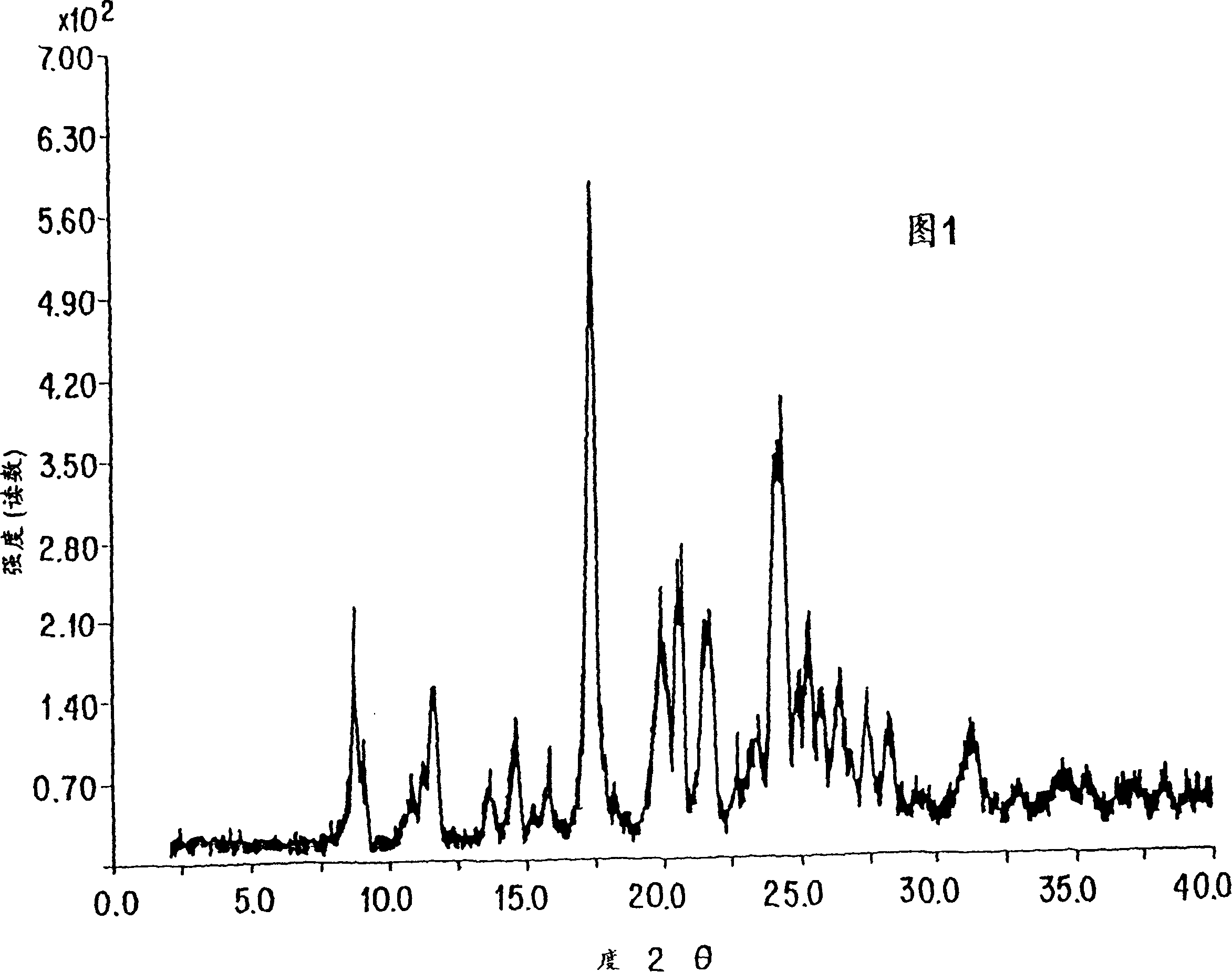
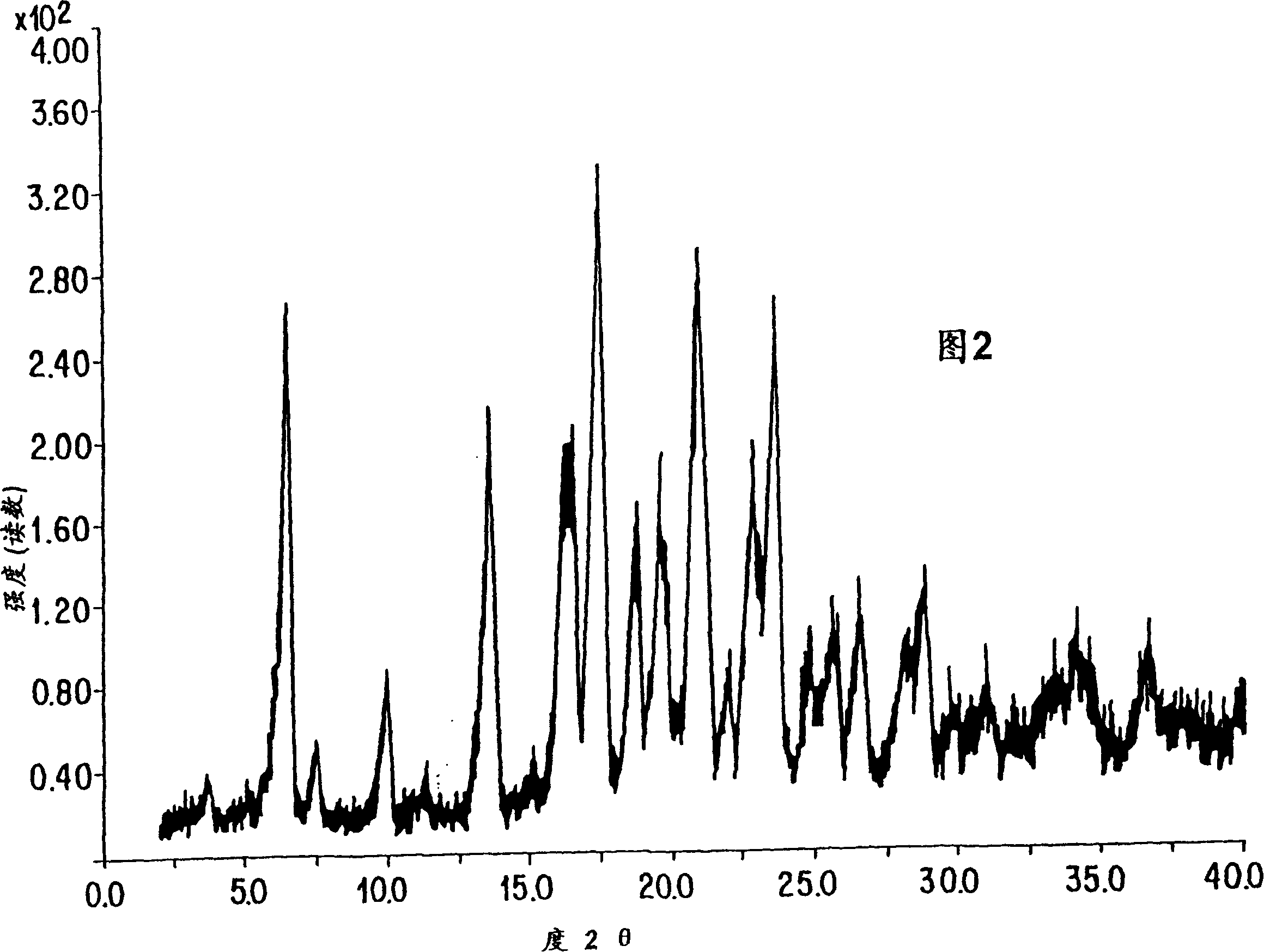
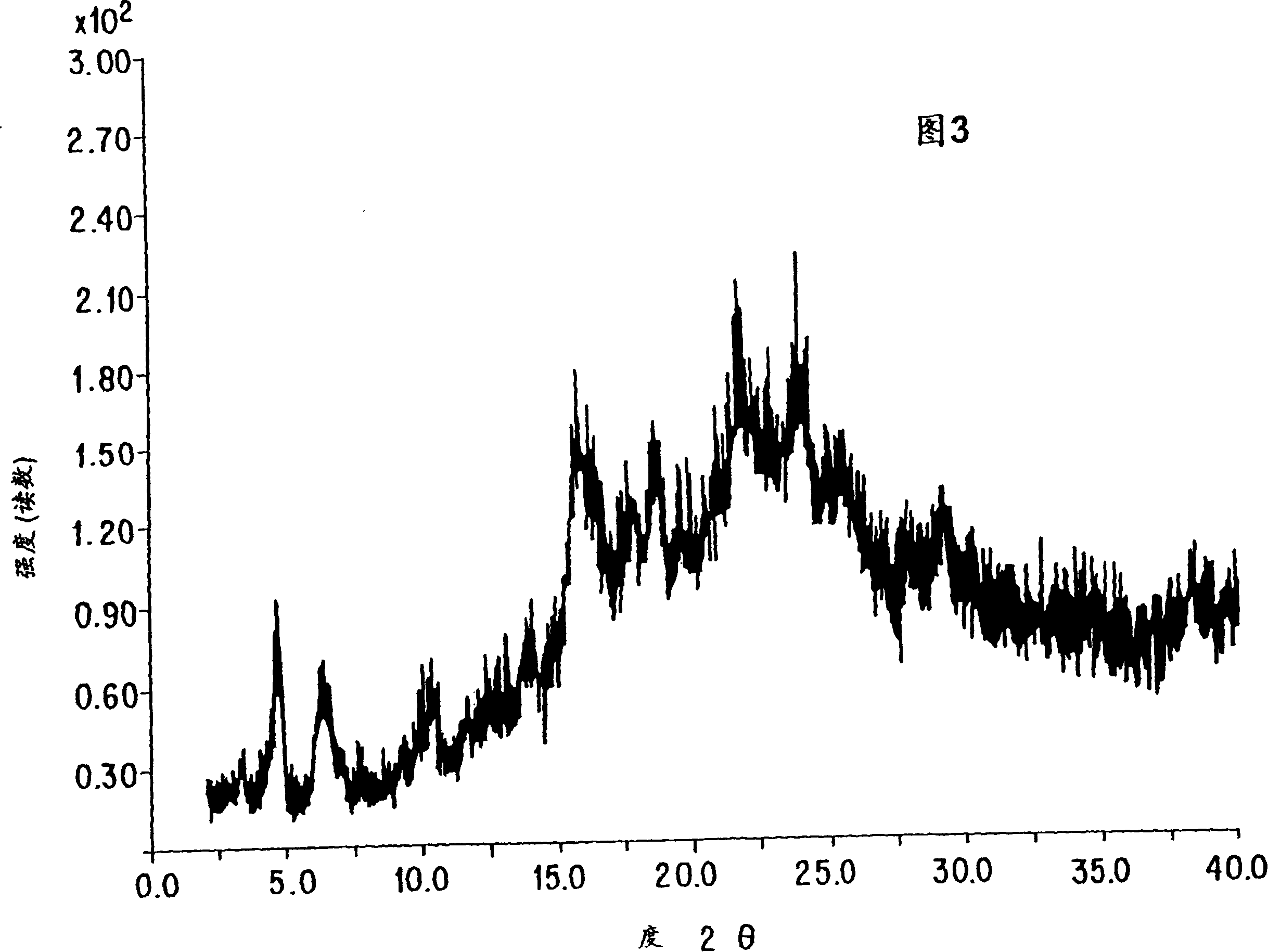
Process for the preparation of leukotriene antagonists
A vinyl and crystallization technology, applied in the field of preparing 1-cyclopropylacetic acid precursor, can solve problems such as low yield, not particularly suitable for large-scale production, and unsatisfactory pharmaceutical preparations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0060] Scheme 3 depicts an improved process for the preparation of compounds of formula (I) contemplated by the present invention. In step (3a), the dilithium salt of 1-(mercaptomethyl)cyclopropylacetic acid is coupled with a sulfonate of formula (II). Thus, 1-(mercaptomethyl)cyclopropylacetic acid (VIII) was first converted to the dilithium dianion. This transformation is carried out by contacting the former with a lithium base such as n-butyllithium in hexane or heptane and the like. The reaction is carried out in an inert organic solvent such as THF, toluene or a mixture thereof at a temperature below 0°C, usually at about -5°C or lower.
[0061] The sulfonate (II) is then added to the solution of the dilithium dianion. The sulfonate can be added directly as a solid, or as a solution in an inert organic solvent such as THF or toluene, preferably THF. Due to the limited stability of the sulfonate (II) in solution, the sulfonate solution is preferably prepared just prior t...
Embodiment 1
[0080] 1,1-cyclopropyldimethanol cyclic sulfite
[0081] Method A:
[0082]To a 1 liter round bottom flask equipped with a stirrer, thermocouple, nitrogen inlet and syringe pump was added dichloromethane (645ml) and 1,1-cyclopropyldimethanol (10.64g; 97.93mmol). The mixture was stirred for 10 minutes to ensure complete dissolution. N,N-Diisopropylethylamine (34.21ml; 195.86mmol) was added and the solution was cooled to 0-5°C. Thionyl chloride (7.01 ml; 96.04 mmol) was added subsurface via a Teflon tube via a syringe pump over 60 minutes. The reaction solution was transferred to a separatory funnel containing cold (0-5°C) phosphate buffer (pH = 7.2, 650ml). After equilibration the layers were separated. The product solution in dichloromethane was washed with 2 wt % sodium chloride solution (650 ml), then the product solution was azeotropically dried and concentrated to 50 ml at 35-40° C. under atmospheric pressure. Assay yield of title compound = 13.07 g (90%)
[0083] Me...
Embodiment 2
[0087] 1-(Hydroxymethyl)cyclopropylacetonitrile
[0088] Method A:
[0089] A solution of the cyclic sulfite of Example 1 in dichloromethane (61 ml; 158.9 mg / ml; 9.69 g ) The solution was concentrated to about 20 ml by distillation at atmospheric pressure. Isopropyl acetate (2 x 30ml) was added and distillation continued to a final volume of 13ml. Dimethylformamide (21 ml) was added to the solution above 55°C and the solution was cooled to room temperature.
[0090] Add 40ml of cyclic sulfite (9.28g; 62.6mmol) in DMF:IPAc (isopropyl acetate) (4:1 ) in the above solution. Sodium cyanide (4.61 g; 94 mmol) and sodium iodide (3.75 g; 25.0 mmol) were added at room temperature. The reaction mixture was heated to 70±3°C and maintained at this temperature until the reaction was complete. The reaction mixture was allowed to cool to room temperature and diluted with cold (0-5°C) isopropyl acetate (187ml). The dark yellow slurry (218ml) was transferred to a separatory funnel conta...
PUM
| Property | Measurement | Unit |
|---|---|---|
| solubility (mass) | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com