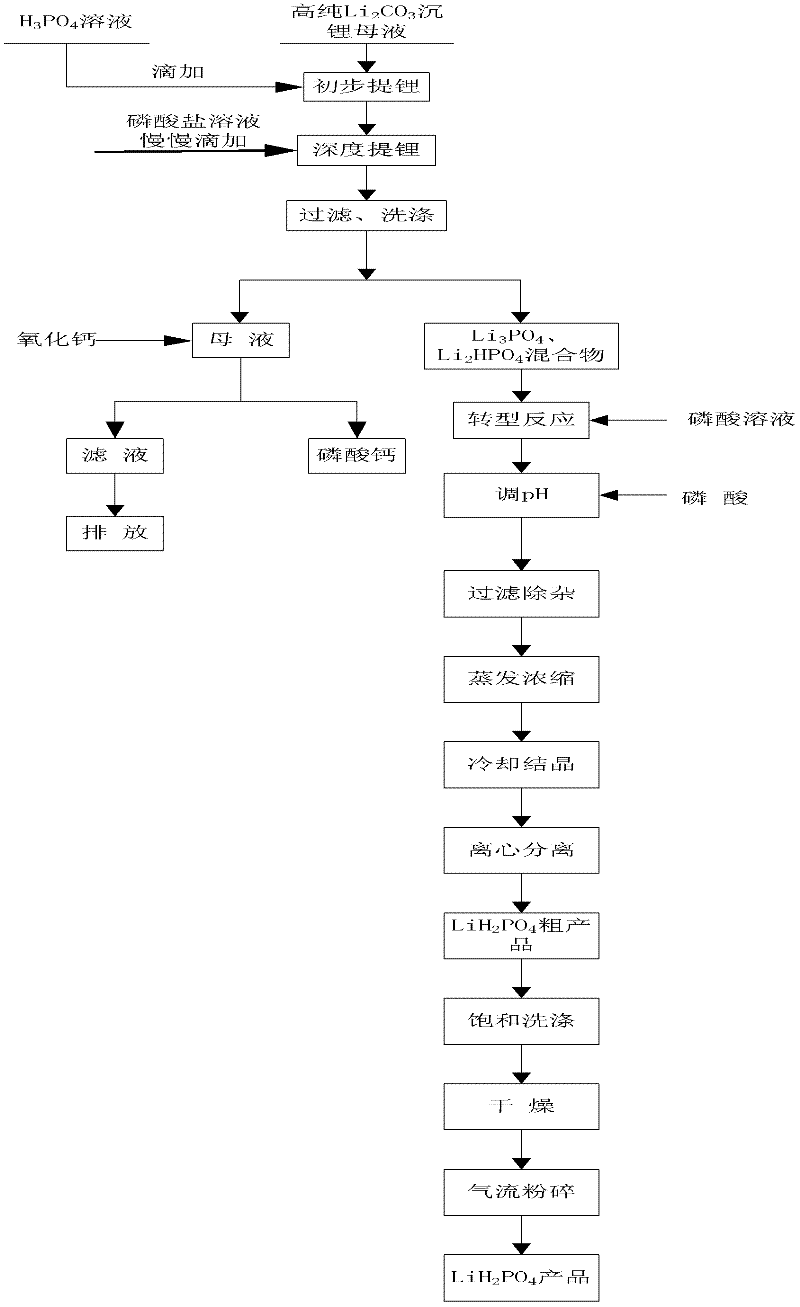
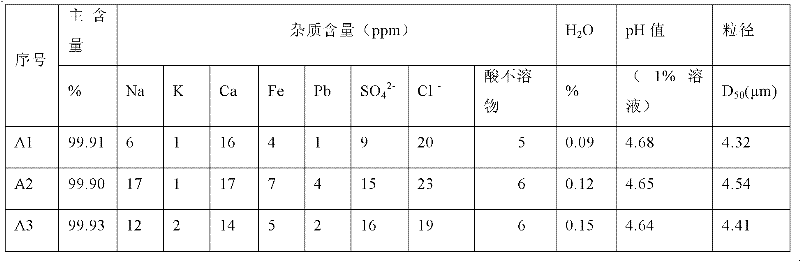
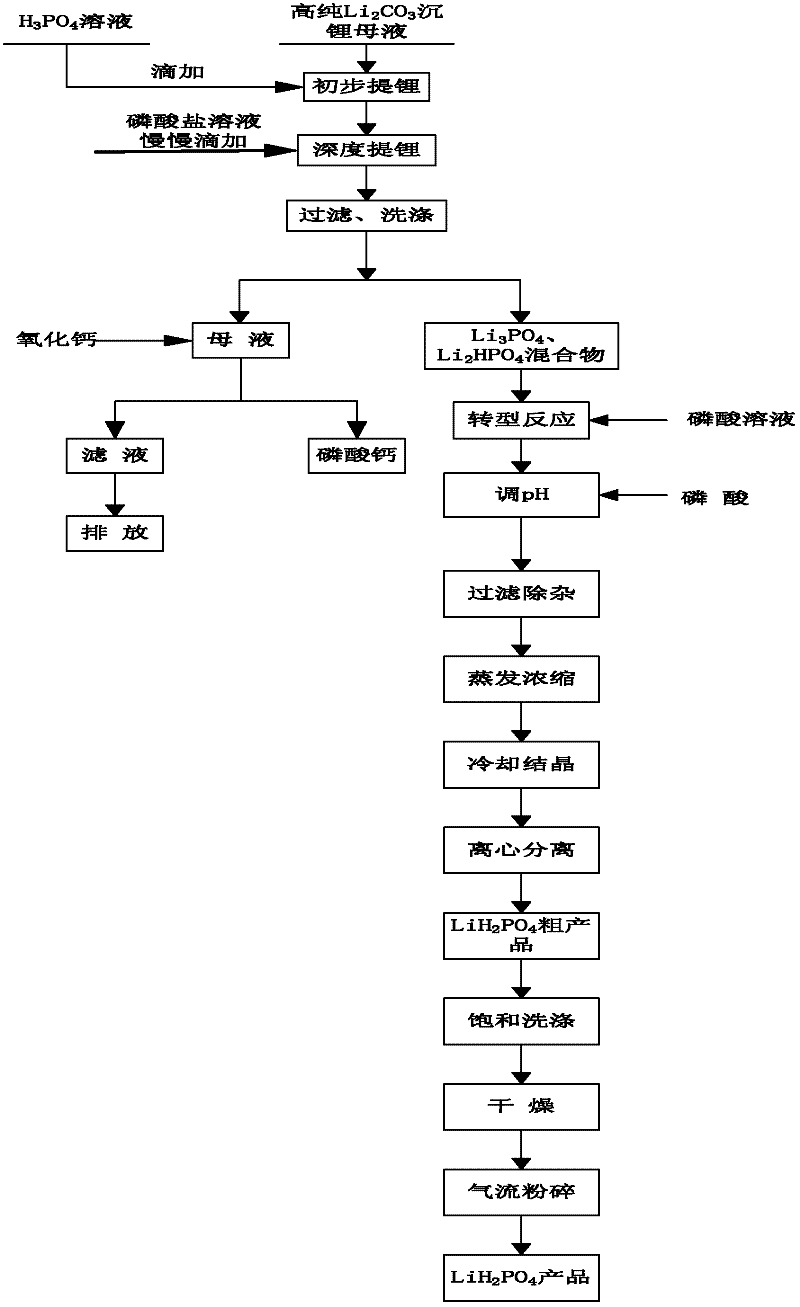
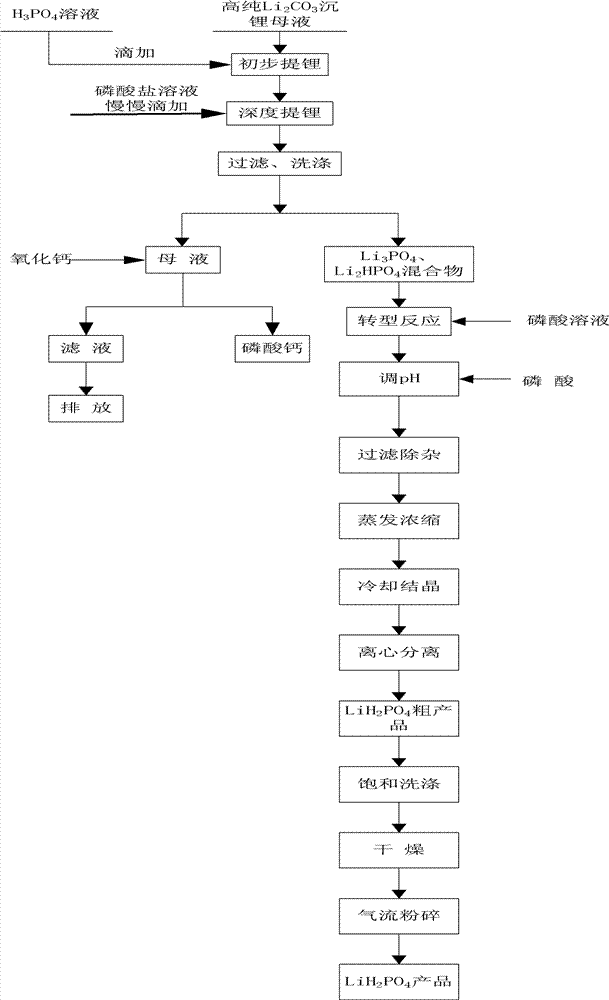
Method for preparing battery grade lithium dihydrogen phosphate with high-purity lithium carbonate lithium depositing mother solution
ActiveCN102351160AFine and uniformBright white colorPhosphorus compoundsPhosphate productEvaporation
The invention relates to a method for preparing battery grade lithium dihydrogen phosphate with a high-purity lithium carbonate lithium depositing mother solution. The method comprises the steps of: conducting preliminary lithium extraction and deep lithium extraction to a lithium carbonate lithium depositing mother solution with phosphoric acid and phosphate so as to obtain a mixture of lithium phosphate and dilithium hydrogen phosphate, reacting the mixture with phosphoric acid to generate a lithium dihydrogen phosphate solution, then carrying out concentration and evaporation, cooling and crystallization, centrifugation, saturation washing, drying, air-stream crushing and packaging, thus obtaining the battery grade lithium dihydrogen phosphate. The method of preparing battery grade lithium dihydrogen phosphate in the invention fully makes use of the mother solution generated during high-purity lithium carbonate production, and has the advantages of simple process, easy operation, low production cost, over 90% of lithium recovery rate, and stable quality of the obtained battery grade lithium dihydrogen phosphate product, and is also suitable for preparing the lithium ion battery positive material lithium iron phosphate. Therefore, the method provided in the invention boasts broad market prospects, as well as good economic and social benefits.
Owner:GANFENG LITHIUM CO LTD
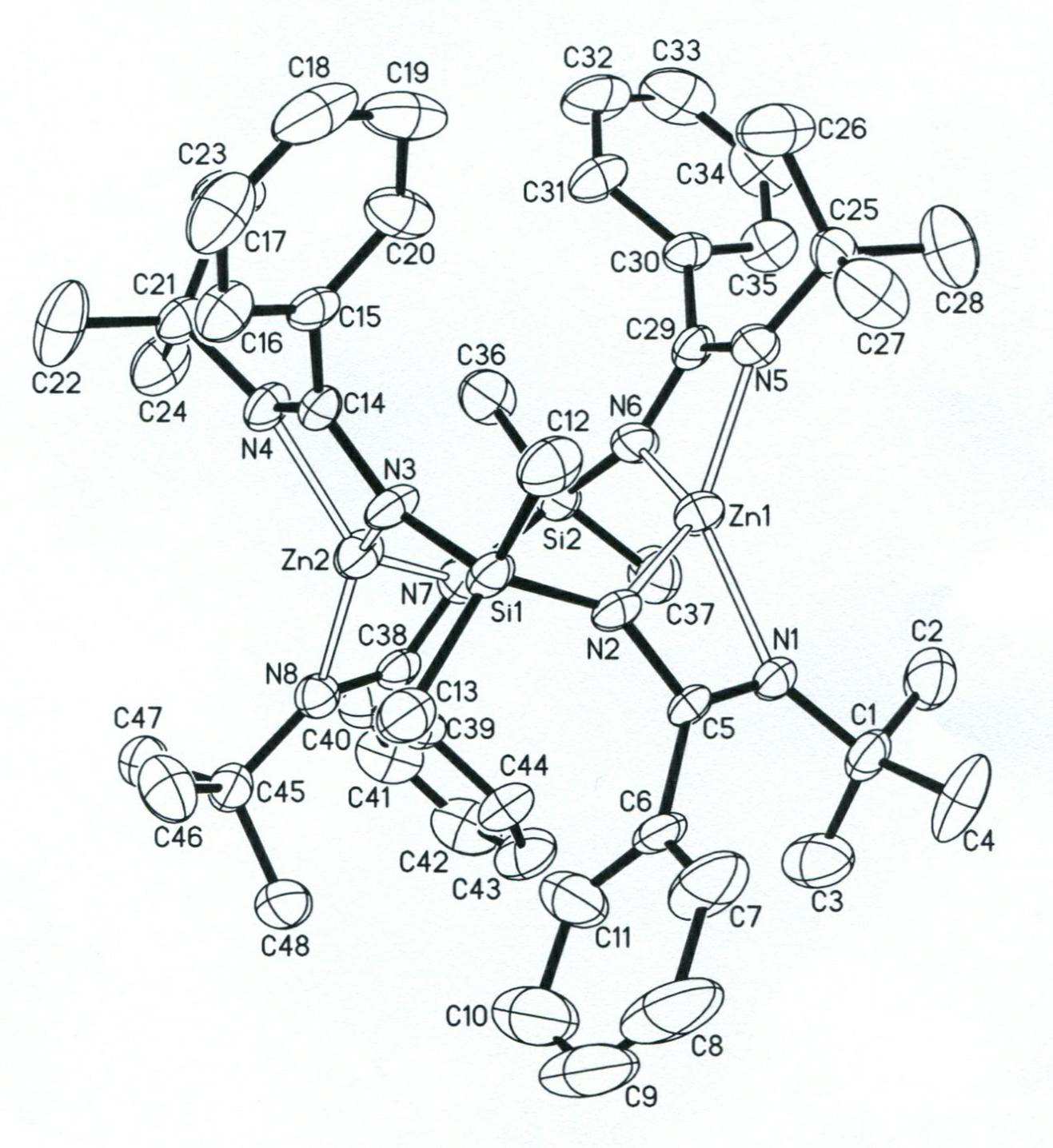
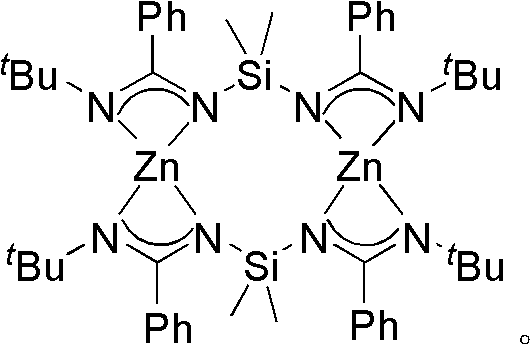
Bridged diamidino group-IV metal catalyst and method for preparing same
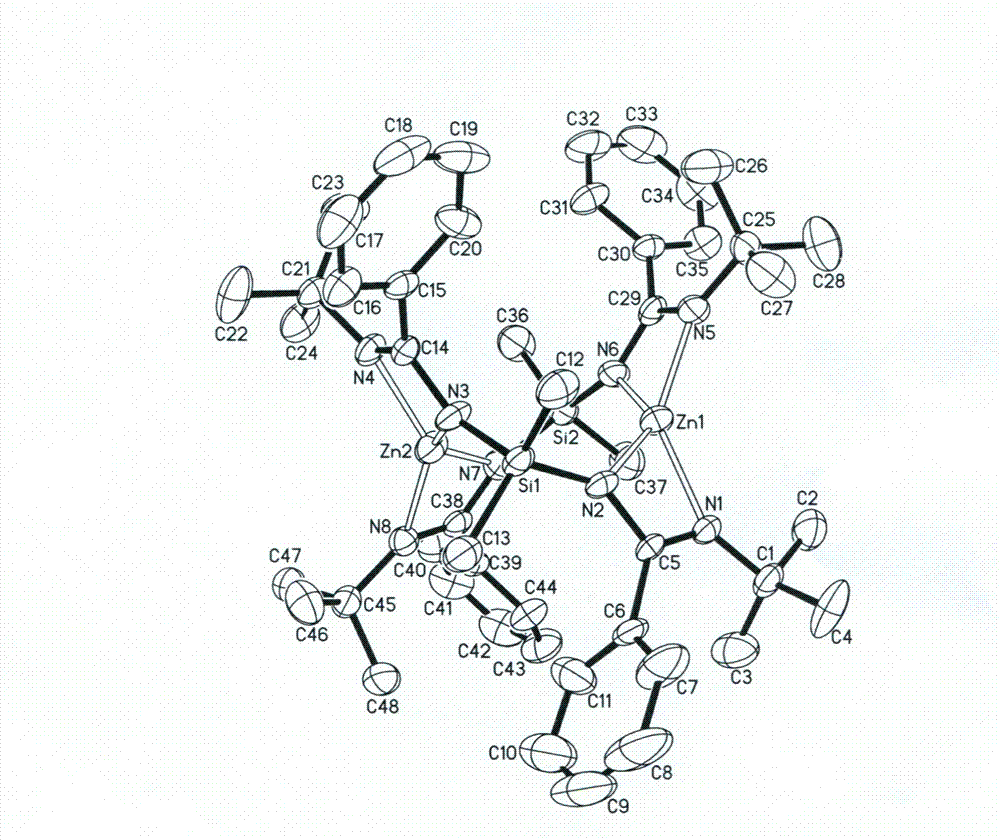
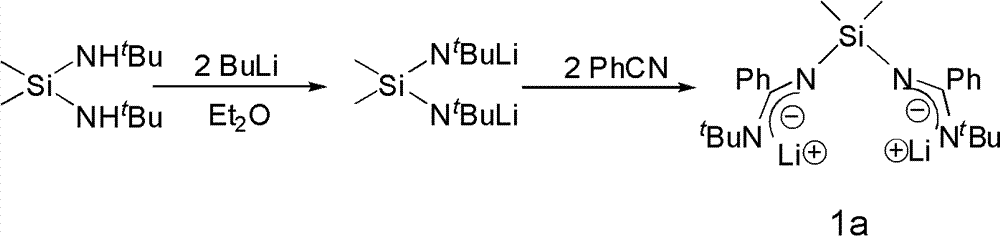
The invention provides a bridged diamidino group-IV metal catalyst, which relates to an olefin polymerization catalyst, in particular to a compound taking metals of a group IV as central atoms and a seven-element skeleton with an N-C-N-Si-N-C-N characteristic as a ligand. A method for preparing the bridged diamidino group-IV metal catalyst comprises the following steps: in the protection of nitrogen, taking bridging diamine as an initial raw material and converting the bridging diamine into a dilithium salt by utilizing butyl lithium; adding cyanophenyl into the dilithium salt to produce an addition reaction, forming a bridged diamidino ligand after migrating a silicon base twice, and performing a complex reaction on the polydentate ligand and group-IV metal chloride of the group IV to prepare bridged diamidino group-IV metal chloride; and reacting lithium methide with the bridged diamidino group-IV metal chloride to displace helium atoms to produce a methyl substitute. The synthesis method has the advantages of universal applicability, moderate reaction condition, simple and easily-obtained materials, low cost, simple steps and relatively higher productivity. The compound has good catalytic effect on the polyreaction of olefin.
Owner:SHANXI UNIV
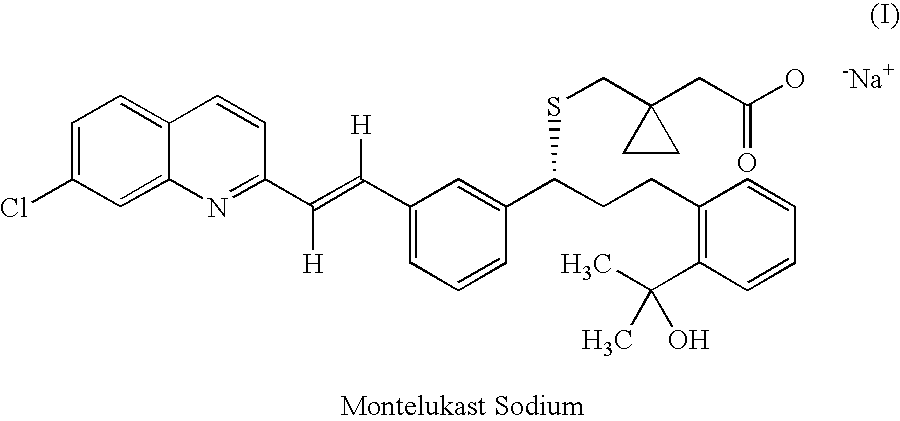
Process for the preparation of leukotriene antagonists
InactiveCN101081834AOrganic chemistryOrganic compound preparationLeukotriene AntagonistsMethyl group
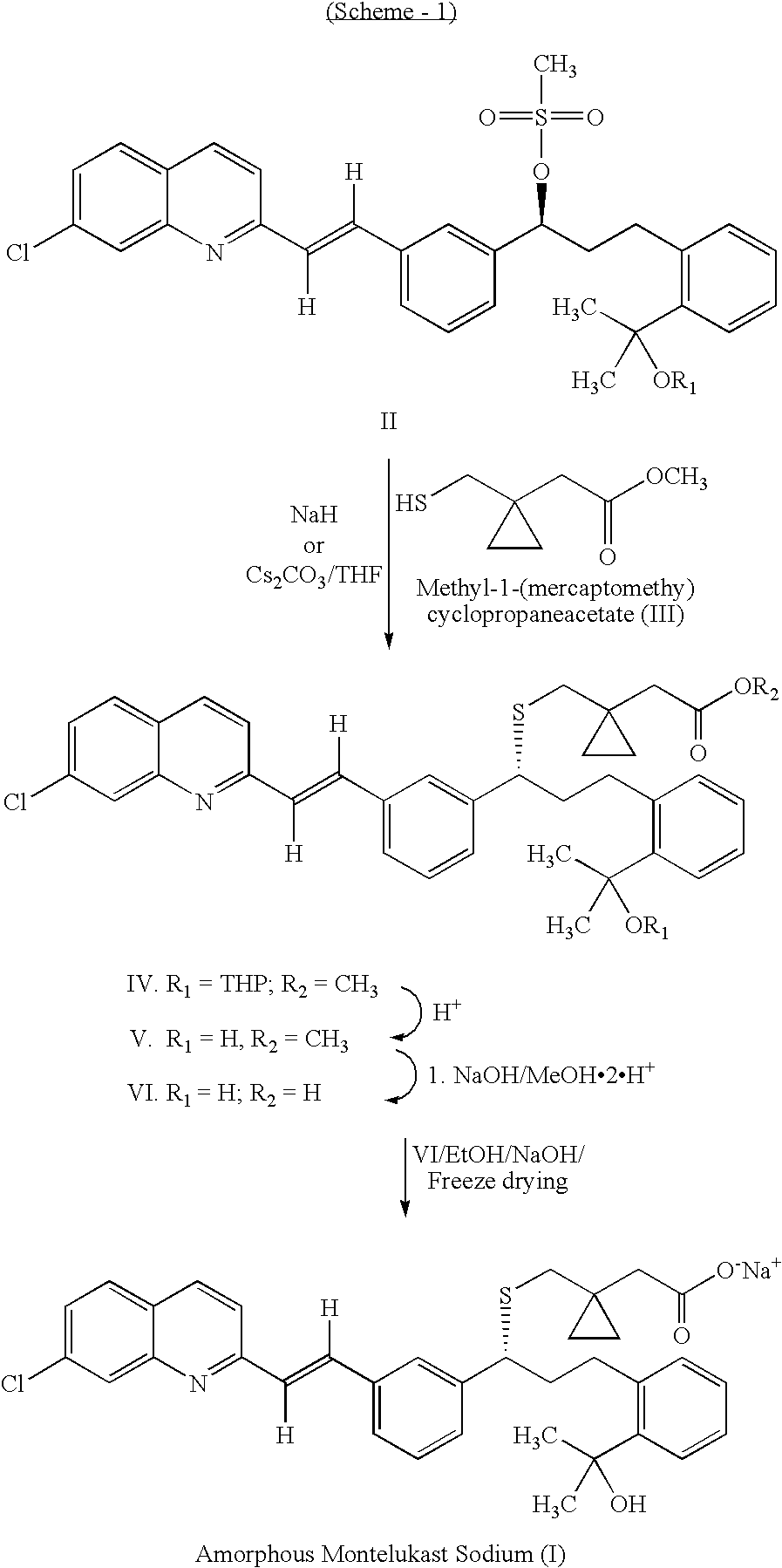
The present invention relates to a process for the preparation of a compound of formula (I) or a sodium salt thereof, wherein HET is 7-chloroquinolin-2-yl or 6,7-difluoroquinolin-2-yl, which comprises: reacting the dilithium dianion of 1-(mercapto-methyl)cyclopropaneacetic acid with a compound of formula (II), wherein HET is as defined above and L is arylsulfonyl or alkylsulfonyl. The invention further provides the dicyclohexylamine salt of a compound of formula (I), an intermediate falling within (II) and a 1-(Mercaptomethyl)cyclopropaneacetic acid intermediate.
Owner:SCHERING AG
Dilithium-initiated double-end functionalized triblock styrene-diene-styrene polymer and preparation method thereof
InactiveCN110591025AIncrease polarityPost-functionalization is easy to implementElastomerThermoplastic elastomer
The invention relates to a dilithium-initiated double-end functionalized triblock styrene-diene-styrene polymer and a preparation method thereof. The polymer comprises the following components in percentages by mass calculated under the condition that the total amount of the triblock styrene-diene-styrene polymer is 100%: 20%-40% of styrene and the balance of diene; and the microstructure of polydiolefin comprises the following components in percentages by content calculated under the condition that the total mole amount of the diene is 100%: 7%-60% of a vinyl structure and the balance of a 1,4-structure. A bifunctional initiator method is adopted in the invention, and the production process of a traditional triblock styrene-diene-styrene polymer is simplified; an alkynyl functionalized 1,1-diphenylethylene derivative or an amino functionalized 1, 1-diphenylethylene derivative are simultaneously and efficiently introduced at two ends of a polymer chain; and by the introduced amine functional group, the defect of weak polarity of a traditional thermoplastic elastomer can be overcome, the compatibility between the amine functional group and the other components is improved, and thealkyne functional group provides an efficient reaction site for post-functionalization, so that preparation of a high-performance thermoplastic elastomer material can be finally realized.
Owner:DALIAN UNIV OF TECH
Polymeric ion traps for suppressing or minimizing transition metal ions and dendrite formation or growth in lithium-ion batteries
ActiveUS20190013551A1Robust manufacturingMinimizing and suppressing dendrite formationNon-aqueous electrolyte accumulator electrodesLi-accumulatorsTrappingMalonate
Electrochemical cells that cycle lithium ions and methods for suppressing or minimizing dendrite formation are provided. The electrochemical cells include a positive electrode, a negative electrode, and a separator disposed therebetween. At least one transition metal ion-trapping moiety, including one or more polymers functionalized with one or more trapping groups, may be included within the electrochemical cell as a coating, pore filler, substitute pendant group, or binder. The one or more trapping groups may be selected from the group consisting of: crown ethers, siderophores, bactins, ortho-phenanthroline, iminodiacetic acid dilithium salt, oxalates malonates, fumarates, succinates, itaconates, phosphonates, and combinations thereof, and may bind to metal ions found within the electrochemical cell to minimize or suppress formation of dendrite protrusions on the negative electrode.
Owner:GM GLOBAL TECH OPERATIONS LLC
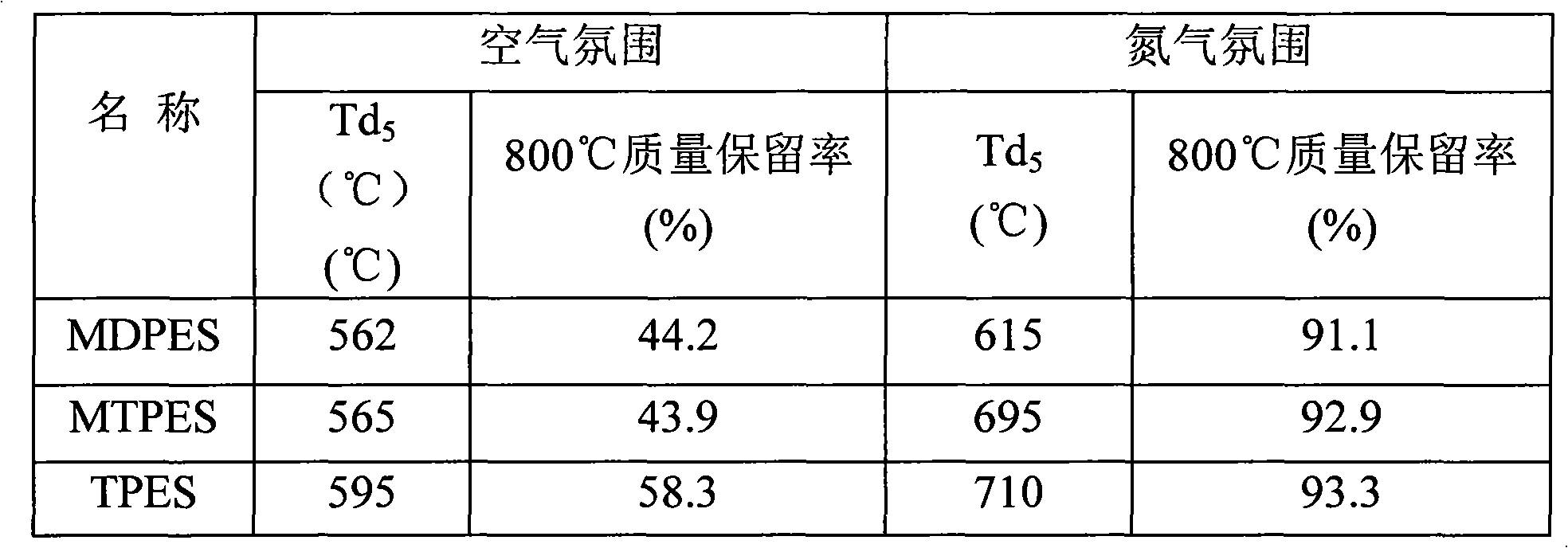
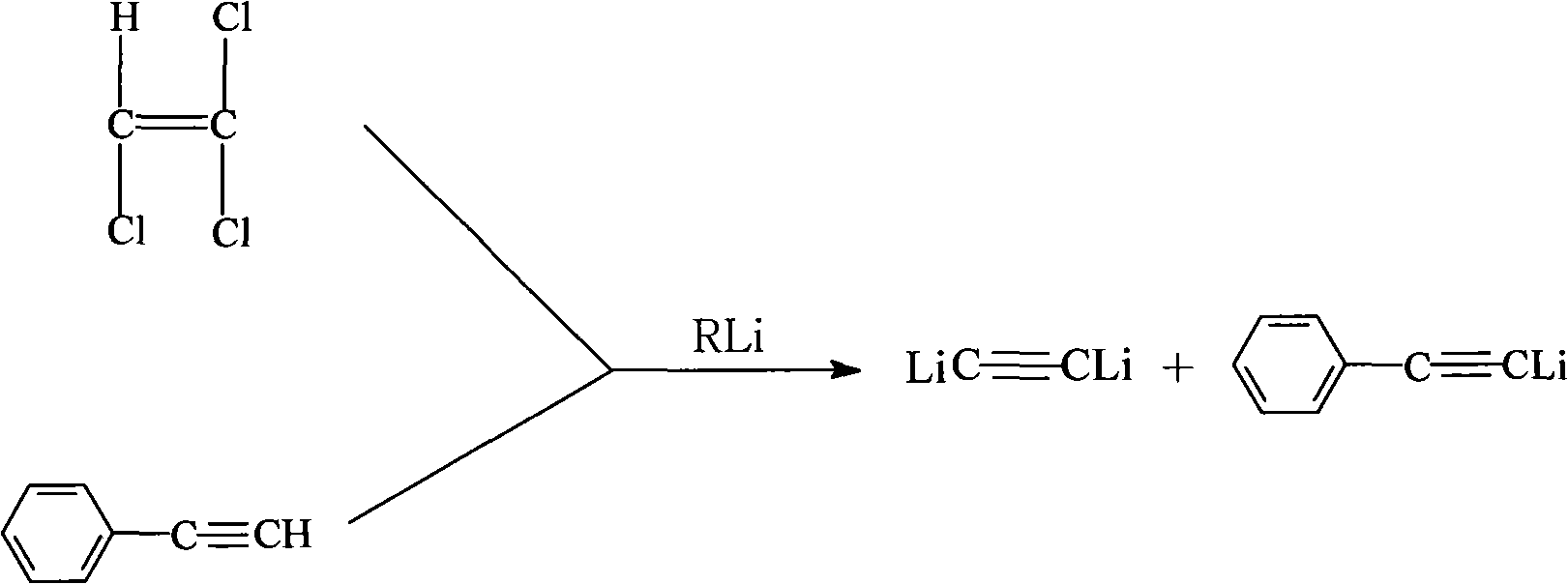
Novel high-temperature resistant phenylacetylene terminated poly (acetenyl-silane) and preparation method thereof
The invention discloses a novel phenylacetylene terminated poly (acetenyl-silane) polymer and a preparation method thereof. The polymer is prepared from dihalogenosilane, phenylacetylene, trichloroethylene and organic lithium serving as raw materials and prepared by two-step reaction under the protection of inert gas. The method comprises the following steps of: 1, under the protection of the inert gas, making a mixture of the trichloroethylene and the phenylacetylene react with the organic lithium to obtain acetenyl dilithium and phenylacetylene lithium; and 2, under the protection of the inert gas, performing coupling reaction on the mixture of the acetenyl dilithium and the phenylacetylene lithium generated in the first step and the dihalogenosilane to obtain a final target product. The raw materials used in the polymer are easily obtained, the process flow is simple, and the operation process is feasible. The polymer prepared by the method is subjected to cross-linking reaction under heat or chemical initiation to form a thermosetting material with high-temperature resistance and excellent thermal oxidation resistance, and the thermosetting material is further heated to form a ceramic structure. The polymer prepared by the method can be used as a matrix resin, a high-temperature resistant coating and a ceramic precursor of an advanced composite material.
Owner:EAST CHINA UNIV OF SCI & TECH
Method for preparing battery grade lithium dihydrogen phosphate with high-purity lithium carbonate lithium depositing mother solution
The invention relates to a method for preparing battery grade lithium dihydrogen phosphate with a high-purity lithium carbonate lithium depositing mother solution. The method comprises the steps of: conducting preliminary lithium extraction and deep lithium extraction to a lithium carbonate lithium depositing mother solution with phosphoric acid and phosphate so as to obtain a mixture of lithium phosphate and dilithium hydrogen phosphate, reacting the mixture with phosphoric acid to generate a lithium dihydrogen phosphate solution, then carrying out concentration and evaporation, cooling and crystallization, centrifugation, saturation washing, drying, air-stream crushing and packaging, thus obtaining the battery grade lithium dihydrogen phosphate. The method of preparing battery grade lithium dihydrogen phosphate in the invention fully makes use of the mother solution generated during high-purity lithium carbonate production, and has the advantages of simple process, easy operation, low production cost, over 90% of lithium recovery rate, and stable quality of the obtained battery grade lithium dihydrogen phosphate product, and is also suitable for preparing the lithium ion battery positive material lithium iron phosphate. Therefore, the method provided in the invention boasts broad market prospects, as well as good economic and social benefits.
Owner:GANFENG LITHIUM CO LTD
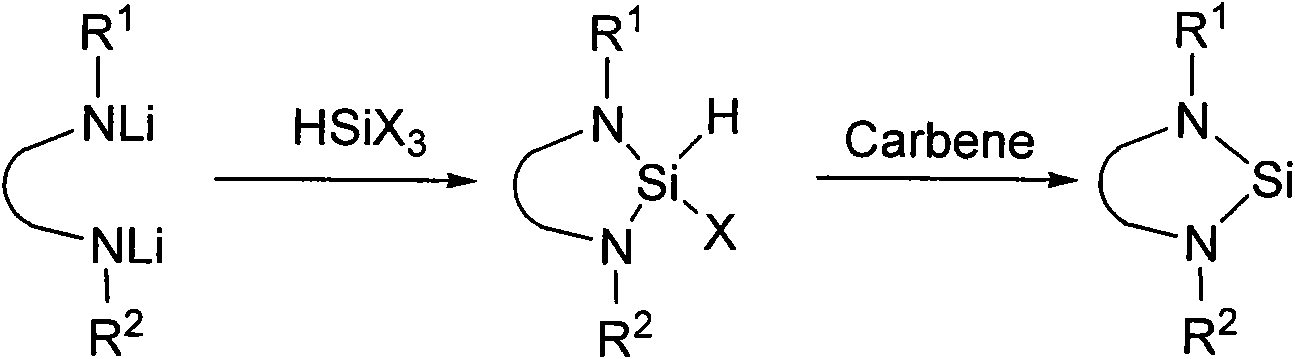
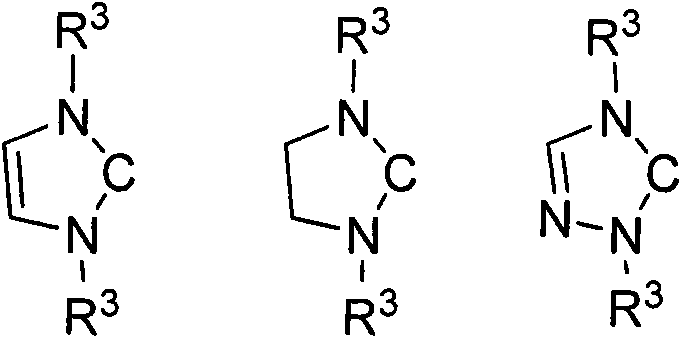
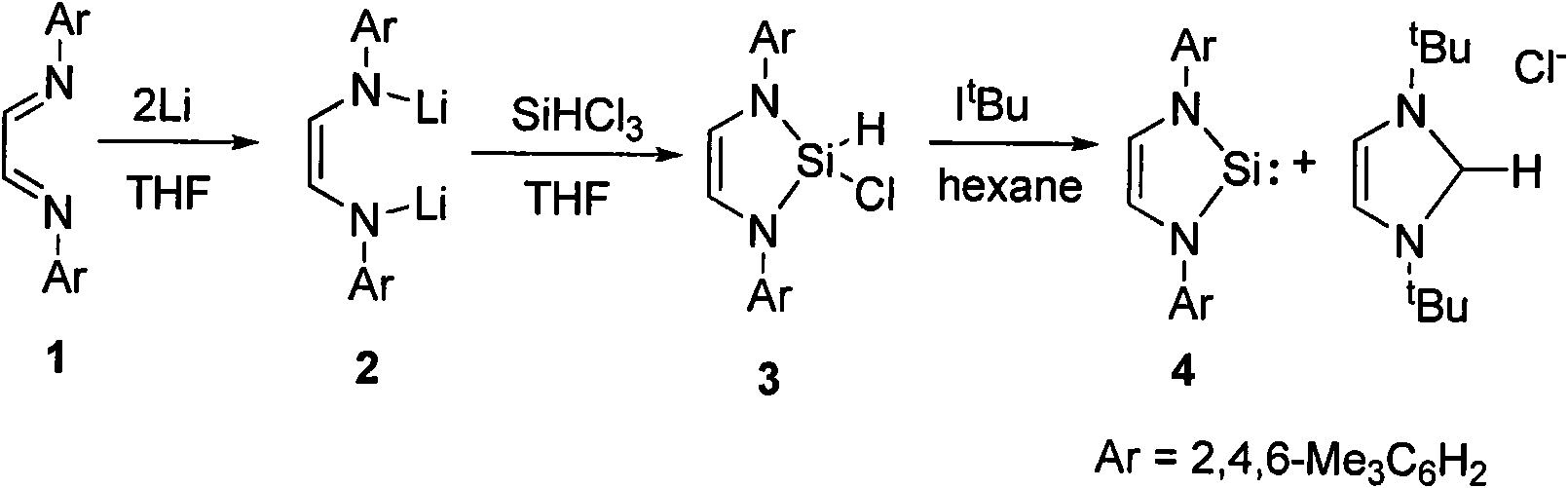
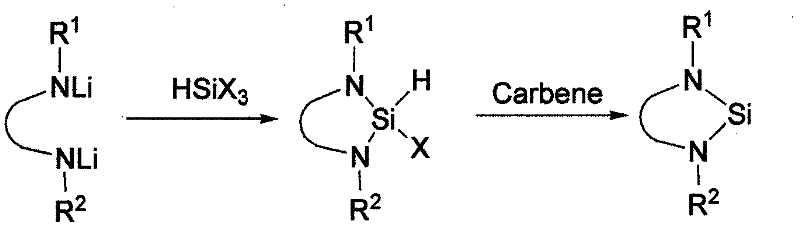
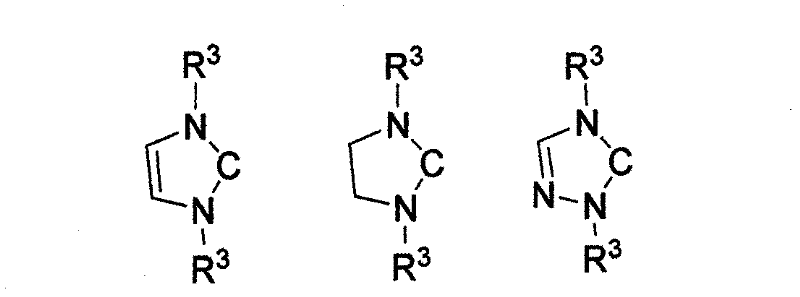
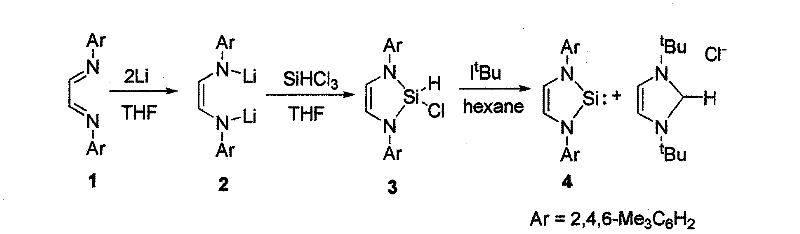
Method for preparing silylene by carbene-induced halogenated silane dehydrohalogenation
The invention discloses a method for preparing silylene by carbene-induced halogenated silane dehydrohalogenation, and relates to a method for synthesizing silylene. Dilithium salt of [NN] bidentate ligand and halogenosilane or substituted halogenosilane organic silicon reagent react to form substituted halogenated silane; and the molar ratio of the substituted halogenated silane to stable carbene is 1:0.5-10, the reaction temperature is between 78 DEG C below zero and 100 DEG C, the substituted halogenated silane and the stable carbene are mixed in a common organic solvent to generate imidazole salt of silylene and carbene, the generated imidazole salt is separated from the solution of a product through filtration after the reaction is finished, and the silylene or derivatives of the silylene are obtained through recrystallization and chromatographic separation. Through the reaction of different organic halogenosilane hydrides and the carbene, the method discovers that the carbene can effectively reduce a silicon compound under the mild condition to generate bivalent silicon intermediate. The imidazole salt as the reaction product can be converted into the carbene more easily so as to realize recycle.
Owner:NANKAI UNIV
Phenylo boric acid-silane-ethynyl polymer and preparation method thereof
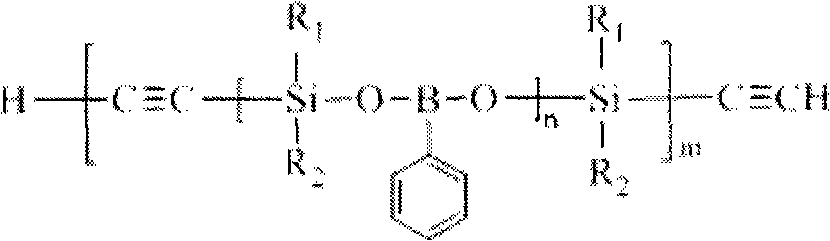
The invention discloses phenylo boric acid-silane-ethynyl polymer and a preparation method thereof. The polymer is prepared by taking dihalo-silane, phenylo boric acid, trichloroethylene and organic lithium as raw materials in three-step reaction under the protection of inert gas. In the first step, the phenylo boric acid and the dihalo-silane react to form a polymer with a silane-phenylo boric acid-silane repetitive structure and halogen-sealed end. In the second step, the trichloroethylene and the organic lithium react to form an ethynyl dilithium. Finally, the ethynyl dilithium and the polymer formed in the first step generate coupling reaction to obtain the final product after hydrolyzation. The used raw materials are easy to obtain, the process flow is simple, and the operation process is feasible. The prepared polymer generates crosslinking reaction to form thermosets with good high temperature resistance and thermal oxidation resistance property under the thermal or chemical initiation, and is further heated to form a ceramic structure. The prepared polymer can be used as matrix resin, high temperature-resistance coating and ceramic precursor of the advanced composite material.
Owner:EAST CHINA UNIV OF SCI & TECH
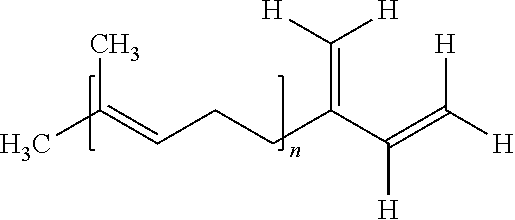
Dilithium initiators
Disclosed herein are highly active dilithio initiators prepared from high molecular weight dienes (C≧6) and methods for the preparation of such compounds. These dilithio initiators result in greater control over polymer microstructure and provide useful polymers and oligomers with low vinyl incorporation.
Owner:FINA TECH
Method for the preparation of montelukast acid and sodium salt thereof in amorphous form
ActiveUS20070082925A1Eliminate the problemTime and cost-effectiveBiocideOrganic chemistryAcetic acidMontelukast
A method for the preparation of montelukast acid sodium salt thereof in amorphous form has been described. The method comprises of following steps: (a) generating the dilithium dianion of 1-(mercaptomethyl)cyclopropane acetic acid, by reacting with alkyl lithium, (b) coupling the said dianion with wet mesylate to get montelukast acid in crude form, (c) obtaining DCHA salt in crude form by adding dicyclohexylamine (DCHA) to crude acid obtained in the above step (b), (d) purifying and converting the said DCHA salt in crude form, to montelukast acid in pure form, and (e) reacting the pure montelukast acid in a polar protic solvent with a source of sodium ion followed by evaporating the solvent and triturating of the residue with non-polar water immiscible solvent.
Owner:MOREPEN LAB LTD
Method for synthesizing zirconium carbide composite ceramic precursor thermoset resin
InactiveCN105778059AGood processing technologySuitable for forming processComposite ceramicTransfer molding
The invention aims to disclose a preparing method for zirconium-containing thermoset ceramic precursor polyzirconocene ethynylene resin.The method includes the steps of preparing acetenyl dilithium salt, preparing phenyl dilithium salt, mixing the two solutions and filtering out the salt.Requirements of a liquid phase impregnation technology (such as a resin transfer molding technology, a high-pressure impregnation technology and an autoclave technology) can be met, and the polyzirconocene ethynylene resin is suitable for serving as matrix of a high-performance ceramic matrix composite material.
Owner:TAISHAN MEDICAL UNIV
Li2Na4V10O28 and preparation method and use thereof
InactiveCN101456586ANot easy to structural deformationStable charge and discharge efficiencyCell electrodesVanadium compoundsLithium hydroxideFiltration
The invention discloses dilithium tetrasodium vanadate and a preparation method and application thereof. The chemical formula of the dilithium tetrasodium vanadate is Li2Na4V10O28. The preparation method for the compound comprises: firstly, dissolving ammonium metavanadate, lithium hydroxide and sodium hydroxide into a water solvent respectively according to the mass ratio of fed substances of 1:0.2-0.4:0.4-0.8, using nitric acid to adjust the pH value of the solution to be approximately between 3 and 5 after mixing, transferring the solution after full mixing into a reaction kettle, sealing the reaction kettle, heating the solution to be between 110 and 200 DEG C and maintaining at the temperature for 8 to 13 hours, and making the solution undergo filtration and standing to obtain precursor crystals; secondly, mechanically grinding and uniformly crushing the precursor crystals, calcining the precursor crystals for 6 to 9 hours at a temperature of between 200 and 270 DEG C under the atmosphere of inert gas, and obtaining the dilithium tetrasodium vanadate. The dilithium tetrasodium vanadate has novel compositions and a novel structure, and is a brand-new lithium ion battery anode active material.
Owner:ZHEJIANG UNIV OF TECH +1
Method for growing large-size lithium lead phosphate single crystals through hydrothermal method
InactiveCN107475768AIncrease profitSmall sizePolycrystalline material growthFrom normal temperature solutionsHydrogen phosphateLead phosphate
The invention discloses a method for growing large-size lithium lead phosphate single crystals through a hydrothermal method. The method includes the steps that a lead source and lithium dihydrogen phosphate are put into an autoclave as hydrothermal reactants, a lithium dihydrogen phosphate solution and / or a dilithium hydrogen phosphate solution with the lithium ion concentration of 1-5 mol / L serve / serves as a mineralizer, and a temperature-difference hydrothermal method is adopted to make the hydrothermal reactants subjected to a combination reaction to obtain the lithium lead phosphate single crystals after growth. According to the method, the lead source and lithium dihydrogen phosphate are directly subjected to the combination reaction in the autoclave under the temperature-difference hydrothermal condition to obtain the lithium lead phosphate single crystals after growth, and the basic reaction materials do not need to be pressed or sintered, so that the process is simpler; besides, the lithium dihydrogen phosphate solution and / or the dilithium hydrogen phosphate solution are / is adopted as the mineralizer, other impurities will not be introduced, the material utilization rate is high, the concentration compatibility of the mineralizer is high, and the large-size lithium lead phosphate single crystals can be obtained.
Owner:桂林百锐光电技术有限公司 +1
Method and system for aromatic macrocyclic compounds (phthalocyanines) as cathode additives for inhibition of transition metal dissolution and stable solid electrolyte interphase formation
ActiveUS11456457B2Negative electrodesPositive electrodesAluminum phthalocyanine chlorideCobalt phthalocyanine
Owner:ENEVATE CORP
Dilithium initiators
Owner:FINA TECH INC
Olefin copolymer and preparation method and application thereof
ActiveCN106256843AReduce rolling resistanceAvoid low coupling efficiencyRolling resistancePolymer science
The invention relates to the field of polymers, and particularly discloses an olefin copolymer and a preparation method and application thereof. The preparation method of the olefin copolymer comprises the steps that 1, in an inert gas environment, a tin-containing organic dilithium initiator, a conjugated diene monomer and a mono vinyl arene monomer are subjected to a first polymerization reaction in an inert solvent; 2, when the total monomer conversion rate of the conjugated diene monomer and the mono vinyl arene monomer reaches 70%-85%, a first polymerization reaction product and an isoprene monomer are subjected to a second polymerization reaction. According to preparation method, a tin-containing organic dilithium compound is used as the initiator, preparation is conducted in the mode of adding the raw materials step by step, and rubber prepared through the method has low rolling resistance and has a wide application prospect in the tire industry.
Owner:CHINA PETROLEUM & CHEM CORP +1
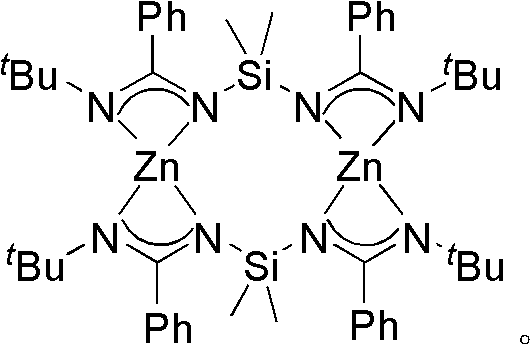
Bridged diamidine-based zinc catalyst and preparation method and application thereof
The invention provides a bridged diamidine-based zinc catalyst, which relates to a lactide ring opening polymerization catalyst, in particular to a catalyst of a diamidine-based ligand zinc composition having the N-C-N-Si-N-C-N characteristic and taking zinc as a central atom. A preparation method of the catalyst comprises the following steps of: transforming butyl lithium into a dilithium salt by taking bridged diamine as an initial raw material under the protection of nitrogen gas; adding cyanophenyl for undergoing an addition reaction; transferring a silicon base twice to form a bridged diamidine-based ligand; and reacting the ligand with zinc dichloride to obtain the catalyst. The synthesis method has the advantages of general applicability, mild reaction condition, simple and readily-available materials, low cost, simple steps and high yield. A compound has high catalytic activity on a ring opening polymerization reaction of lactide and good application prospect.
Owner:SHANXI UNIV
Method for preparing silylene by carbene-induced halogenated silane dehydrohalogenation
The invention discloses a method for preparing silylene by carbene-induced halogenated silane dehydrohalogenation, and relates to a method for synthesizing silylene. Dilithium salt of [NN] bidentate ligand and halogenosilane or substituted halogenosilane organic silicon reagent react to form substituted halogenated silane; and the molar ratio of the substituted halogenated silane to stable carbene is 1:0.5-10, the reaction temperature is between 78 DEG C below zero and 100 DEG C, the substituted halogenated silane and the stable carbene are mixed in a common organic solvent to generate imidazole salt of silylene and carbene, the generated imidazole salt is separated from the solution of a product through filtration after the reaction is finished, and the silylene or derivatives of the silylene are obtained through recrystallization and chromatographic separation. Through the reaction of different organic halogenosilane hydrides and the carbene, the method discovers that the carbene can effectively reduce a silicon compound under the mild condition to generate bivalent silicon intermediate. The imidazole salt as the reaction product can be converted into the carbene more easily so as to realize recycle.
Owner:NANKAI UNIV
Alkylphosphine oligomer and its synthesis method
ActiveCN104610352BThe synthesis process is simple and continuousShort reaction timeGroup 5/15 element organic compoundsSynthesis methodsReaction temperature
The present invention discloses an alkylphosphine oligomer and a synthesis method thereof, wherein the structure general formula of the alkylphosphine oligomer is represented by the figure. The synthesis method comprises: adding a solvent and a stabilizer to p-dibromobiphenyl, adding a dehalogenating agent to a reactor under a cooling condition, increasing the reaction temperature to produce a dilithium salt, cooling the reaction system, adding alkyl phosphine dichloride in a dropwise manner, increasing the reaction temperature, adding a reagent, carrying out a quenching reaction, completing the reaction, and carrying out solvent removing, alcohol washing, water washing and drying to obtain the alkylphosphine oligomer. The product of the present invention has advantages of strong char formation ability and good stability. The synthesis method has advantages of continuous reaction process, short reaction time, simple synthesis process, and efficient reaction.
Owner:CHINA PETROLEUM & CHEM CORP +1
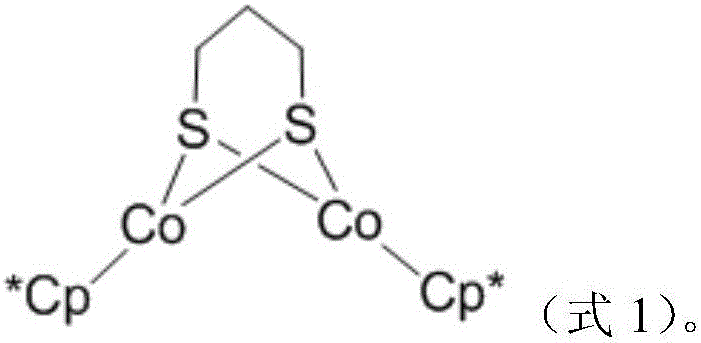
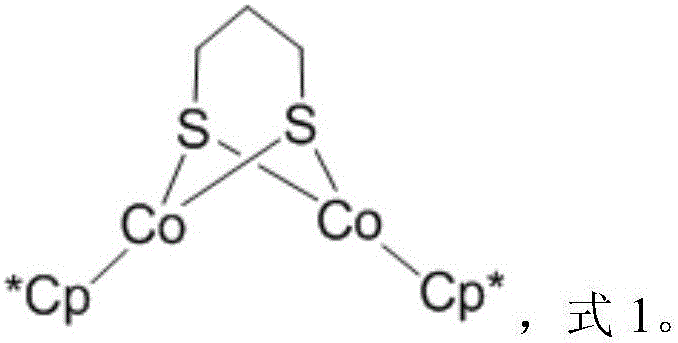
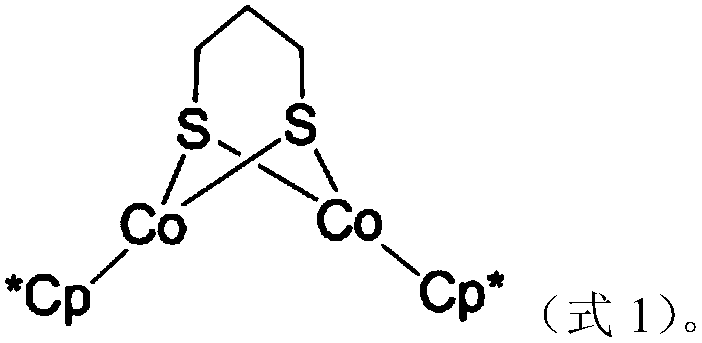
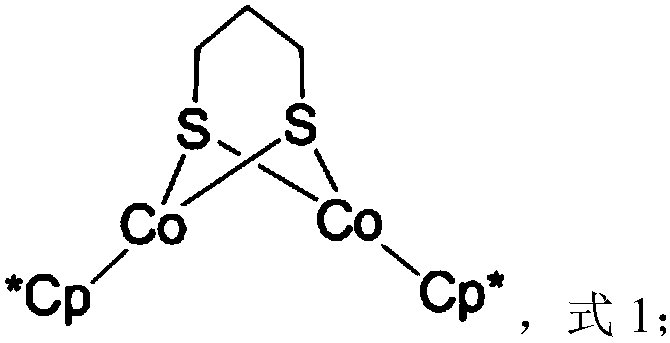
Dinuclear cobalt compound and application thereof in underground catalytic reforming viscosity reduction of heavy oil
The invention provides a dinuclear cobalt compound and application thereof in underground catalytic reforming viscosity reduction of heavy oil. Specifically, the dinuclear cobalt compound is prepared by the method of: firstly reacting Cp*Li with anhydrous cobalt chloride, then reacting the product with 1, 3 propanedithiol dilithium salt, and then conducting aftertreatment to obtain the dinuclear cobalt compound. The invention also provides application of the dinuclear cobalt compound as a catalyst in underground catalytic reforming viscosity reduction of heavy oil, wherein the catalytic reforming viscosity reduction utilizes catalytic cracking reaction or hydrogen donor catalytic cracking reaction. Testing proves that the dinuclear cobalt compound provided by the invention has a good effect in underground catalytic reforming viscosity reduction of heavy oil.
Owner:PETROCHINA CO LTD
Bridged diamidine-based zinc catalyst and preparation method and application thereof
The invention provides a bridged diamidine-based zinc catalyst, which relates to a lactide ring opening polymerization catalyst, in particular to a catalyst of a diamidine-based ligand zinc composition having the N-C-N-Si-N-C-N characteristic and taking zinc as a central atom. A preparation method of the catalyst comprises the following steps of: transforming butyl lithium into a dilithium salt by taking bridged diamine as an initial raw material under the protection of nitrogen gas; adding cyanophenyl for undergoing an addition reaction; transferring a silicon base twice to form a bridged diamidine-based ligand; and reacting the ligand with zinc dichloride to obtain the catalyst. The synthesis method has the advantages of general applicability, mild reaction condition, simple and readily-available materials, low cost, simple steps and high yield. A compound has high catalytic activity on a ring opening polymerization reaction of lactide and good application prospect.
Owner:SHANXI UNIV
Ultraviolet light absorber, and preparation method and composition thereof
ActiveCN112225881AGood effectExcellent intramolecular electronsPolyurea/polyurethane coatingsRadiation-absorbing paintsElectron delocalizationUltraviolet lights
The invention discloses an ultraviolet light absorber, and a preparation method and a composition thereof, which belong to the field of ultraviolet light absorption. The preparation method comprises the following steps of reacting a 1, 4-dilithium-based butadiyne solution with aromatic ether to obtain a butadiyne-based polyaromatic hydrocarbon alkyl compound. According to the butadiyne-based polyaromatic hydrocarbon alkyl compound, conjugated polyacetylene molecules are used as a main chain, and a conjugated polyacetylene structure has a one-dimensional carbon chain structure formed by singlebonds and triple bonds alternately, so that cylindrical electron delocalization is formed, the butadiyne-based polyaromatic hydrocarbon alkyl compound has excellent intramolecular electron and chargetransport properties, and the ultraviolet absorption wavelength of the compound can be subjected to red shift; oxygen atoms rich in electrons are introduced to the ortho-position of a benzene ring andstabilized through alkyl or alkylene or aromatic hydrocarbon, the optimal reaction condition is explored, and the ultraviolet light absorber with the stable effect can be obtained.
Owner:JINLING INST OF TECH
A binuclear cobalt compound and its application in heavy oil underground catalytic upgrading and viscosity reduction
The invention provides a dinuclear cobalt compound and application thereof in underground catalytic reforming viscosity reduction of heavy oil. Specifically, the dinuclear cobalt compound is prepared by the method of: firstly reacting Cp*Li with anhydrous cobalt chloride, then reacting the product with 1, 3 propanedithiol dilithium salt, and then conducting aftertreatment to obtain the dinuclear cobalt compound. The invention also provides application of the dinuclear cobalt compound as a catalyst in underground catalytic reforming viscosity reduction of heavy oil, wherein the catalytic reforming viscosity reduction utilizes catalytic cracking reaction or hydrogen donor catalytic cracking reaction. Testing proves that the dinuclear cobalt compound provided by the invention has a good effect in underground catalytic reforming viscosity reduction of heavy oil.
Owner:PETROCHINA CO LTD
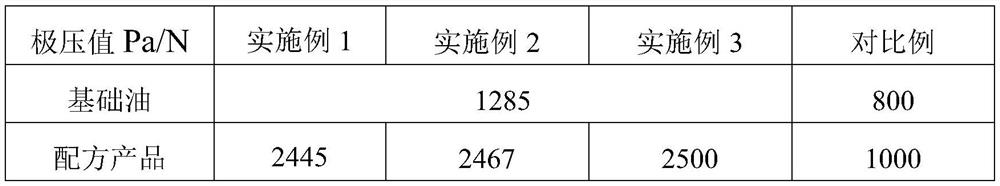
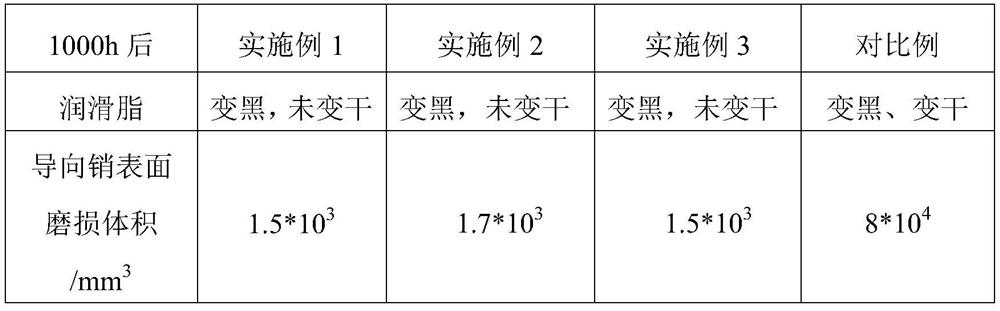
Special lubricating grease for caliper guide pin
ActiveCN114276854AStrong resistance to extreme pressureIncrease the extreme pressure valueLubricant compositionChemical reactionAntioxidant
The invention discloses special lubricating grease for a caliper guide pin, which is prepared from the following components in parts by mass: 80 to 90 parts of base oil, 0.1 to 0.5 part of nanoscale sulfide additive, 1.65 to 3 parts of extreme pressure agent, 1 to 2 parts of corrosion inhibitor, 5.5 to 7 parts of thickening agent, 0.1 to 0.5 part of peptizing agent and 0.1 to 0.5 part of antioxidant, the nanoscale sulfide additive is one or more of copper sulfide nanoparticles, nickel sulfide nanoparticles and zinc sulfide nanoparticles, and the thickening agent is formed by compounding lithium 12-hydroxystearate, dilithium azelate and palmitic acid. The nano-scale sulfide additive is added in the formula, nano-particles are large in surface area and active in chemical property, the nano-particles and the abraded surface are prone to chemical reaction, a sediment film and a chemical reaction film are formed, and the fretting wear of the caliper guide pin can be obviously improved.
Owner:DONGFENG MOTOR GRP
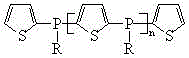
A kind of alkyl phosphine oligomer and its synthesis method
ActiveCN104610358BThe synthesis process is simple and continuousShort reaction timeGroup 5/15 element organic compoundsSynthesis methodsReaction temperature
The present invention discloses an alkylphosphine oligomer and a synthesis method thereof, wherein the structure general formula of the alkylphosphine oligomer is represented by the figure. The synthesis method comprises: adding a solvent and a stabilizer to thiophene, adding a dehydrogenation agent to a reactor under a cooling condition, increasing the reaction temperature to produce a dilithium salt, cooling the reaction system, adding alkyl phosphine dichloride in a dropwise manner, increasing the reaction temperature, adding a reagent, carrying out a quenching reaction, completing the reaction, and carrying out solvent removing, alcohol washing, water washing and drying to obtain the alkylphosphine oligomer. The product of the present invention has advantages of strong char formation ability and good stability. The synthesis method has advantages of continuous reaction process, short reaction time, simple synthesis process, and efficient reaction.
Owner:SINOPEC DALIAN RES INST OF PETROLEUM & PETROCHEMICALS CO LTD +1
Anode of lithium ion cell and lithium ion cell
ActiveCN100416893CReduce contentInhibition of dissolutionElectrode carriers/collectorsActive material electrodesHydrogen phosphateManganese
A positive electrode of Li ion battery features that it contains Li salt chosen from lithium phosphate, dilithium hydrogen phosphate, lithium sulfate, lithium molybdate, lithium titanate, etc. A Li ion battery with high high-temp cycle performance and storage nature is composed of said positive electrode, diaphragm, negative electrode and non-water electrolyte.
Owner:BYD CO LTD
Preparation method and application of ferrocenyl silicone ceramic
InactiveCN110342937AHas a hyperbranched topologyImprove solubilityOrganic-compounds/hydrides/coordination-complexes catalystsSolubilityButyl lithium
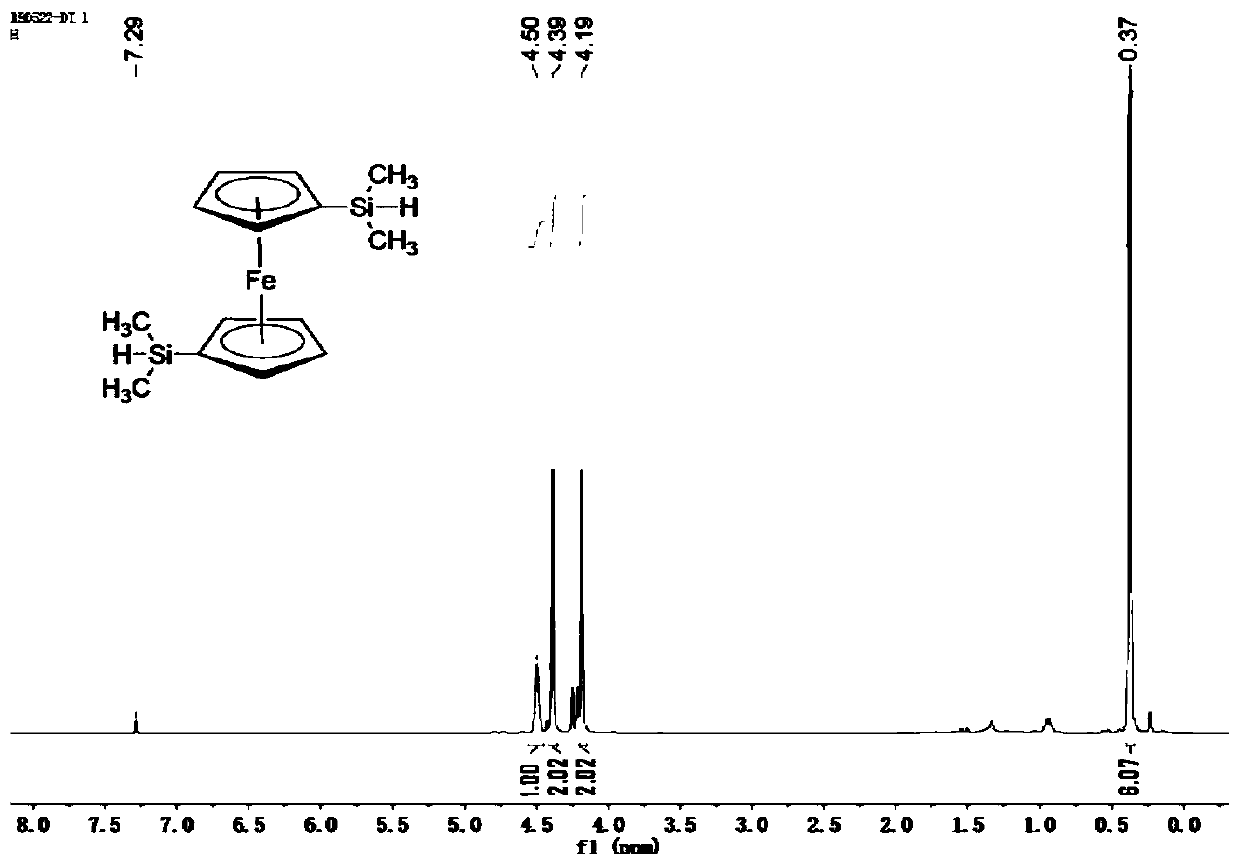
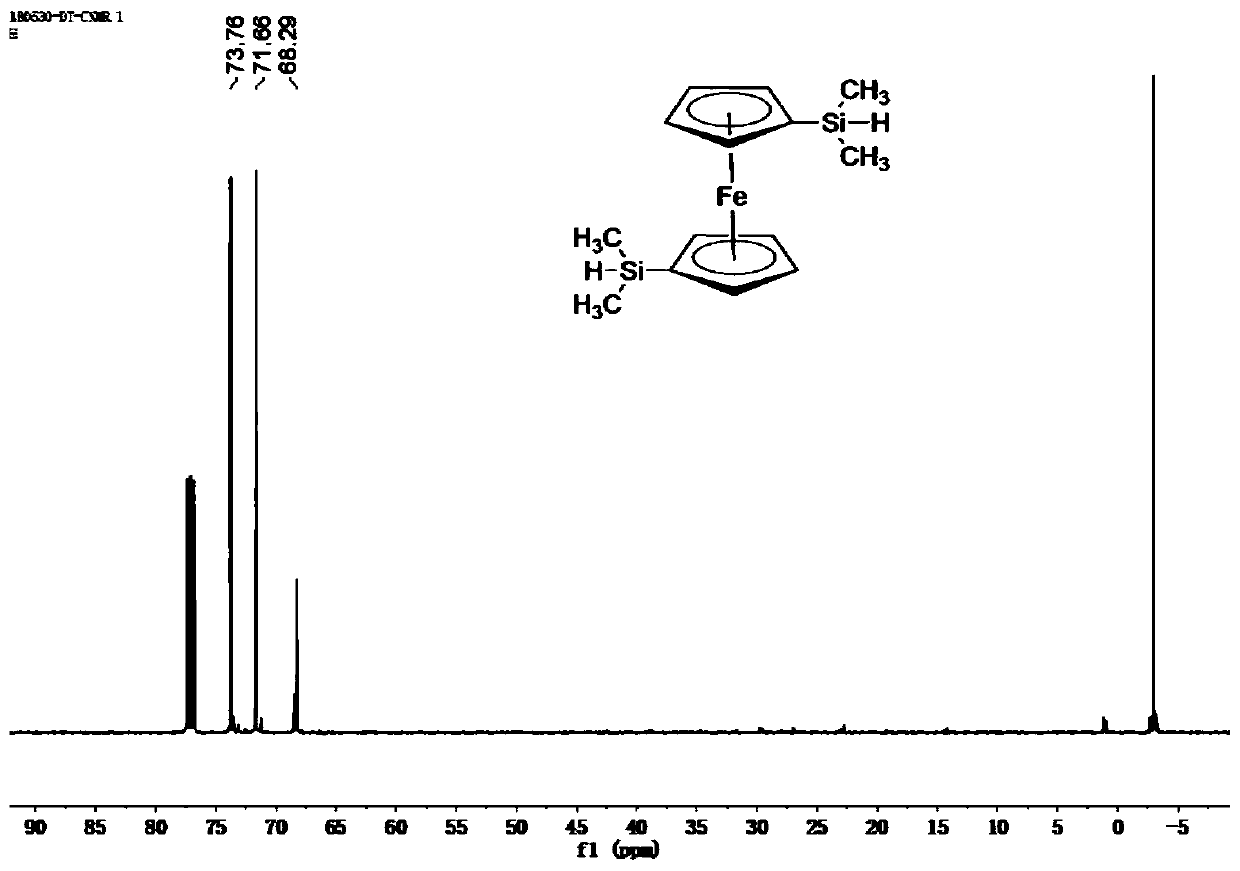
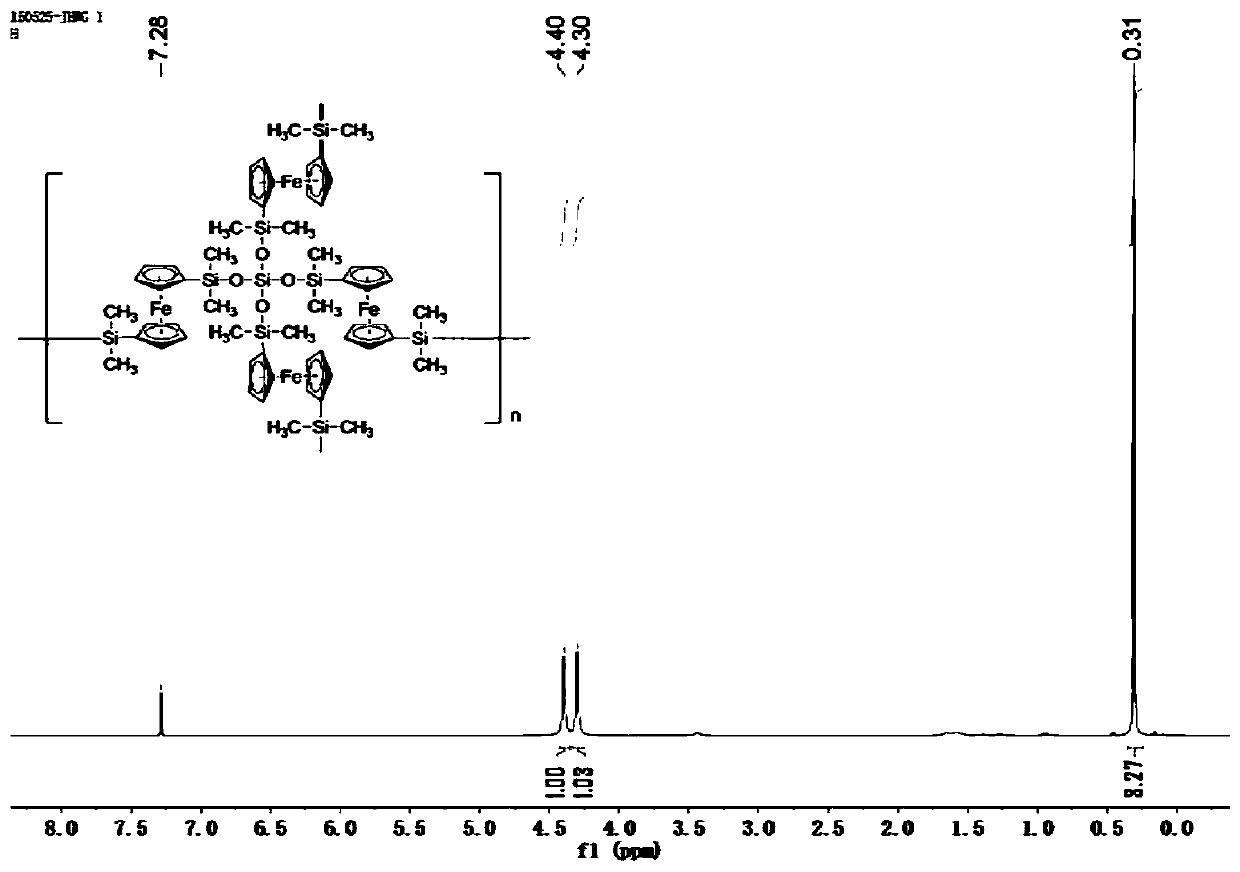
The invention belongs to the field of ceramic materials, and particularly relates to a preparation method and application of a ferrocenyl silicone ceramic. The preparation method comprises the steps:synthesizing a high-activity ferrocenyl dilithium salt from n-butyl lithium and ferrocene through the lithiation property of n-butyl lithium at a low temperature, performing a reaction further betweenthe ferrocenyl dilithium salt and dimethyl monochlorosilane so as to form monomer ferrocenyldimethylsilane, then performing a Piers-Rubinsztajn reaction for polymerization with tetraethyl orthosilicate so as to obtain a silicone polymer precursor, and then performing high temperature sintering so as to obtain the ferrocenyl silicone ceramic (Fe-Si-C). The prepared ferrocenyl silicone ceramic (Fe-Si-C) precursor has a hyperbranched topological structure, good solubility and high ceramic yield; and due to introduction of transition metal Fe, the ceramic not only has certain electromagnetic properties, but also has good catalytic properties, and can be applied to electromagnetic materials and catalytic materials.
Owner:UNIV OF JINAN
Alkylphosphine oligomer and synthesis method thereof
ActiveCN104610358AImprove flame retardant performancePromote charcoalGroup 5/15 element organic compoundsSynthesis methodsReaction temperature
The present invention discloses an alkylphosphine oligomer and a synthesis method thereof, wherein the structure general formula of the alkylphosphine oligomer is represented by the figure. The synthesis method comprises: adding a solvent and a stabilizer to thiophene, adding a dehydrogenation agent to a reactor under a cooling condition, increasing the reaction temperature to produce a dilithium salt, cooling the reaction system, adding alkyl phosphine dichloride in a dropwise manner, increasing the reaction temperature, adding a reagent, carrying out a quenching reaction, completing the reaction, and carrying out solvent removing, alcohol washing, water washing and drying to obtain the alkylphosphine oligomer. The product of the present invention has advantages of strong char formation ability and good stability. The synthesis method has advantages of continuous reaction process, short reaction time, simple synthesis process, and efficient reaction.
Owner:SINOPEC DALIAN RES INST OF PETROLEUM & PETROCHEM +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com