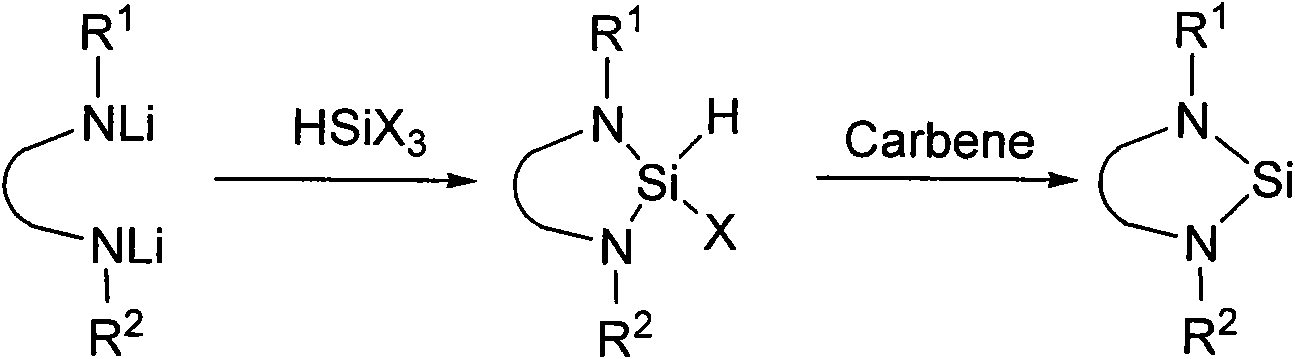
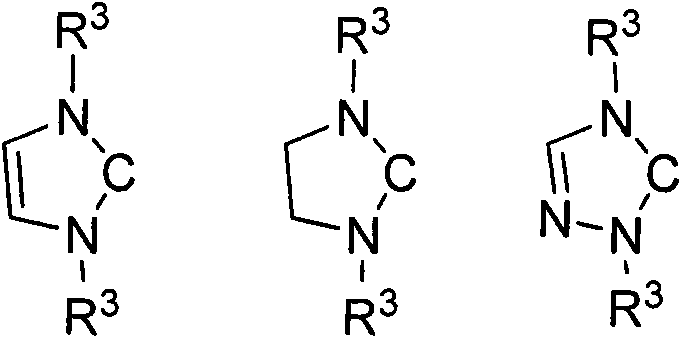
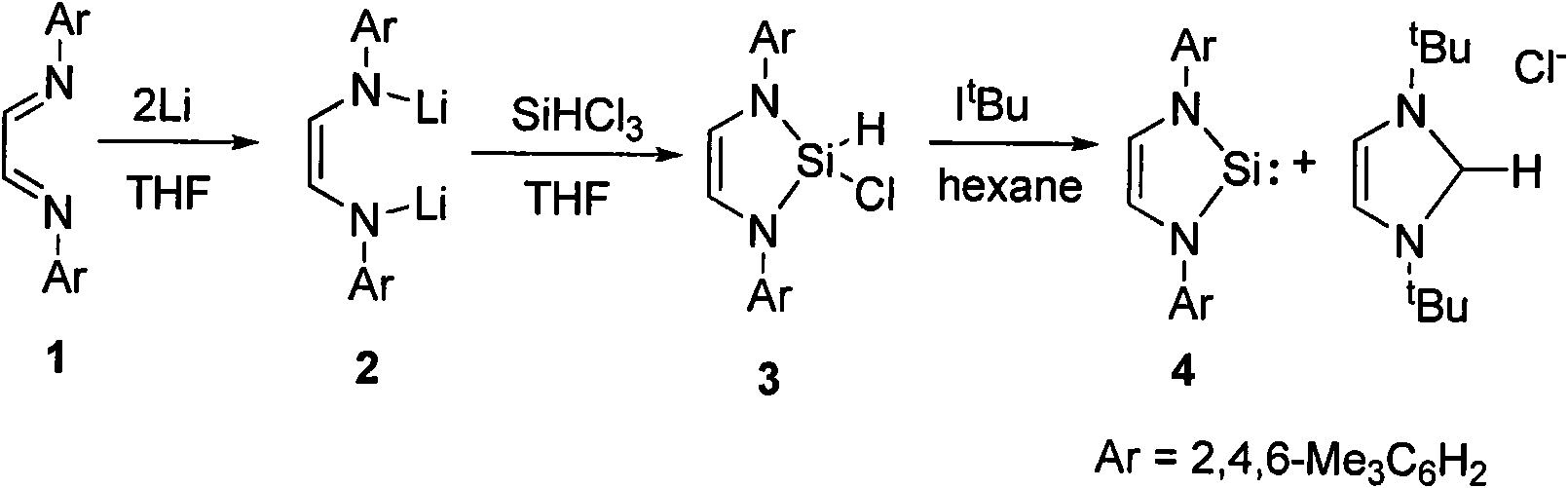
Method for preparing silylene by carbene-induced halogenated silane dehydrohalogenation
A technology of halosilane and dehydrohalogenation, applied in chemical instruments and methods, compounds of Group 4/14 elements of the periodic table, organic chemistry, etc., can solve problems such as danger, complex products, and many reaction by-products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Embodiment 1: the synthesis of compound 3:
[0029] Compound 1 (2.92g, 10mmol) was dissolved in 30mL of tetrahydrofuran, and 0.175g (25mmol) of lithium metal was added under the protection of argon. The reaction was stirred for two days and turned orange red. The unreacted lithium was filtered off, and the filtrate was cooled to -78 ℃, slowly added 10 mmol trichlorosilane dropwise with a syringe, warmed to room temperature and stirred for 12 hours, sucked dry, extracted with 50 ml n-hexane, concentrated the filtrate to obtain white crystal 3 (2.49 g, 70%). m.p.125-127℃. 1 HNMR (CDCl 3 ): δ=2.35(s, 6H, p-CH 3 ); 2.46(s, 12H, o-CH3); 5.78(s, 2H, olef.H); 6.41(s, 2H, Si-H); 6.99(s, 4H, Aryl-H). 13 C(CDCl 3 ): δ=18.65 (p-CH 3 ); 20.86 (o-CH3); 117.92 (HC=CH); 128.87, 129.41, 135.97, 136.71. (Aromaten-C).Elemental analysis (%) calcd for C 20 H2 4 N 2 Si: C, 67.29; H, 7.06; N, 7.85. Found: C, 66.93; H, 7.35; N, 7.42.
Embodiment 2
[0030] Embodiment 2: the synthesis of compound 4:
[0031] Dissolve 1.78g (5mmol) of compound 3 in 20mL of n-hexane, add I t Bu (0.9g, 5mmol) in n-hexane solution (20mL), stirred at room temperature for 10 hours, filtered, and the filtrate was concentrated and placed in a -40°C refrigerator to obtain yellow crystals 4 (1, 30g, 81.2%).m.p.110-112 ℃. 1 H NMR (C 6 D. 6 ): δ=2.17(s, 6H, p-CH 3 ); 2.25(s, 12H, o-CH3); 6.29(s, 2H, olef.H); 6.83(s, 4H, Aryl-H). 13 C{ 1 H}NMR (C 6 D. 6 ): δ=18.49 (p-CH 3 ); 20.96 (o-CH3); 124.41 (HC=CH); 129.30, 134.83, 135.78, 140.25 (Aromaten-C). 29 Si { 1 H}NMR (C 6 D. 6 ): 77.8. Elemental analysis (%) calcd for C 20 h 24 N 2 Si: C, 74.95; H, 7.55; N, 8.74. Found: C, 74.92; H, 7.41; N, 8.68.
Embodiment 3
[0032] Embodiment 3: the preparation of compound 6:
[0033] Lithium salt 5 (2.26g, 5.0mmol) was dissolved in 60mL of n-hexane, cooled to -78°C, and 1.1 equivalent of SiHCl was added dropwise to the solution 3 (0.75 g, 5.5 mmol). The solution gradually rose to room temperature, and white lithium chloride was produced. The solution was continuously stirred at room temperature for 12 hours, and then the lithium chloride was filtered off. The filtrate was concentrated in vacuo to 20 mL and then placed at -40°C for freezing and crystallization to obtain 2.1 g of white crystalline compound 6 with a yield of 83.5%.
[0034] Characterization:
[0035] Mp: 83-85°C. 1 H NMR (CDCl 3 , 300MHz): δ1.07 (d, 3H, J=6.82, dipp-iPr-CH 3 ), 1.12 (d, 3H, J=6.89, dipp-iPr-CH 3 ), 1.19 (d, 3H, J=6.89, dipp-iPr-CH 3 ), 1.35 (d, 3H, J=6.83, dipp-iPr-CH 3 ), 3.59 (sept, 1H, J = 6.84, dipp-iPr-CH), 3.91 (sept, 1H, J = 6.82, dipp-iPr-CH), 6.35 (s, 1H, Si-H), 6.71 (t , 2H, J=7.35, Ar-H), 6.87(t,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com