Preparation method of high-stability adefovir dipivoxil
An adefovir dipivoxil, high-stability technology, applied in the field of drug synthesis, can solve the problems of thermal instability, poor stability and small particle size of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
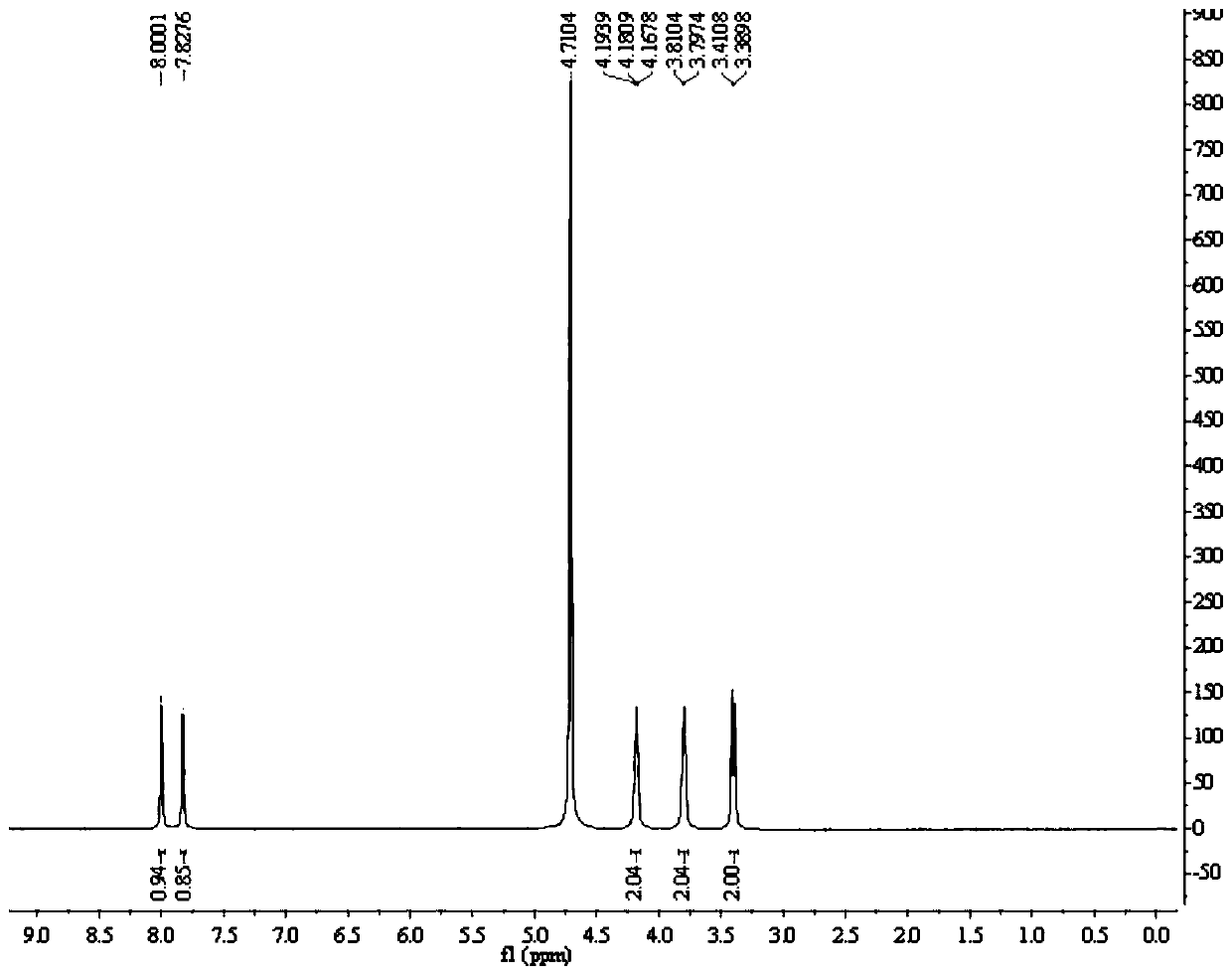
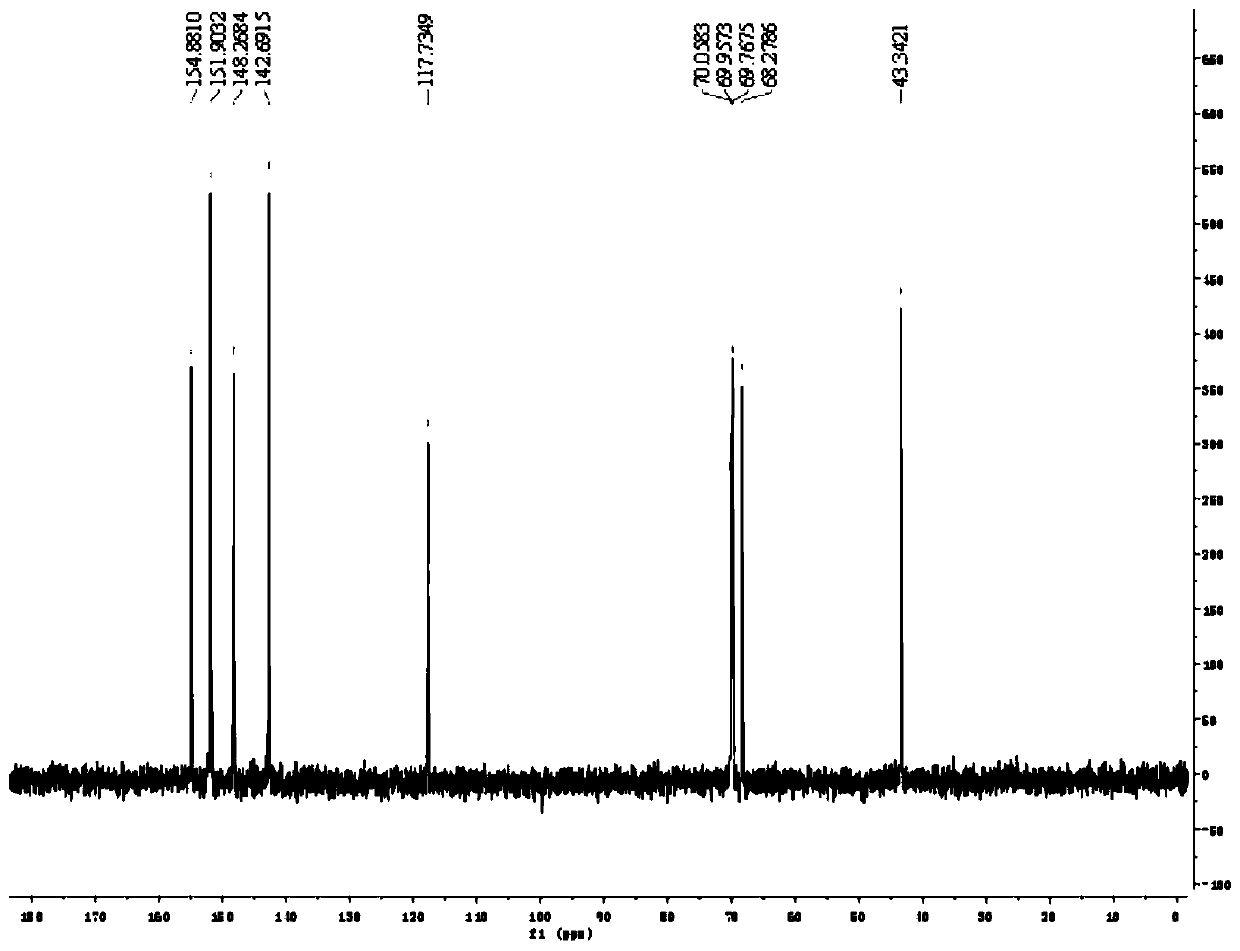
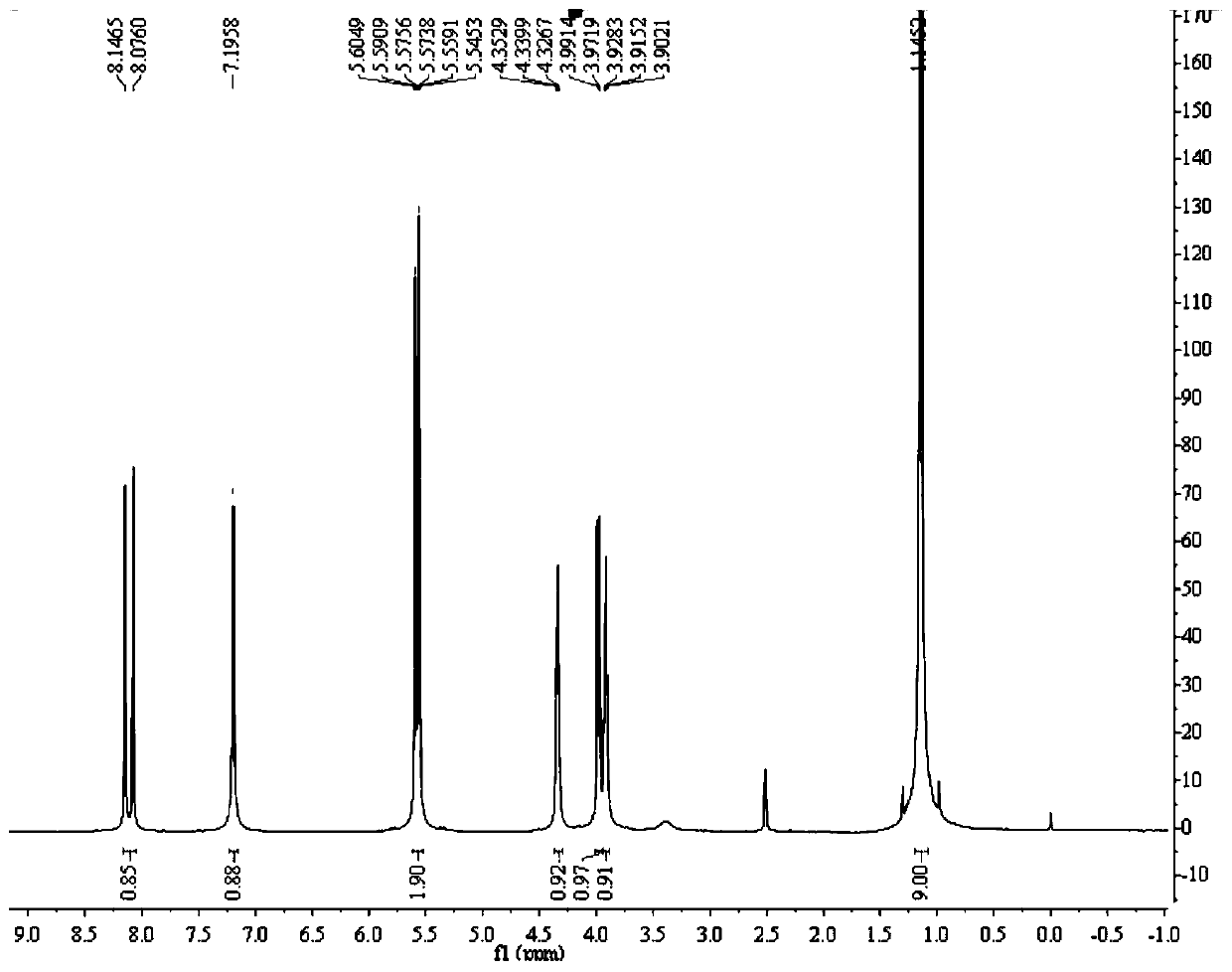
[0052] Add 5.20kg (2w / w) of acetonitrile into a 50L reactor, add 2.60kg (1eq) of compound 1 and 4.24kg (3.5eq) of bromotrimethylsilane under stirring, raise the temperature to 65±5°C, and keep the reaction for 2 hours. Concentrate the organic phase under reduced pressure at 45±5°C until no liquid distills out, add 7.80kg (3w / w) of purified water to dissolve the organic phase, raise the temperature to 55±5°C and stir for 0.5 hours; add 30% NaOH aqueous solution dropwise to the 50L reaction kettle Adjust the pH to 2.5-3.5, raise the temperature to 75±5°C and keep stirring for 2 hours; cool down to 3±3°C and keep stirring for 3 hours; centrifuge, and filter the cake with 2.60kg (1w / w) of purified water and 1.30kg of ethanol (0.5 w / w) washing; add 10.40kg (4w / w) of purified water to the 50L reactor, add filter cake under stirring, heat up to 75±5°C, beat for 2 hours; cool down to 3±3°C for 3 hours; centrifuge , the filter cake was washed with 2.60kg (1w / w) of purified water; the f...
Embodiment 2
[0058] Add 5.20kg (2w / w) of DMF to the 50L reactor, add 2.60kg (1eq) of compound 1 and 1.72kg (2eq) of trimethylchlorosilane under stirring, raise the temperature to 55±5°C, and keep the reaction for 2 hours. Concentrate the organic phase under reduced pressure at 45±5°C until no liquid distills out, add 7.80kg (3w / w) of purified water to dissolve the organic phase, raise the temperature to 55±5°C and stir for 0.5 hours; add 30% NaOH aqueous solution dropwise to the 50L reaction kettle Adjust the pH to 2.5-3.5, raise the temperature to 75±5°C and keep stirring for 2 hours; cool down to 3±3°C and keep stirring for 3 hours; centrifuge, and filter the cake with 2.60kg (1w / w) of purified water and 1.30kg of ethanol (0.5 w / w) washing; add 10.40kg (4w / w) of purified water to the 50L reactor, add filter cake under stirring, heat up to 75±5°C, beat for 2 hours; cool down to 3±3°C for 3 hours; centrifuge , the filter cake was washed with 2.60kg (1w / w) of purified water; the filter cake...
Embodiment 3
[0064] Add 5.20kg (2w / w) of tetrahydrofuran into a 50L reactor, add 2.60kg (1eq) of compound 1 and 7.92kg (5eq) of iodotrimethylsilane under stirring, raise the temperature to 75±5°C, and keep the reaction for 2 hours. Concentrate the organic phase under reduced pressure at 45±5°C until no liquid distills out, add 7.80kg (3w / w) of purified water to dissolve the organic phase, raise the temperature to 55±5°C and stir for 0.5 hours; add 30% NaOH aqueous solution dropwise to the 50L reaction kettle Adjust the pH to 2.5-3.5, raise the temperature to 75±5°C and keep stirring for 2 hours; cool down to 3±3°C and keep stirring for 3 hours; centrifuge, and filter the cake with 2.60kg (1w / w) of purified water and 1.30kg of ethanol (0.5 w / w) washing; add 10.40kg (4w / w) of purified water to the 50L reactor, add filter cake under stirring, heat up to 75±5°C, beat for 2 hours; cool down to 3±3°C for 3 hours; centrifuge , the filter cake was washed with 2.60kg (1w / w) of purified water; the f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com