Nanoscale iron phosphate precursor and preparation method thereof, and lithium iron phosphate and preparation method thereof
A technology of lithium iron phosphate and iron phosphate, applied in the direction of nanotechnology, nanotechnology, nanotechnology for materials and surface science, etc., can solve the problems of different precipitation speeds, limited applications, uneven material composition, etc., and achieve stable performance , the effect of high conductivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] S1: Prepare liquid A: 0.1mol / L 100mL NH 4 h 2 PO 4 Solution; B solution: 0.2mol / L 50mL of FeCl 3 Dissolve 0.9mL of furfuryl alcohol in the solution;
[0040] S2: Pour liquid A into a 250mL three-neck flask, stir and keep warm at 93°C for a period of time, then drop liquid B into liquid A at a rate of 1mL / min;
[0041] S3: Add 1mL ammonia water to the system, stir and react for 6h, the precipitate is filtered, washed and dried to obtain spherical FePO 4 Precursor;
[0042] S4: Combine the precursor obtained in S3 with Li 2 CO 3 Put it into a mortar at a ratio of 1:0.5 and add absolute ethanol for wet grinding for 1 hour, then dry it, place the dry material in an Ar atmosphere at 700°C, and calcinate it at a high temperature for 6 hours to obtain lithium iron phosphate.
Embodiment 2
[0044] S1: Prepare liquid A: 0.1mol / L 100mL NH 4 h 2 PO 4 Solution; B solution: 0.2mol / L 50mL of FeCl 3 Dissolve 0.9mL of furfuryl alcohol in the solution;
[0045] S2: Pour liquid A into a 250mL three-neck flask, stir and keep warm at 93°C for a period of time, then drop liquid B into liquid A at a rate of 1mL / min;
[0046] S3: Add 0.5mL ammonia water to the system, stir and react for 6h, the precipitate is filtered, washed and dried to obtain spherical FePO 4 Precursor;
[0047] S4: Combine the precursor obtained in S3 with Li 2 CO 3 Put it into a mortar at a ratio of 1:0.5 and add absolute ethanol for wet grinding for 1 hour, then dry it, place the dry material in an Ar atmosphere at 700°C, and calcinate it at a high temperature for 6 hours to obtain lithium iron phosphate.
Embodiment 3
[0049] S1: Prepare liquid A: 0.1mol / L 100mL NH 4 h 2 PO 4Solution; B solution: 0.2mol / L 50mL of FeCl 3 Dissolve 0.9mL of furfuryl alcohol in the solution;
[0050] S2: Pour liquid A into a 250mL three-neck flask, stir and keep warm at 93°C for a period of time, then drop liquid B into liquid A at a rate of 1mL / min;
[0051] S3: Add 2mL ammonia water to the system, stir and react for 6h, the precipitate is filtered, washed and dried to obtain spherical FePO 4 Precursor;
[0052] S4: Combine the precursor obtained in S3 with Li 2 CO 3 Put it into a mortar at a ratio of 1:0.5 and add absolute ethanol for wet grinding for 1 hour, then dry it, place the dry material in an Ar atmosphere at 700°C, and calcinate it at a high temperature for 6 hours to obtain lithium iron phosphate.
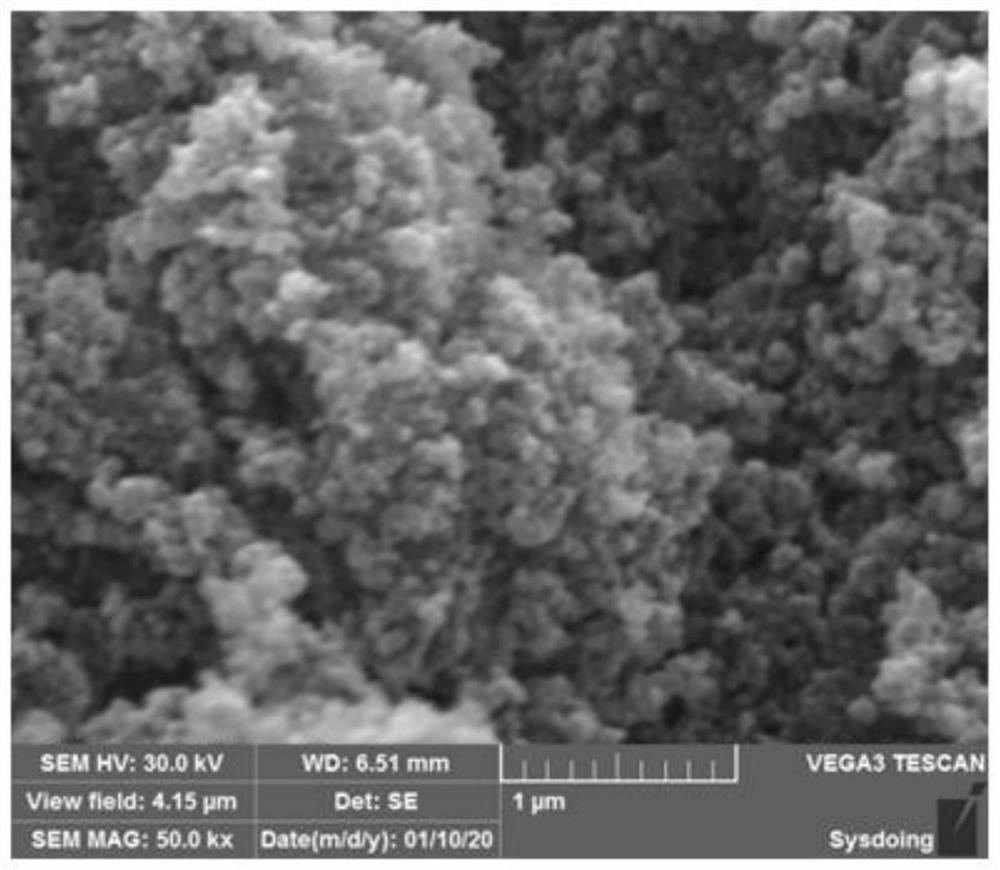
[0053] figure 1 It is the microscopic morphology of the iron phosphate precursor prepared in Example 1, which is amorphous and has a uniform particle size distribution, about 50 nm.
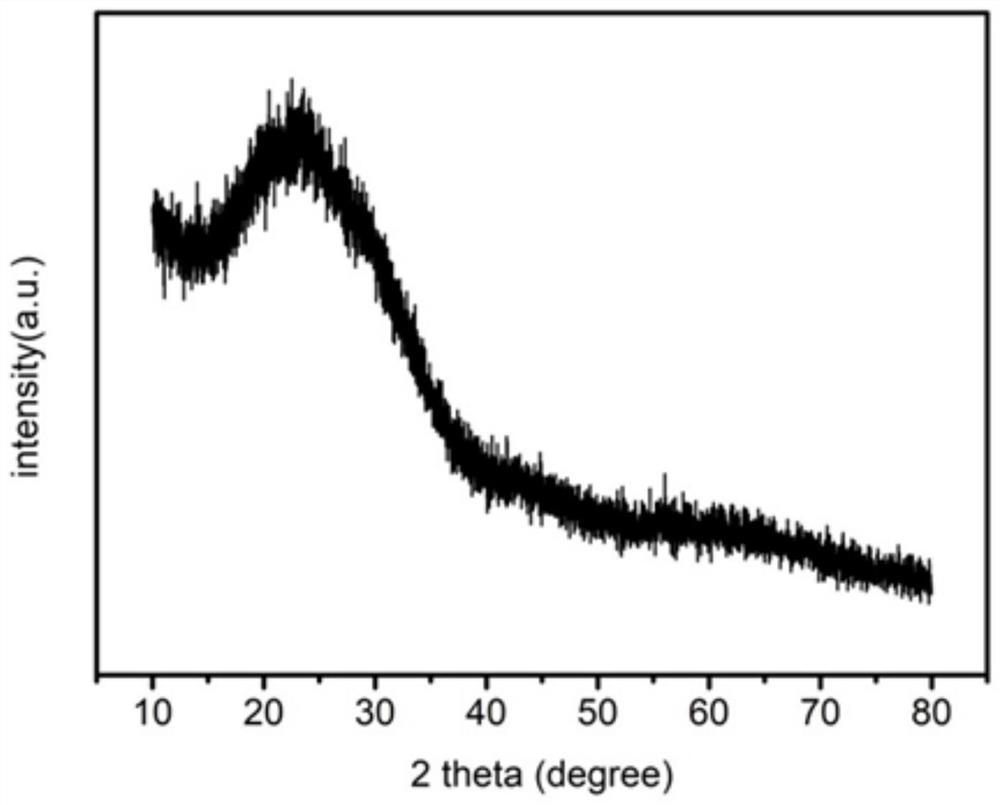
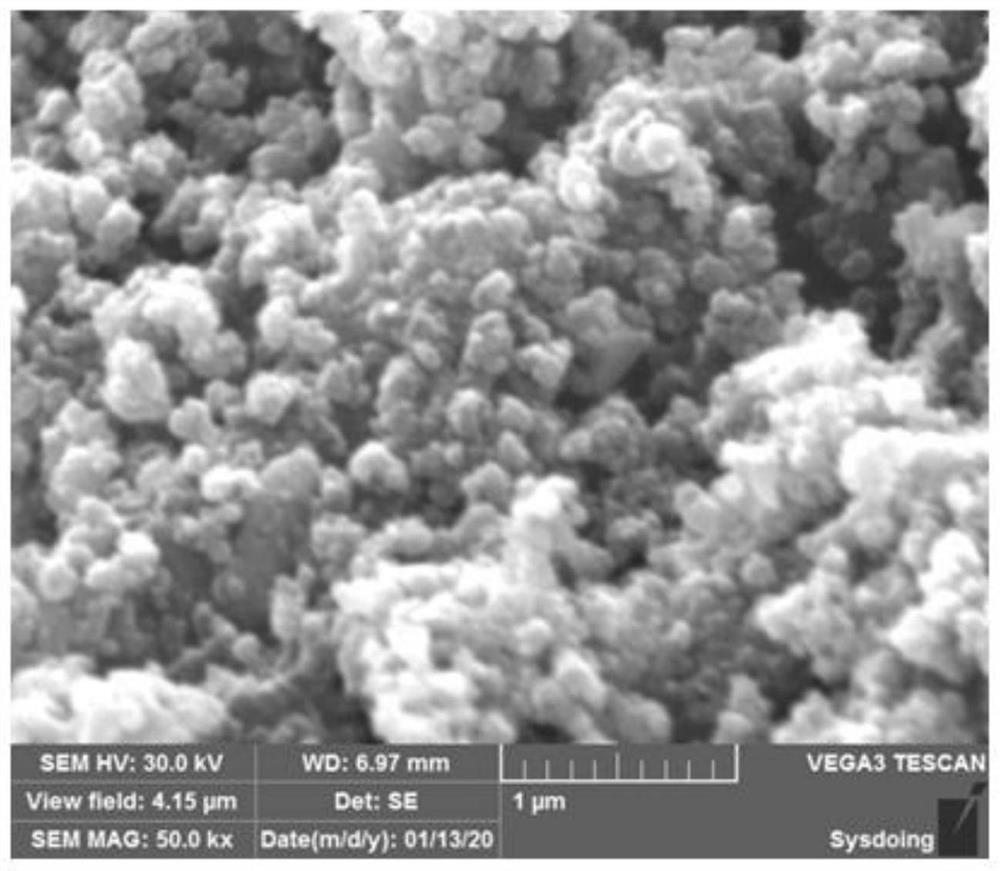
[0054] fi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com