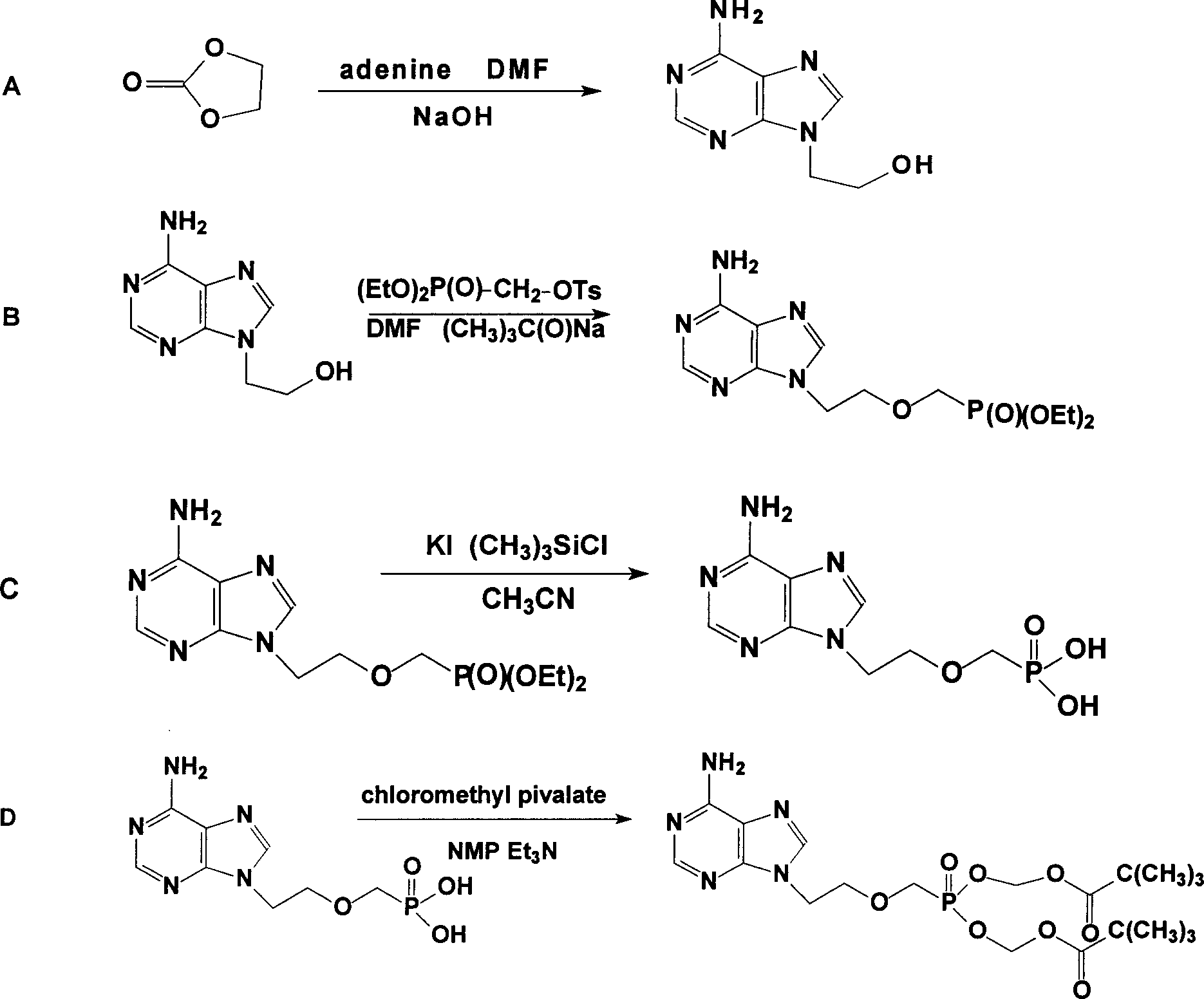
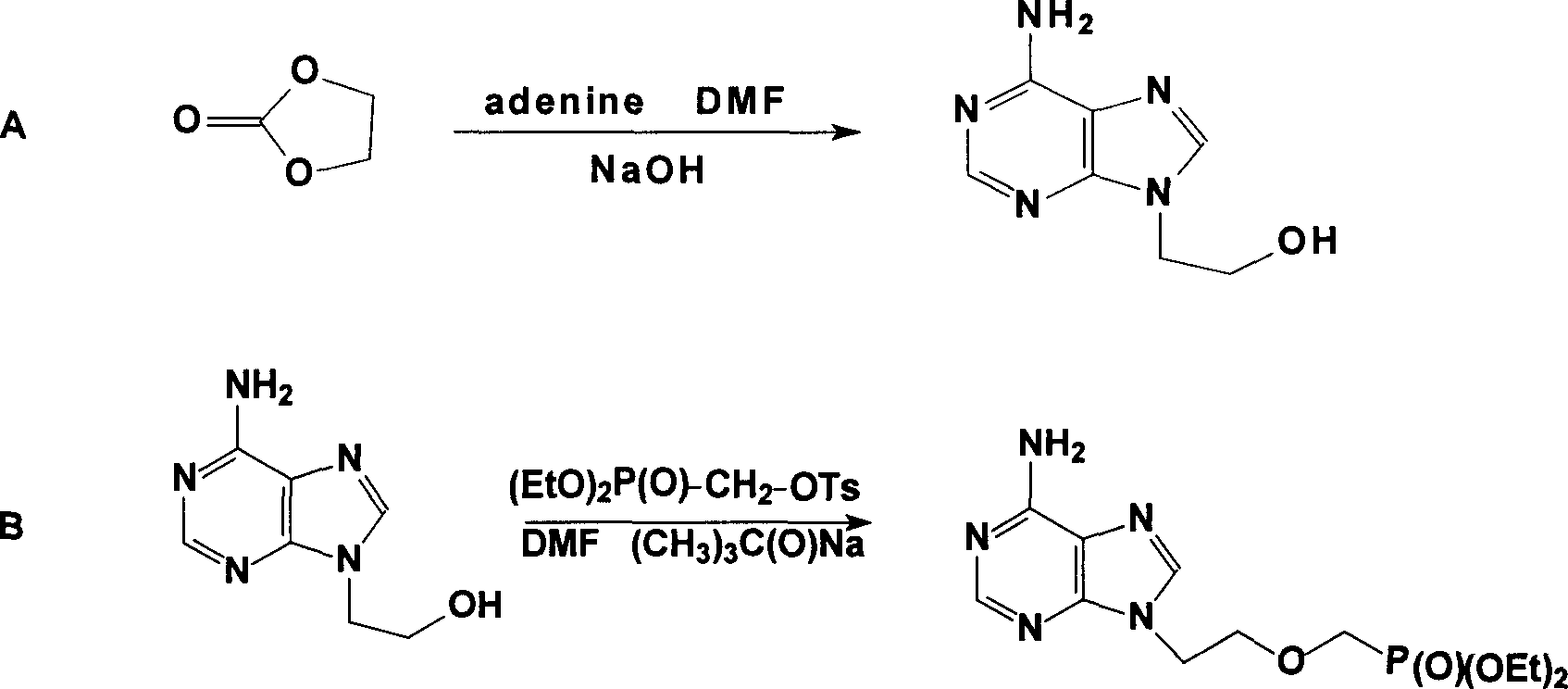
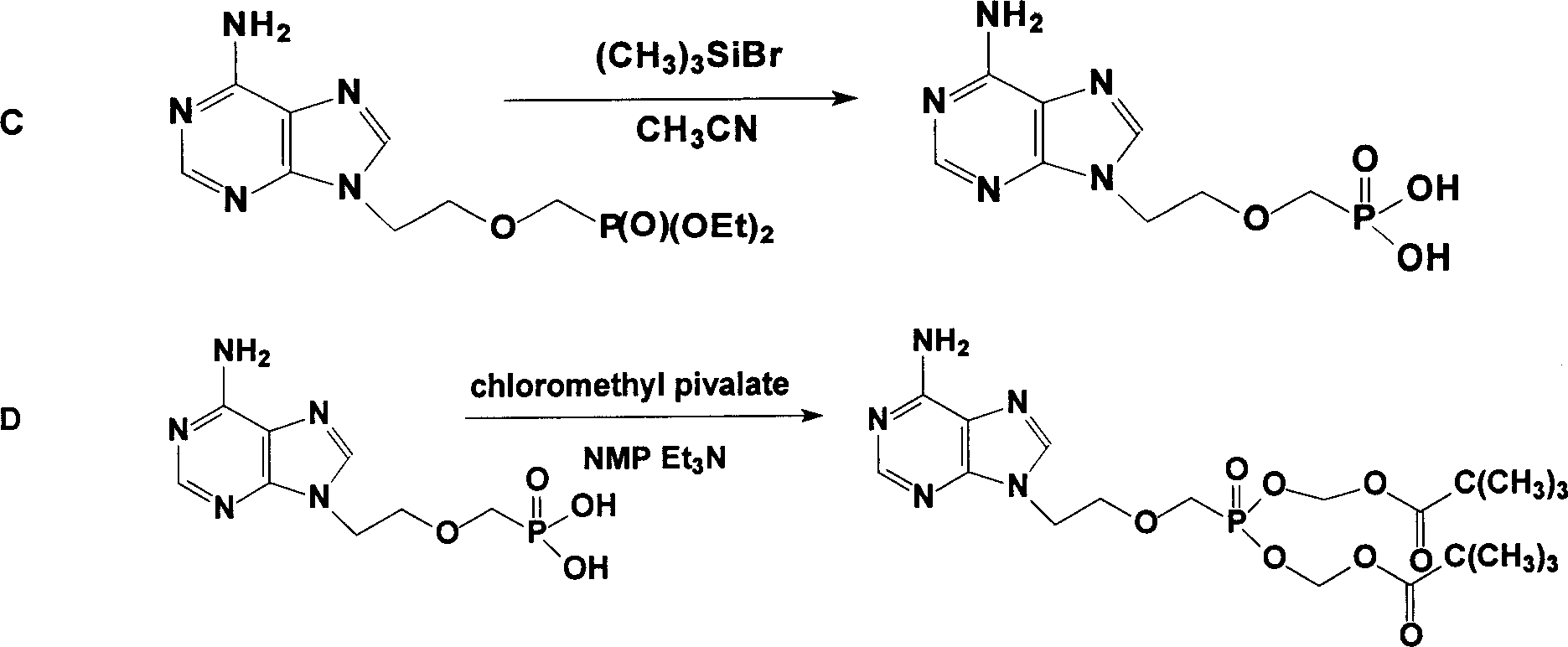
Prepn process of Adefovir dipivalate
A technology of dipentapentyl ester and trimethylchlorosilane, which is applied in the field of preparing nucleotide analogs 9-[methoxy]phosphinyl]methoxy)ethyl]adenine, can solve the problem of expensive, Problems such as high preparation cost and difficulty in purchasing can achieve the effect of reducing production cost and not affecting product quality and yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] The specific operations of steps A and B are as described in published patents, and the specific implementation of step C is: in a 1000ml three-necked bottle equipped with a condenser tube and a drying tube, replace the air with nitrogen and drop into 130g, that is, 0.395mol of diethyl PMEA, 350g or 8.54mol acetonitrile, 289g or 1.74mol potassium iodide and 192.5g or 1.77mol trimethylchlorosilane, heated to 55°C under stirring, refluxed for 2 hours, then heated to 75°C and refluxed for 3 hours, sampling test (TLC) . After passing the test, remove acetonitrile to dryness under negative pressure (the temperature in the bottle does not exceed 80°C). Cool to room temperature, add 260ml of water to fully dissolve, then raise the temperature to 55°C, stir and keep warm for 1 hour, cool to room temperature, and neutralize with 20% (W / W) sodium hydroxide solution to pH=3.0--3.5. Heat up to 75°C for 1 hour, cool to 0--3°C for 3 hours, and filter to dryness. Transfer the filter...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com