Li2Na4V10O28 and preparation method and use thereof
A technology of dilithium and tetrasodium decabonate vanadate and dilithium and tetrasodium decabonate vanadate is applied in the field of new compound dilithium and tetrasodium decabonate vanadate and its preparation, and can solve the problems of low safety in use, low electronic conductivity, Price increase and other issues, to achieve the effect of stable charging and discharging efficiency, small battery interface resistance, and low equipment temperature resistance requirements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
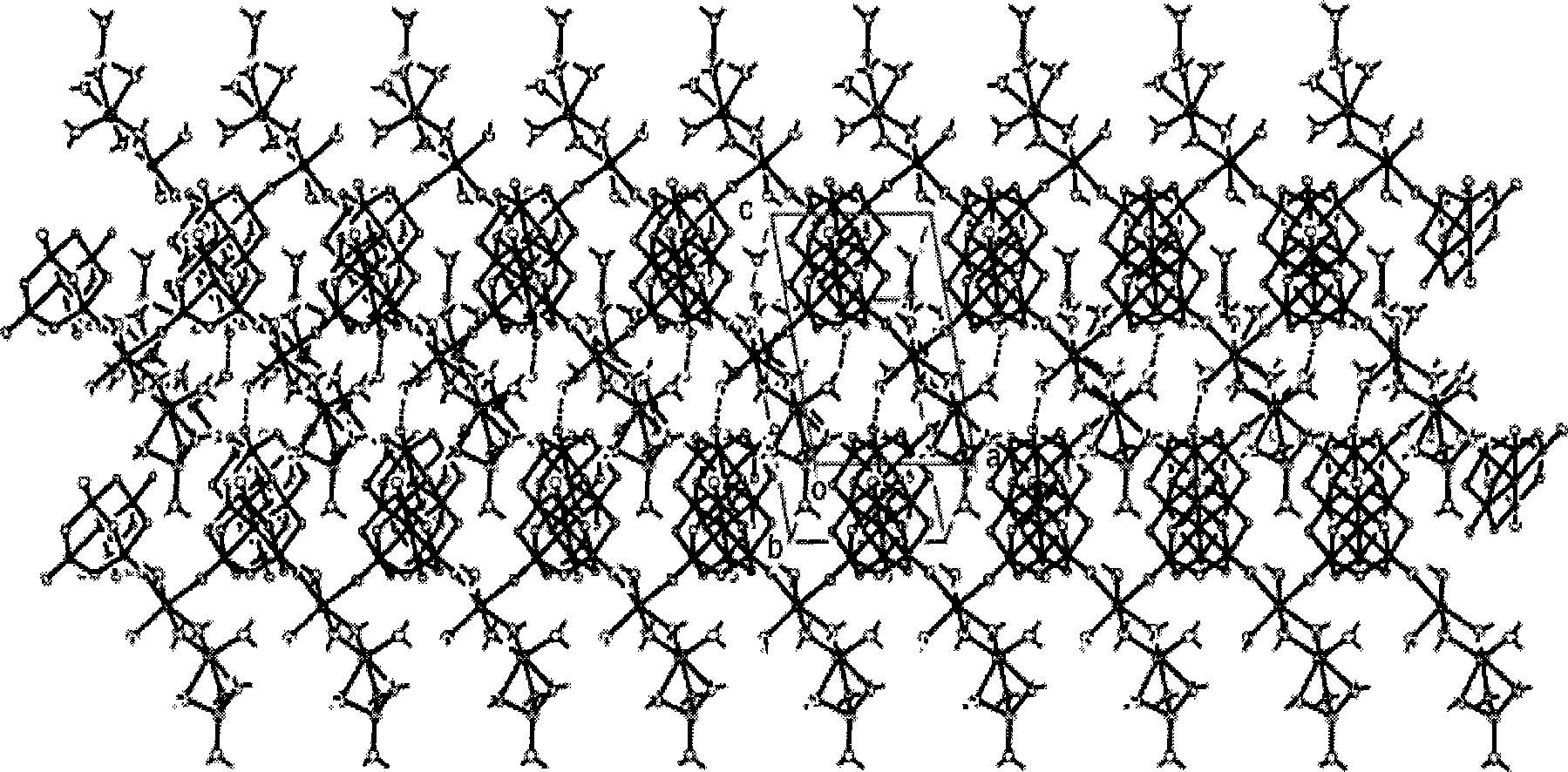
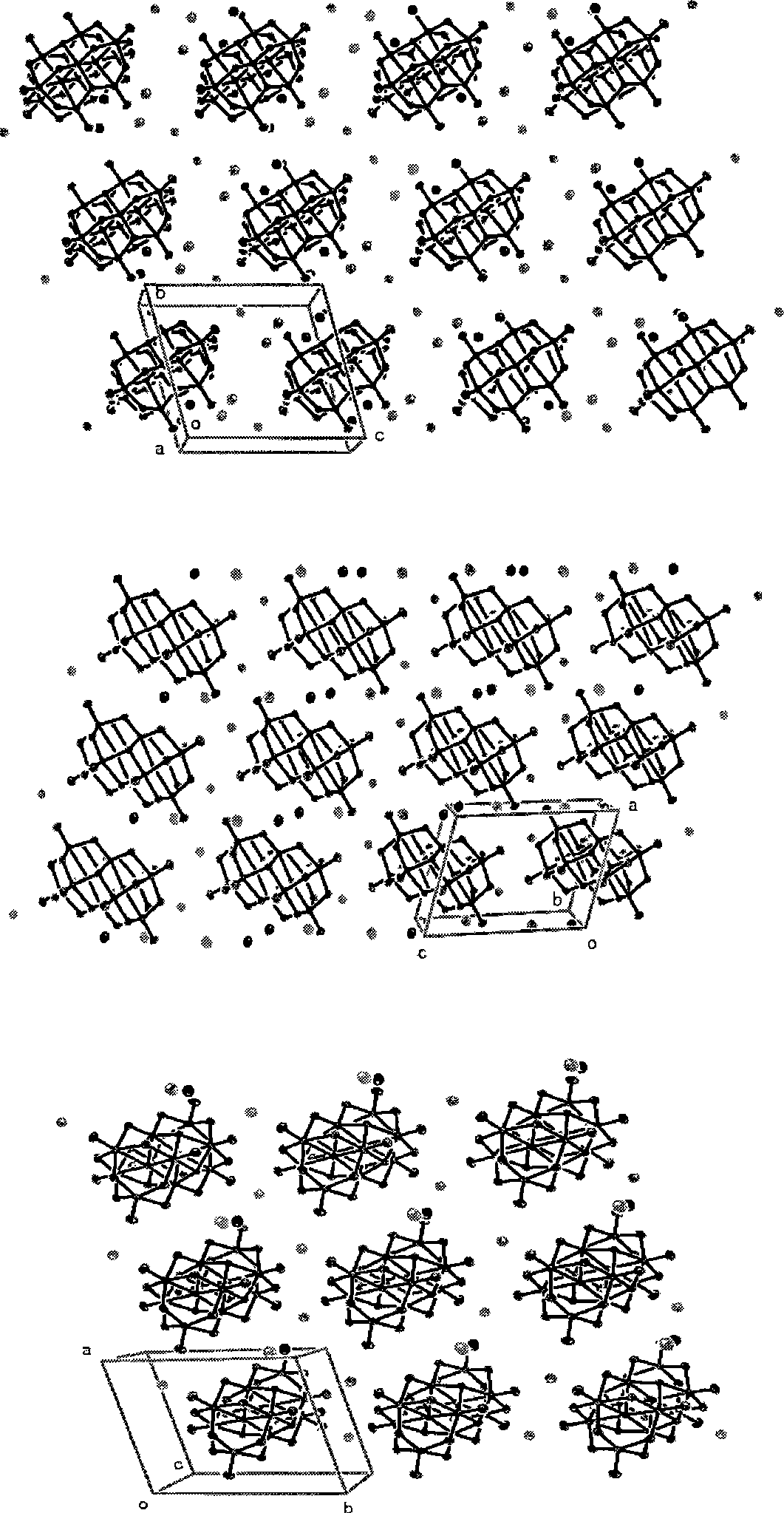
[0035] Ammonium metavanadate, sodium hydroxide and lithium hydroxide are respectively dissolved in the water solvent according to the molar ratio of 1:0.2:0.4, the consumption of ammonium metavanadate is 6.0g, and the total consumption of water solvent is the quality of ammonium metavanadate 30 times. After mixing, adjust the pH to about 4 with dilute nitric acid, move it to a high-pressure stainless steel reaction kettle, heat it at a temperature of about 120°C for 12 hours, filter the solution and let it stand for about 15 days, and produce about 6.60g of orange crystals as the precursor Single crystal [LiNa 2 (H 2 O) 9 ] 2 V 10 o 28 , its molecular space structure is determined, and the structure diagram is as follows figure 1 .
Embodiment 2
[0037] Ammonium metavanadate, sodium hydroxide and lithium hydroxide are respectively dissolved in the water solvent according to the molar ratio of 1:0.3:0.5, the consumption of ammonium metavanadate is 3.0g, and the total consumption of water solvent is the ammonium metavanadate quality 56 times. After mixing, adjust the pH to about 4 with dilute nitric acid, move it to a high-pressure stainless steel reaction kettle, and heat it at a temperature of about 150°C for 10 hours. The solution is filtered and left for about 10 days, and about 2.80g of orange crystals are formed, which is the precursor. Single crystal [LiNa 2 (H 2 O) 9 ] 2 V 10 o 28 , its molecular space structure is determined, and the structure diagram is as follows figure 1 .
Embodiment 3
[0038] Example 3 Pulverization of precursor crystals
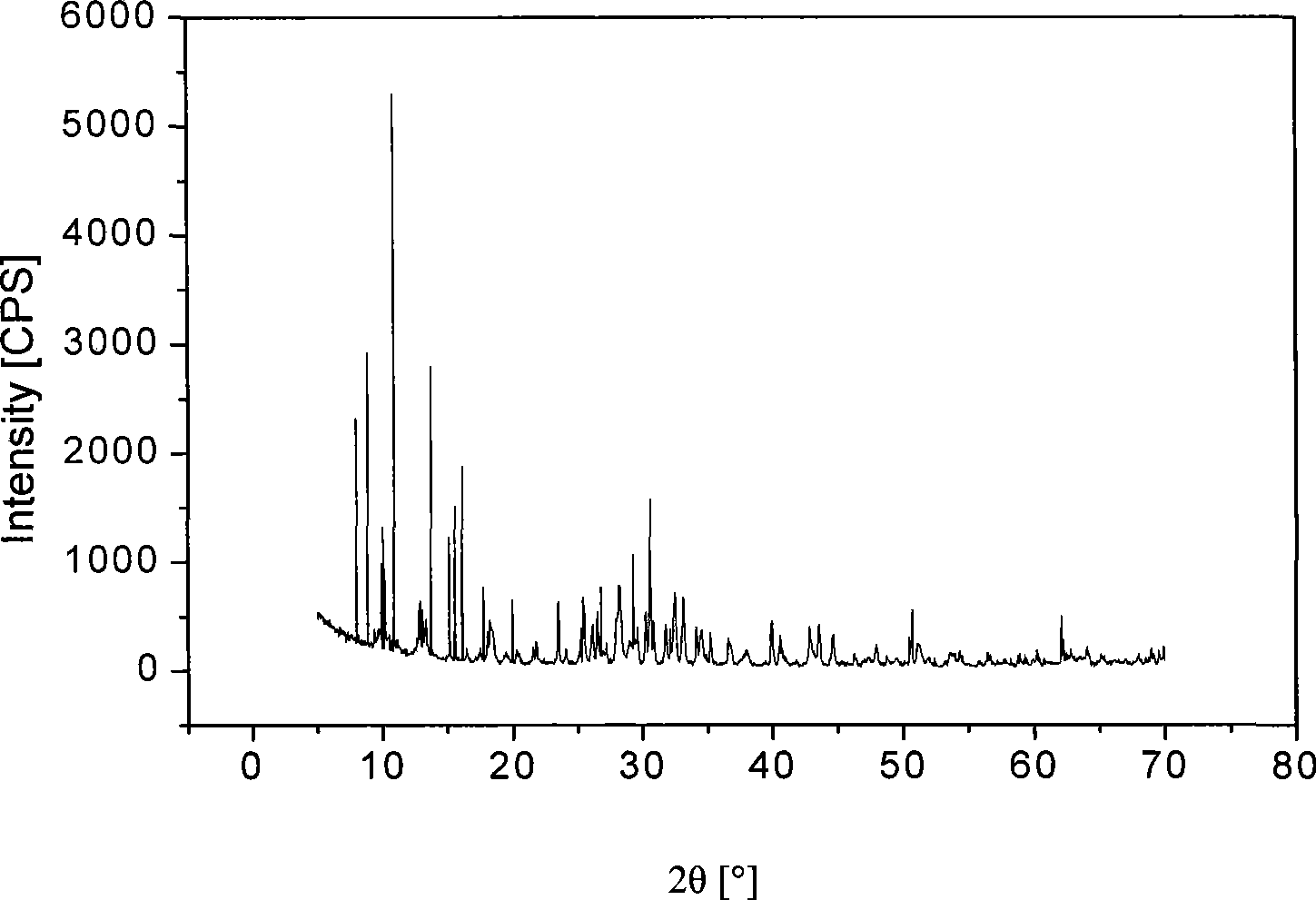
[0039] Weigh about 8 grams of precursor crystals, after 2 hours of ball milling, the XRD spectrum is as follows figure 2 shown.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Discharge capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com