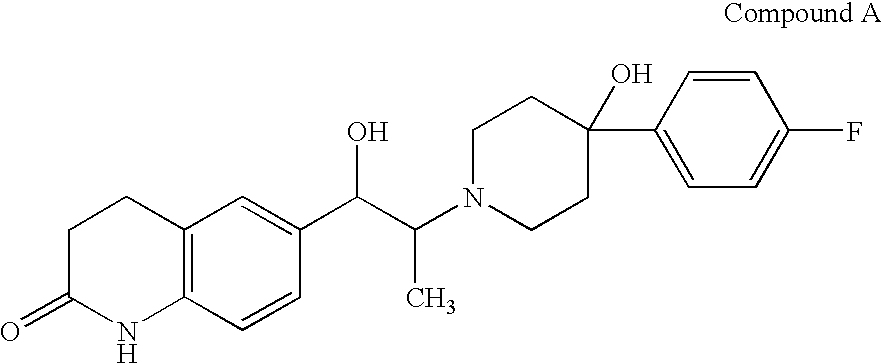
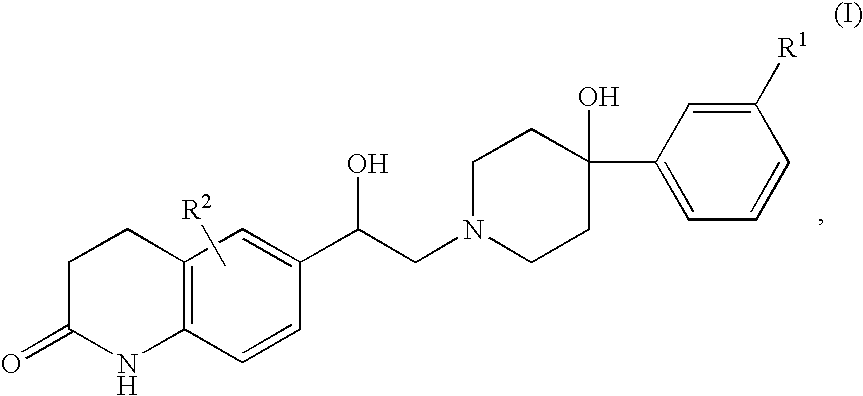
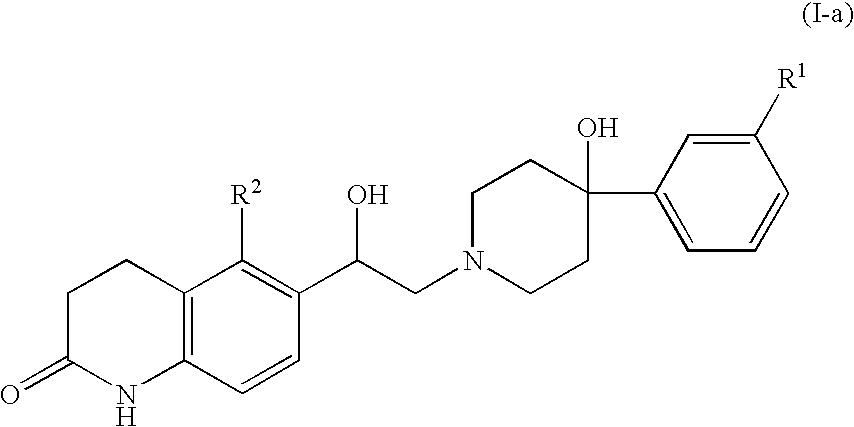
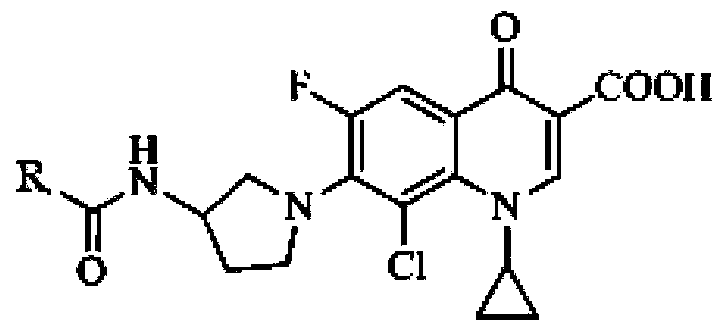
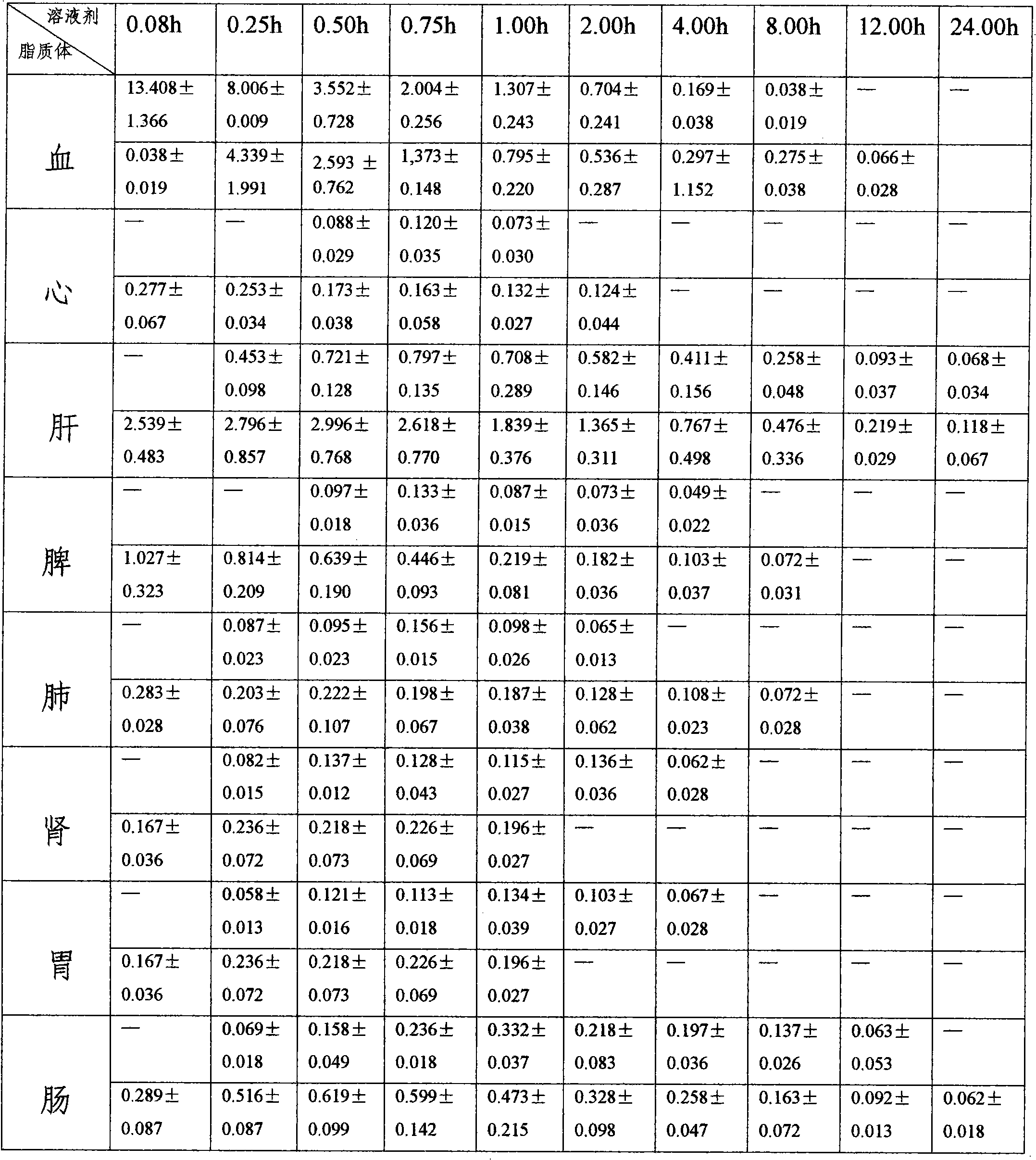
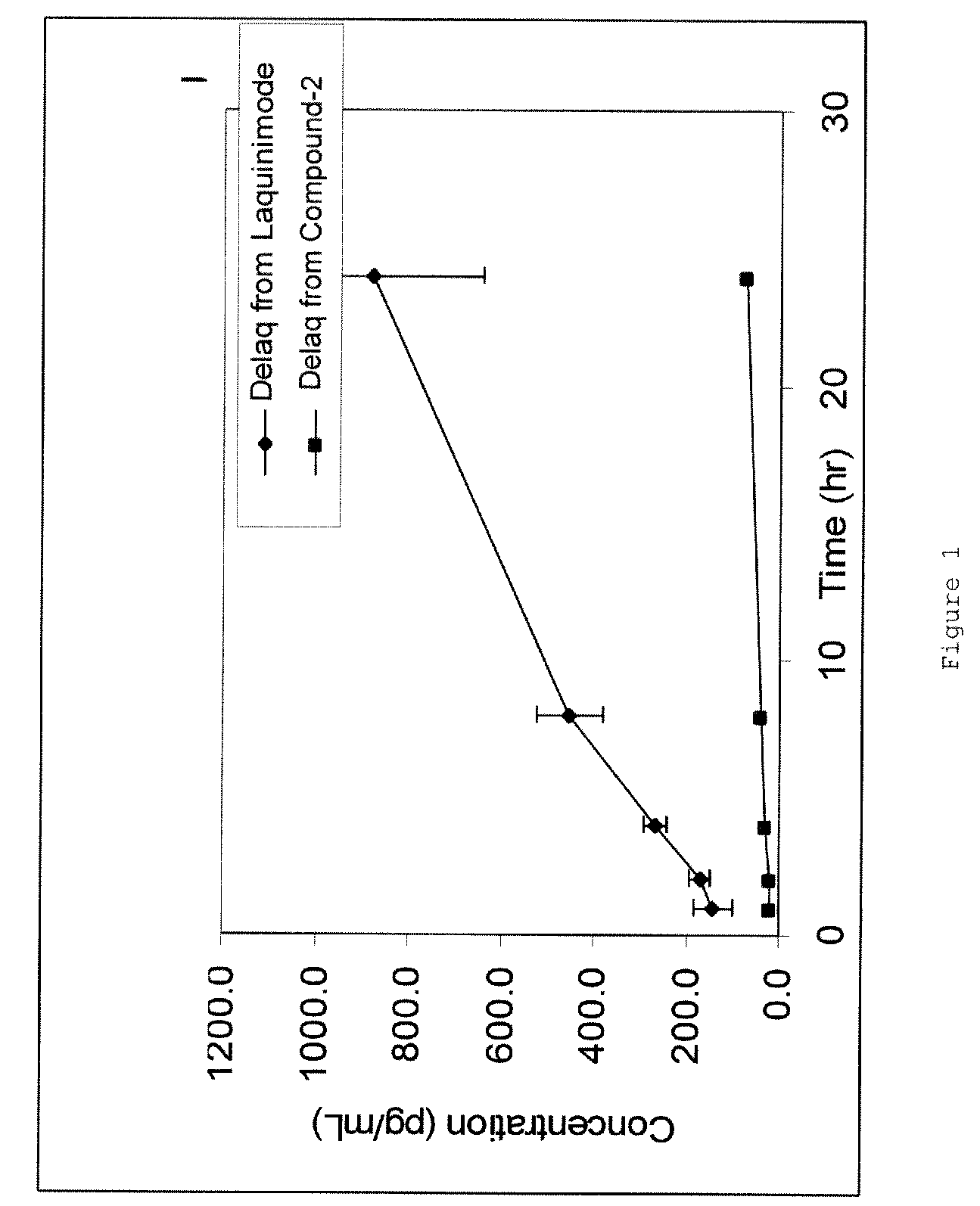
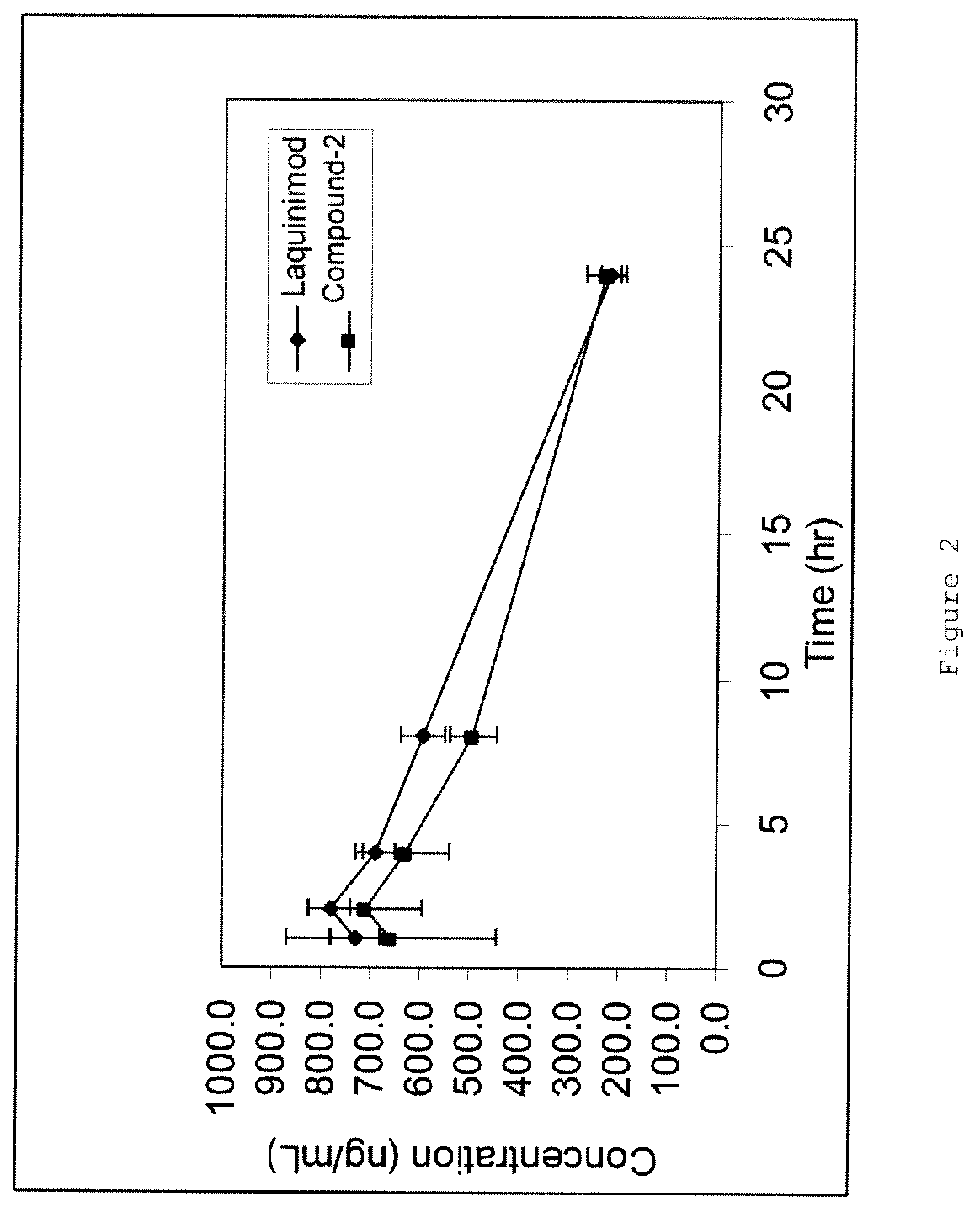
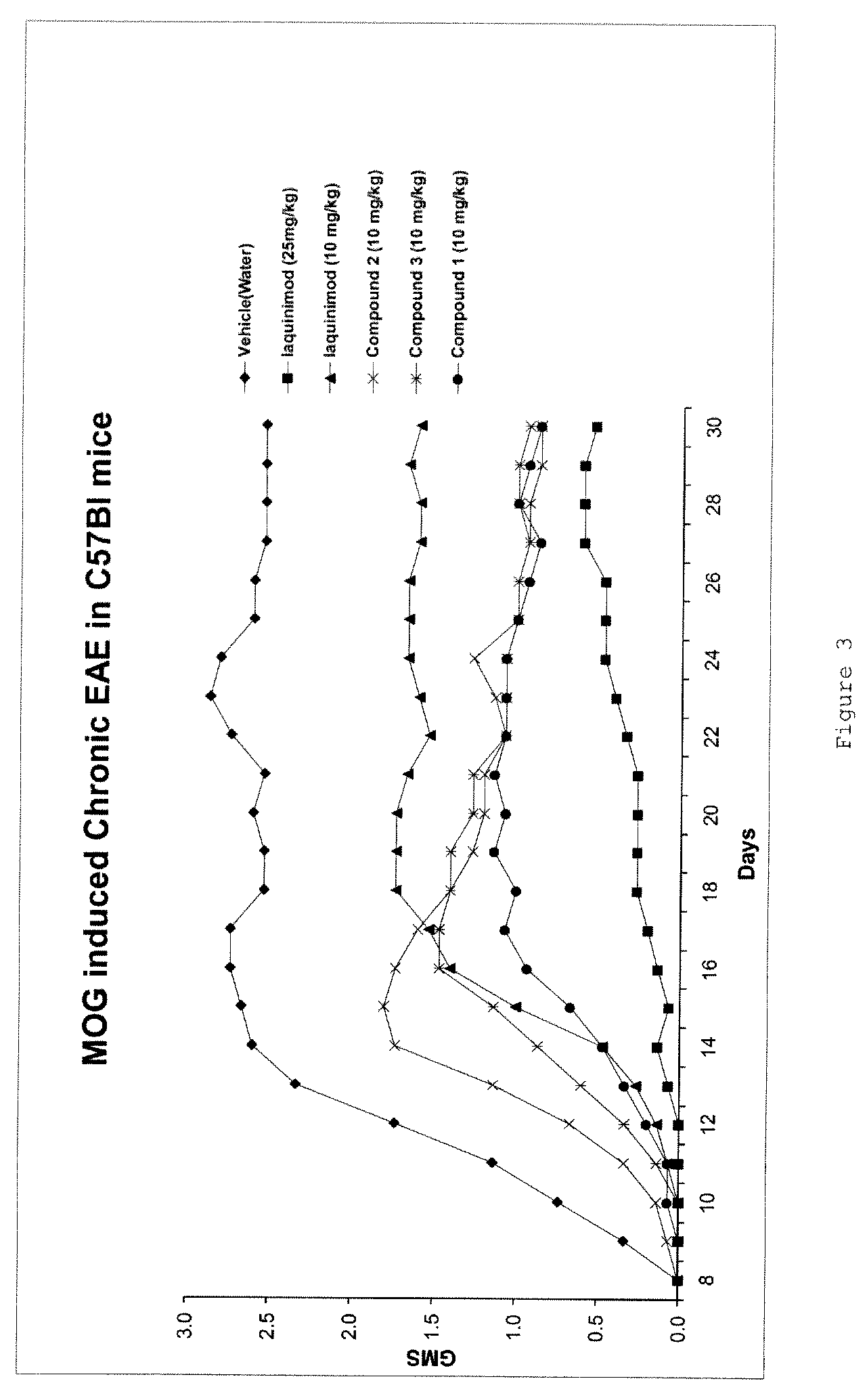
Patents
Literature
270 results about "Ethylone" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
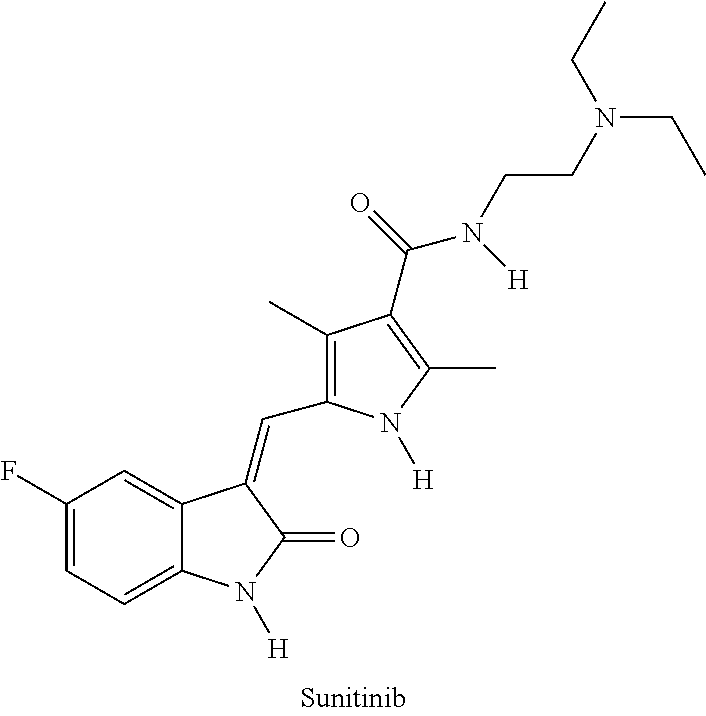
Ethylone, also known as 3,4-methylenedioxy-N-ethylcathinone (MDEC, βk-MDEA), is a recreational designer drug classified as an entactogen, stimulant, and psychedelic of the phenethylamine, amphetamine, and cathinone chemical classes. It is the β-keto analogue of MDEA ("Eve"). Ethylone has only a short history of human use and is reported to be less potent than its relative methylone. In the United States, it began to be found in cathinone products in late 2011.
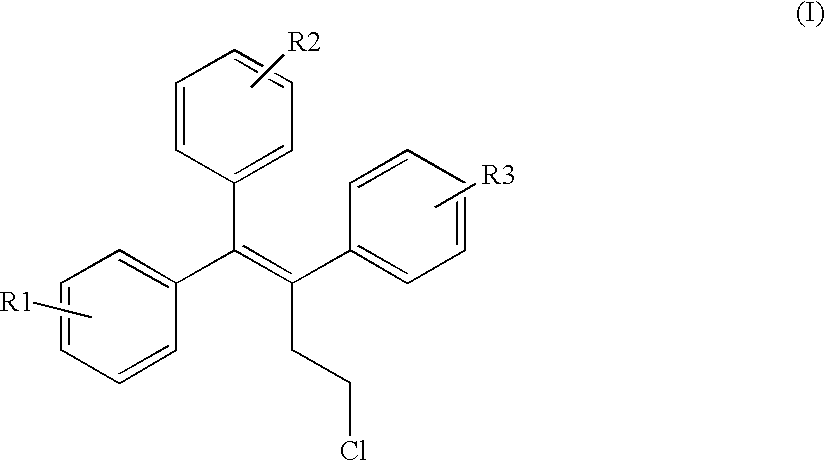
Triphenylalkene derivatives and their use as selective estrogen receptor modulators
InactiveUS6576645B1Efficient productionSufficient amountBiocideNervous disorderHalogenStereoisomerism
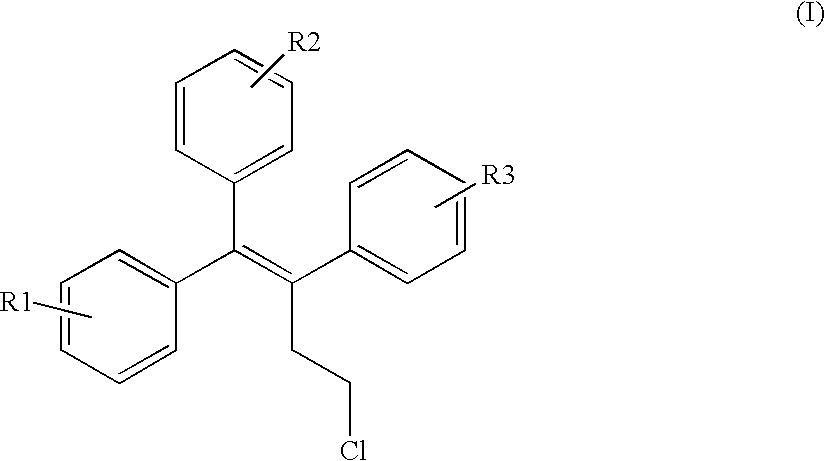
The invention provides novel selective estrogen receptor modulator compounds of the general formula:wherein R1 and R2, which are the same or different area) H, halogen, OCH3, OH; or where X is O, NH or S; and n is an integer from 1 to 4; and R4 and R5, which are the same or different, are a 1 to 4 carbon alkyl, H, -CH2C=CH or -CH2CH2OH; or R4 and R5 form an N-containing five- or six-membered ring or heteroaromatic ring; orc) -Y-(CH2)nCH2-O-R6where Y is O, NH or S and n is an integer from 1 to 4; and R6 is H, -CH2CH2OH, or -CH2CH2Cl; ord) 2,3-dihydroxypropoxy, 2-methylsulfamylethoxy, 2-chloroethoxy, 1-ethyl-2-hydroxyethoxy, 2,2-diethyl-2-hydroxyethoxy or carboxymethoxy; andR3 is H, halogen, OH or -OCH3;stereoisomers thereof and their non-toxic pharmaceutically acceptable salts and esters and mixtures thereof, which compounds exhibit valuable pharmacological properties.
Owner:FORENDO PHARMA LTD
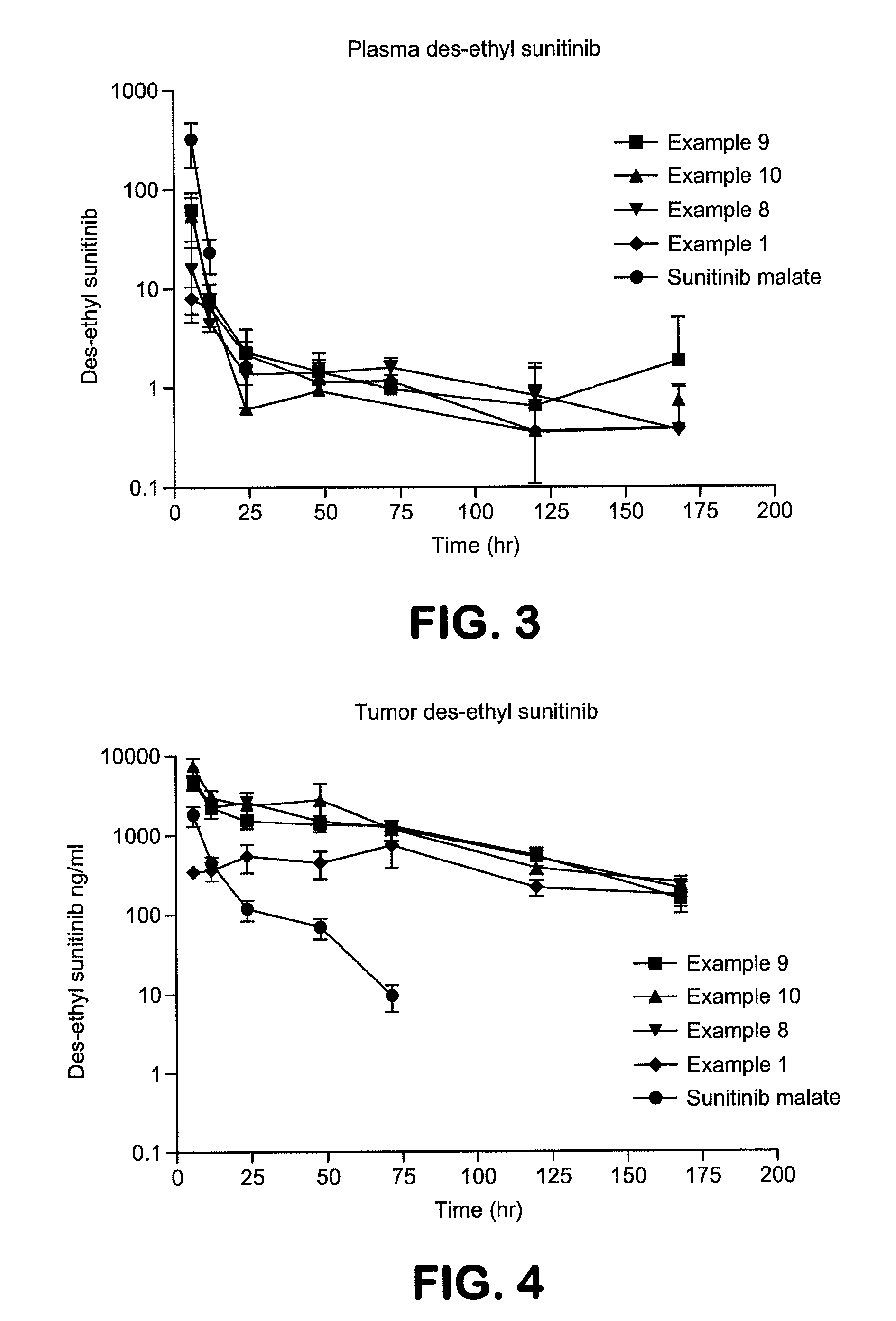
Anti-CTLA4 Antibody and Indolinone Combination Therapy for Treatment of Cancer
InactiveUS20090074787A1Organic active ingredientsAntibody ingredientsDiseaseReceptor tyrosine kinase inhibitor
The invention relates to administration of an anti-CTLA4 antibody, particularly human antibodies to human CTLA4, such as those having amino acid sequences of antibodies 3.1.1, 4.1.1, 4.8.1, 4.10.2, 4.13.1, 4.14.3, 6.1.1, ticilimumab (also referred to as 11.2.1 or CP-675,206), 11.6.1, 11.7.1, 12.3.1.1, 12.9.1.1, and ipilimumab (also referred to as 10D1 or MDX-010), in combination with an indolinone receptor tyrosine kinase inhibitor (RTKI), e.g., N-[2-diethylamino]ethyl]-5-[(Z)-(5-fluoro-2-oxo-1,2-dihydro-3H-indol-3-ylidene)methyl]-2,4-dimethyl-1H-pyrrole-3-carboxamide (compound 1), N-[2-(ethylamino)ethyl]-5-[(Z)-(5-fluoro-2-oxo-1,2-dihydro-3H-indol-3-ylidene)methyl]-2,4-dimethyl-1H-pyrrole-3-carboxamide (compound 2), and 5-[(Z)-(5-fluoro-2-oxo-1,2-dihydro-3H-indol-3-ylidene)methyl]-N-[(2S)-2-hydroxy-3-morpholin-4-ylpropyl]-2,4-dimethyl-1H-pyrrole-3-carboxamide (compound 3), for treatment of cancer. The invention relates to administering a combination of an anti-CTLA4 antibody and an indolinone RTKI such as, inter alia, compound 1. The invention relates to neoadjuvant, adjuvant, first-line, second-line, and third-line therapy of cancer, whether localized or metastasized, and at any point(s) along the disease continuum (e.g., at any stage of the cancer).
Owner:PFIZER PFIZER PRODS
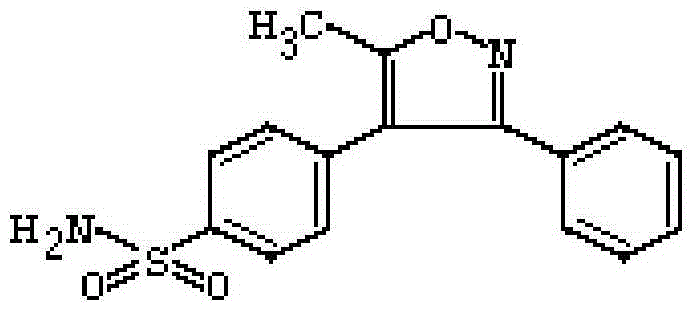
5-ethyl-2-{4-[4-(4-tetrazol-1-yl-phenoxymethyl)-thiazol-2-yl]-piperidin-1-yl}-pyrimidine
Compounds, compositions, and methods relating to 5-ethyl-2-{4-[4-(4-tetrazol-1-yl-phenoxymethyl)-thiazol-2-yl]-piperidin-1-yl}-pyrimidine or a pharmaceutically acceptable salt thereof are provided for the treatment of Type II diabetes and other diseases associated with poor glycemic control.
Owner:CYMABAY THERAPEUTICS
Crystal form of Peimeiqusai disodium and its preparation
ActiveCN1778802AImprove controllabilitySimple and fast operationOrganic active ingredientsOrganic chemistryPhenacylPemetrexed disodium
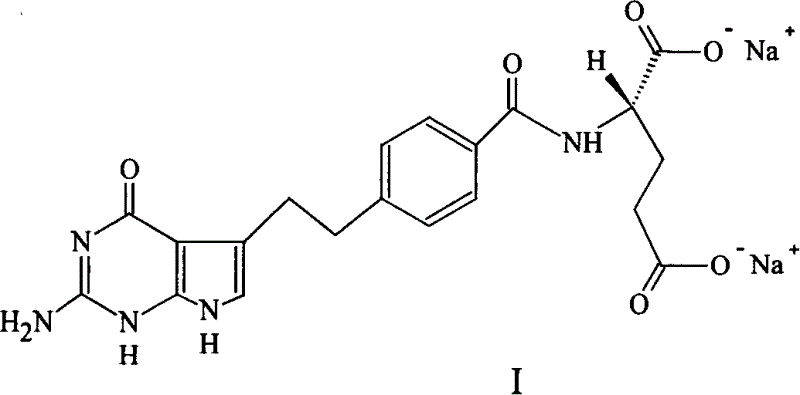
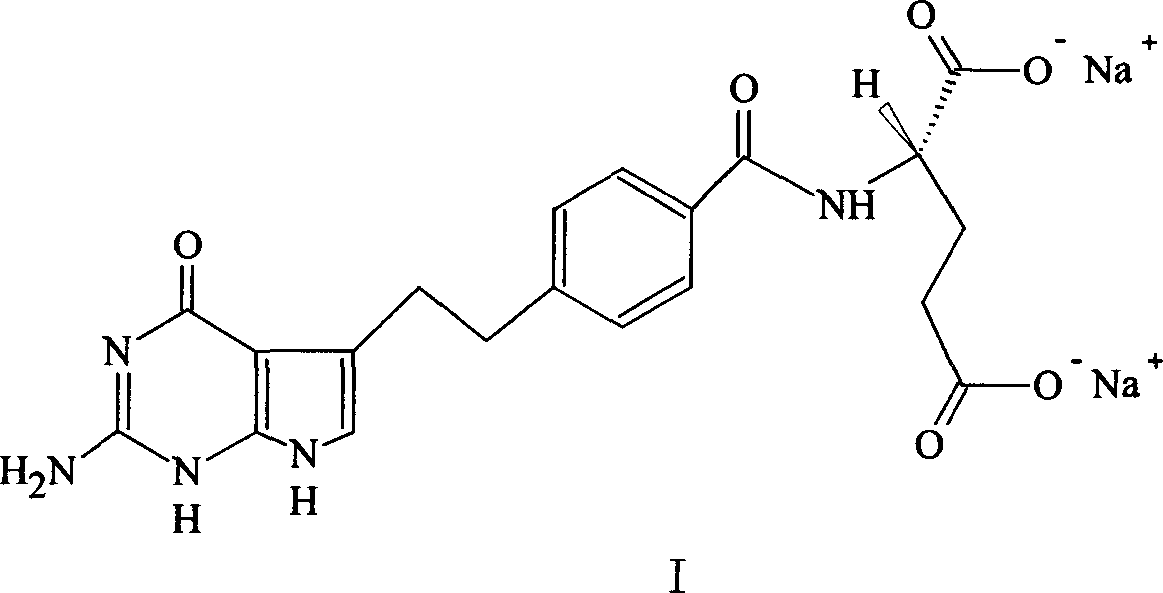
A crystal form of folic acid antagonistic N-(4-(2-(2-amino-4,7-methyl-4-oxo-1H-pyrrolo(2,3-d)pyrimidine-5-radical)ethyl)benzoyl)-L-glutamic acid disodium salt and its production are disclosed.
Owner:重庆凯林制药有限公司 +2
Preparation method for synthesizing apremilast intermediate
ActiveCN104447445AStable and cheapReaction is easy to controlOrganic chemistryOrganic compound preparationPhenyl groupSulfone
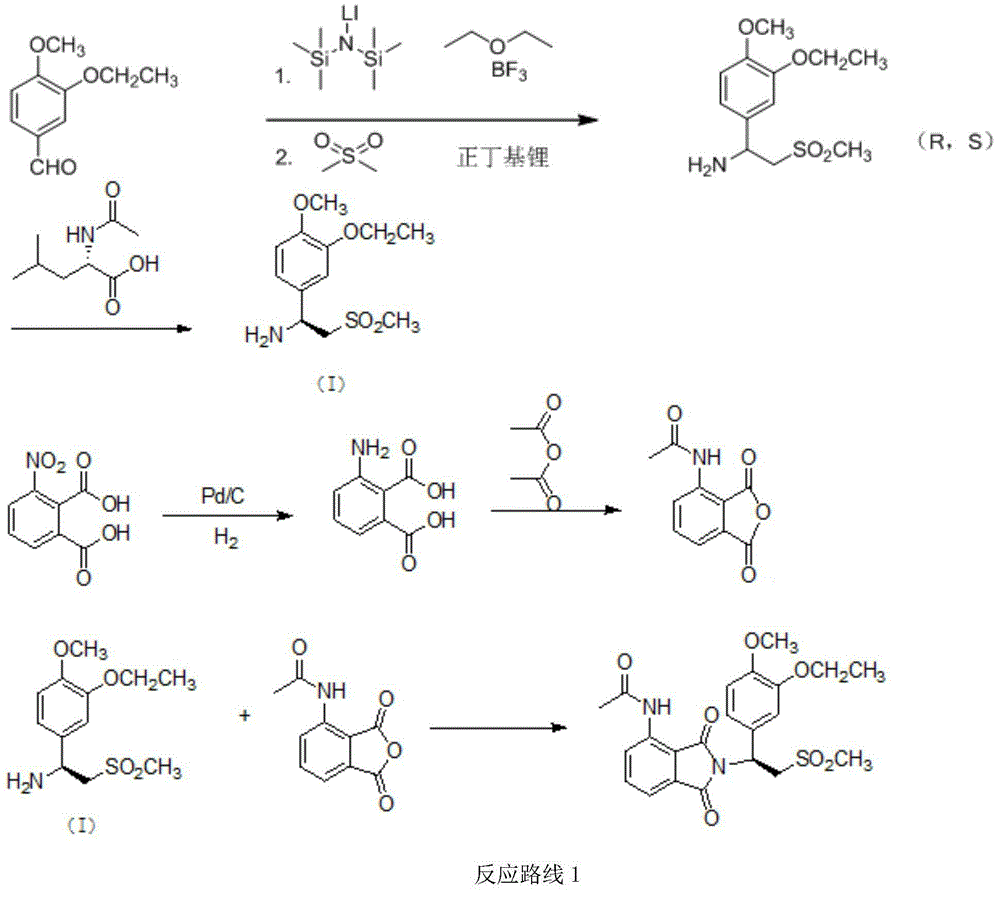
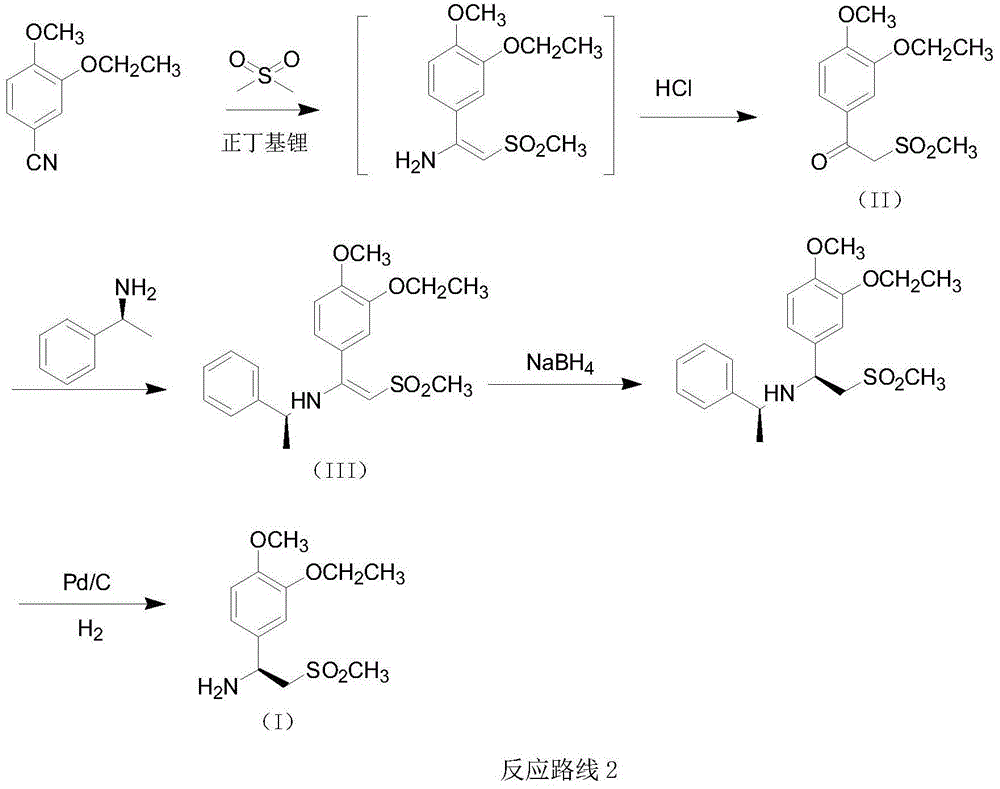
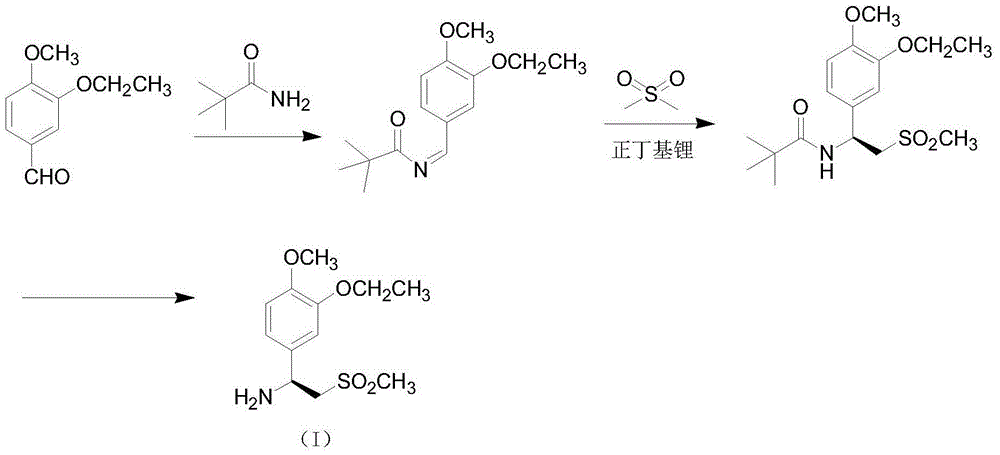
The invention relates to a preparation method for synthesizing an apremilast intermediate. The preparation method comprises the following steps of: carrying out condensation reaction on 3-ethoxyl-4-methoxyl-benzoate and dimethyl sulfone under an alkaline condition to generate 2-(3-ethoxy-4-methoxyphenyl)-1-methylsulfonyl acetone; reacting the compound II and chiral amine in the presence of an acidic catalyst to obtain 1-N-substituted amino-1-(3-ethoxyl-4-methoxyl) phenyl-2-methylsulfonyl ethylene (III), and directly hydrogenating the obtained compound III in the presence of a hydrogenation catalyst without separating the compound III to obtain a product (S)-1-(3-ethoxyl-4-methoxyl) phenyl-2-methanesulfonyl ethylamine (I), namely the apremilast intermediate, wherein the apremilast intermediate can be further prepared into N-acetyl L-leucinate. The invention also provides a preparation method of apremilast. The preparation method disclosed by the invention has the advantages of simple process flow, safety, environmental friendliness and low cost and is favorable to clean industrialized production.
Owner:XINFA PHARMA
Method for synthesizing steroid progestogen
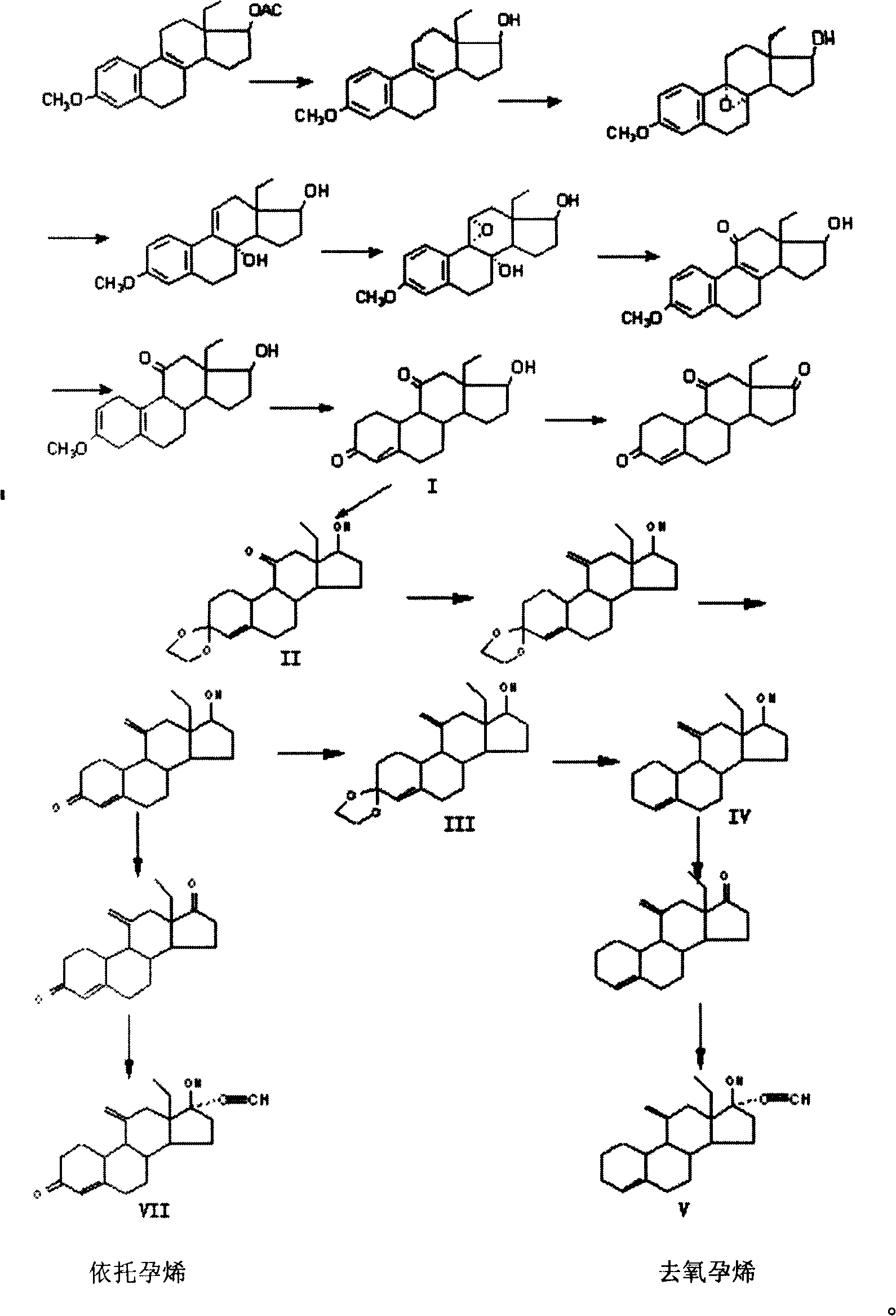
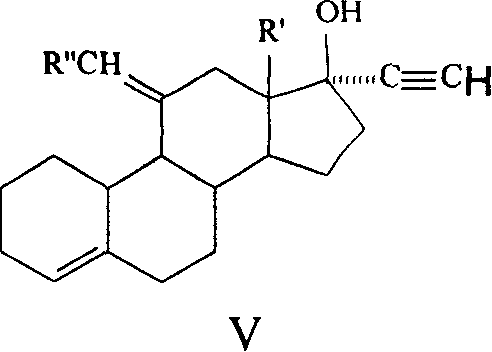
The invention discloses a synthetic method of steroidal gestagen and key medium I, II, III, IV in the drug chemical technological domain, which adopts 13 ethyl- 1,3,5(10),8(9)- estrange tetraenes-17-alcohol as raw material to synthesize deoxidation pregnancy and depending pregnancy.
Owner:JIANGSU CHUANGUO PHARMA CO LTD
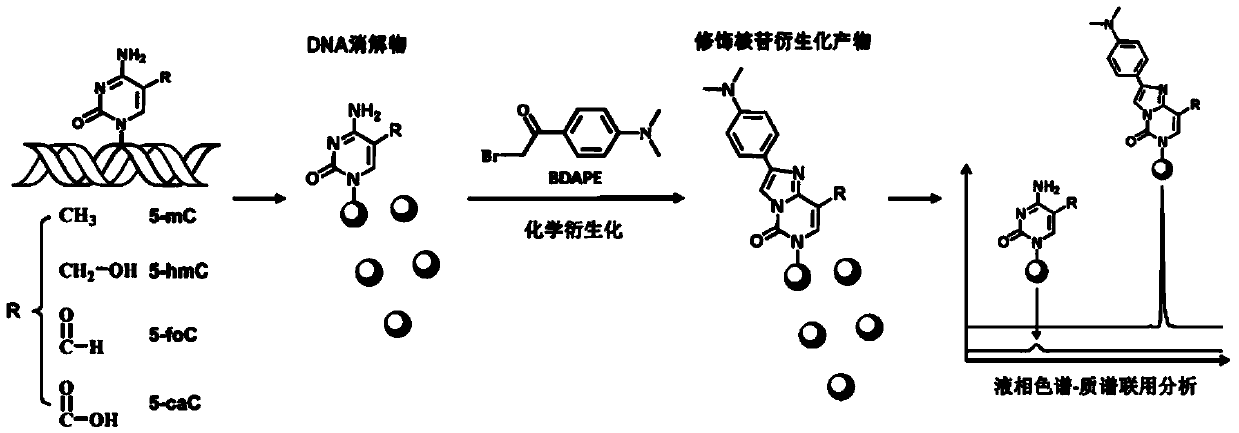
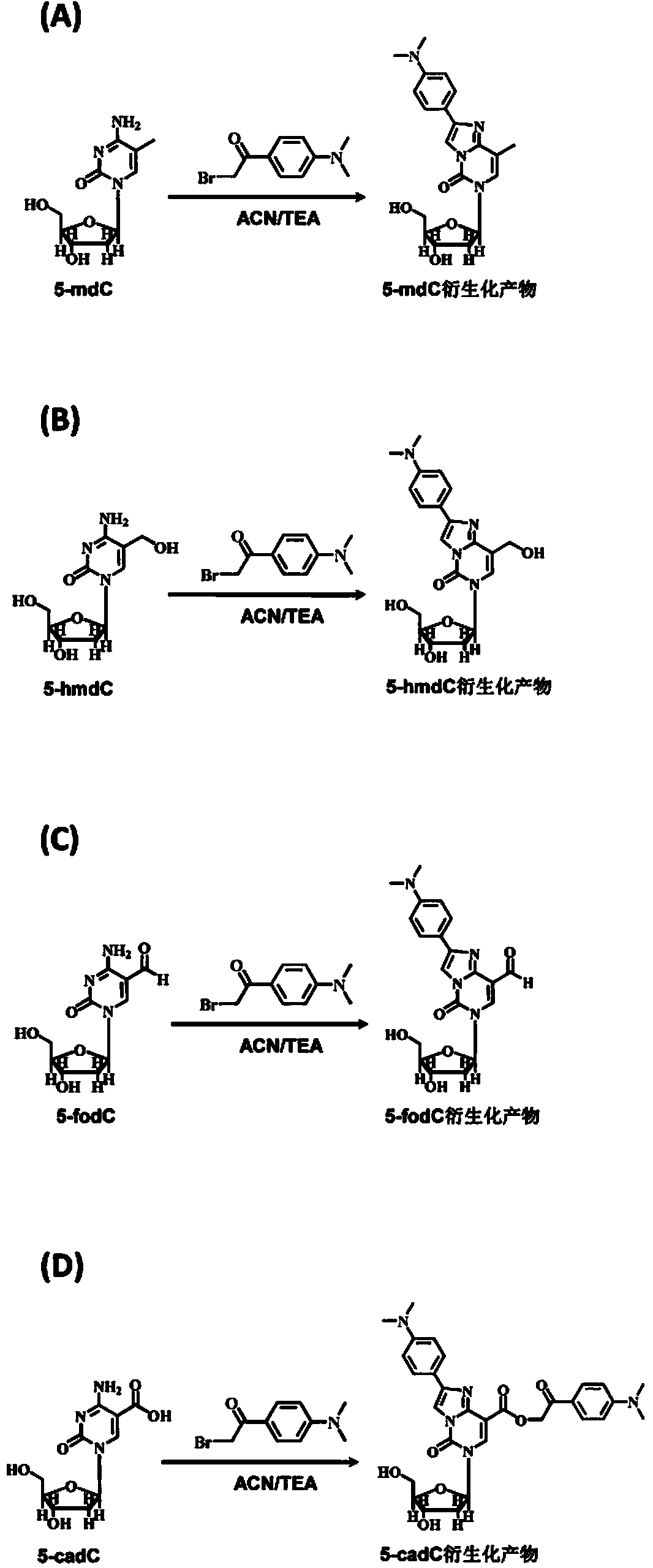
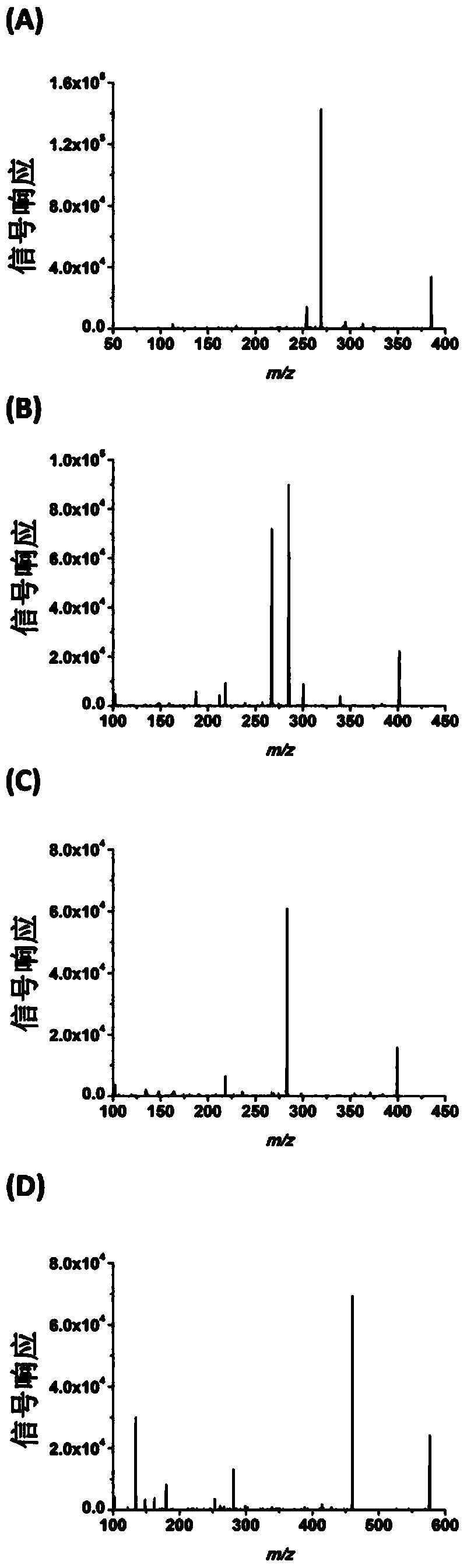
Chemical derivatization method and application thereof to nucleic acid modification detection by liquid chromatogram-mass spectrometer (LC-MS) method
InactiveCN104502492AThe method is easy to operateEasy to separateComponent separationMicrobiological testing/measurementReversed-Phase Liquid ChromatographyKetone
The invention discloses a chemical derivatization method and an application thereof to nucleic acid modification detection by a liquid chromatogram-mass spectrometer (LC-MS) method. A derivatization strategy is performed on 5-methylcystein, 5-hydroxymethylcytosine, 5-aldehyde cytosine and 5-carboxylcytosine in deoxyribonucleic acid (DNA) by means of 2-bromo-1-(4-dimethylamino-phenyl)-ethyl ketone (BDAPE). Retention behaviors of modified nucleosides in reversed phase liquid chromatography are remarkably improved, and meanwhile, the detection sensitivity of the modified nucleosides in mass spectra is greatly improved. The chemical derivatization method has the advantages that the sensitivity and the selectivity are high, the operation is simple and convenient, quantitative detection of cytosine modification with extremely low abundance in the DNA can be achieved without complicated sample pretreatment, and the method can be applied to analysis of diseases and study on mutual relation among the 5-methylcystein, the 5-hydroxymethylcytosine, the 5-aldehyde cytosine and the 5-carboxylcytosine.
Owner:WUHAN UNIV
Preparing method of N-[2-(7- anisyl-1- naphthyl) ethide] acetamide
InactiveCN101759591AMeet the requirements of medicinal valueComply with purityOrganic active ingredientsNervous disorderDehydrogenationAntidepressants drugs
The present invention relates to a preparing method of agomelatine (N-[2-(7- anisyl-1- naphthyl) ethide] acetamide) of melatonin antidepressant drugs of commercial production. Firstly, 7- anisyl-1-tetralone is used as raw material, and is prepared into 2-(1,2,3,4- tetrahydrochysene-1- oxhydryl-7- methoxynaphthalene-1-base) acetonitrile in a cyanophoric way; then, 2-(1,2- dihydro-1-oxhydryl-7- methoxynaphthalene-1-base) acetonitrile is generated in an aromatization dehydrogenation way, and (7-- anisyl-1- naphthyl) acetonitrile is generated in a backflow reaction dehydrogenation way; finally, the agomelatine (N-[2-(7- anisyl-1- naphthyl) ethide] acetamide) is generated by reducing and acetylizing in a one-kettle way. The present invention has the advantages of high product purity, friendly environment, convenient operation and low cost, and is suitable for the commercial production.
Owner:ZHEJIANG HUAHAI PHARMA CO LTD
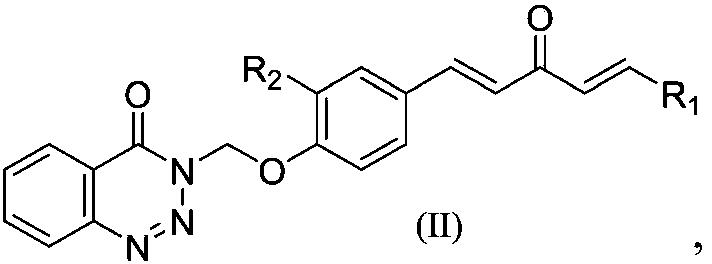
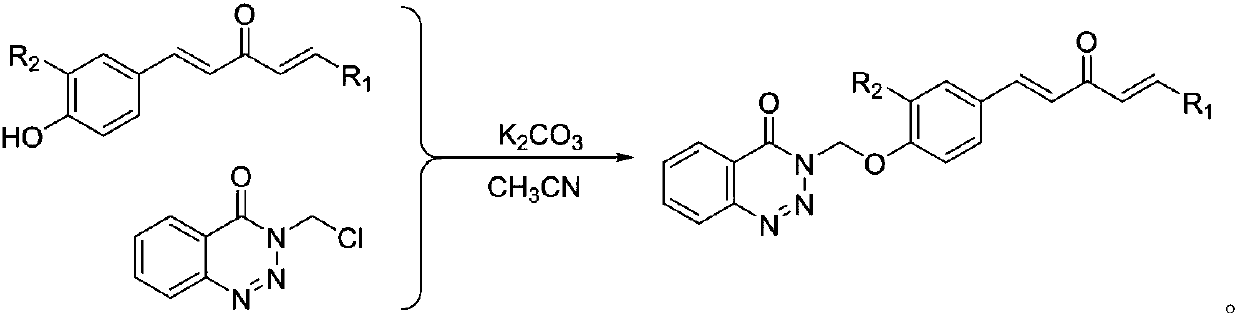
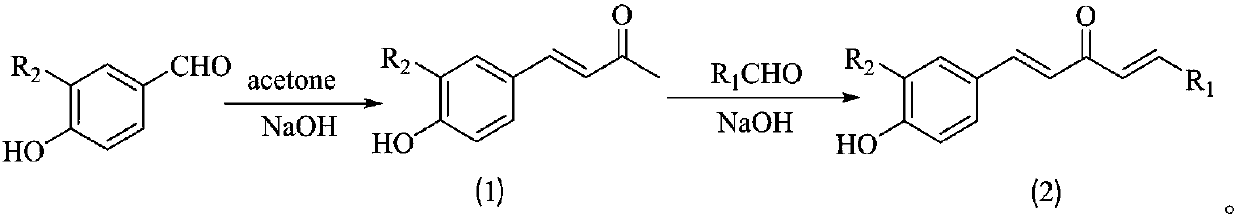
1,4-pentadiene-3-ketone derivative containing benzotriazinone as well as preparation method and application of 1,4-pentadiene-3-ketone derivative
ActiveCN107602493AGood inhibitory effectEnhanced inhibitory effectBiocideOrganic chemistryBenzeneThiazole
The invention discloses a 1,4-pentadiene-3-ketone derivative containing benzotriazinone. The 1,4-pentadiene-3-ketone derivative containing benzotriazinone is characterized by having the general formula as shown in the specification, wherein R1 is phenyl, substituted phenyl (p-fluorophenyl, p-chlorophenyl, o-chlorophenyl, 2-methyoxyphenyl, 4-methyoxyphenyl, 4-methylphenyl, 2,4-dimethyoxyphenyl, 3,4-dimethyoxyphenyl, cinnamaldehyde group, 3-nitrophenyl, 2-chloro-5-nitrophenyl and the like), heterocyclic group (furyl, thienyl, 2-pyridyl, 3-pyridyl, 4-pyridyl, pyrryl and the like) or substituted aromatic heterocyclic group (5-methyl thiazole, 5-methyl-2-thienyl, 4-bromo-2-thienyl and the like), and R2 is a hydrogen atom, methyl (ethyl), methoxyl (ethyoxyl) and the like. The compound disclosedby the invention has relatively high inhibiting activity for citrus canker bacteria, ralstonia solanacearum and tobacco mosaic viruses so as to be used for preparing an agricultural bactericide and anantiviral agent.
Owner:GUIZHOU UNIV
Tsukamurella-tyrosinosolvens and application thereof in catalysis preparation of (S) -alpha - ethyl -2-oxo-1-pyrrolidine acetic acid prepared by catalysis
ActiveCN101748087AHigh stereoselectivityBacteriaMicroorganism based processesTsukamurellaBioconversion
The present invention provides a novel strain, tsukamurella-tyrosinosolvens E105, and an application thereof in the chiral biocatalysis preparation of (S)-alpha-ethyl-2-oxo-1-pyrrolidine acetic acid. The tsukamurella-tyrosinosolvens E105 is stored in China Center for Type Culture Collection (CCTCC) at Wuhan University of Science and Technology (postal code: 430072) on Dec.16th, 2009. The preservation series number is CCTCC NO:M209306. The present invention adopts a microorganism preparation method of (S)-alpha-ethyl-2-oxo-1-pyrrolidine acetic acid for the first time and provides a novel strain of high stereoselectivity and capability of preparing (S)-alpha-ethyl-2-oxo-1-pyrrolidine acetic acid of high optical purity. The present invention can make the optical purity of the target product (S)-alpha-ethyl-2-oxo-1-pyrrolidine acetic acid prepared by the chiral biocatalysis of tsukamurella-tyrosinosolvens E105 cells reach 99.4%, and make productivity reach 48.1%. The novel strain obtained by screening provides helpful references in the aspects of research in the microorganism preparation of the key chiral intermediate of levetiracetam and the technique optimization of bioconversion.
Owner:ZHEJIANG UNIV OF TECH
Amorphous form of tetracyclic compound
An amorphous form of 9-ethyl-6,6-dimethyl-8-(4-morpholin-4-yl-piperidin-1-yl)-11-oxo-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile and a solid dispersion containing the amorphous form can be used extremely advantageously as drugs for oral administration.
Owner:CHUGAI PHARMA CO LTD
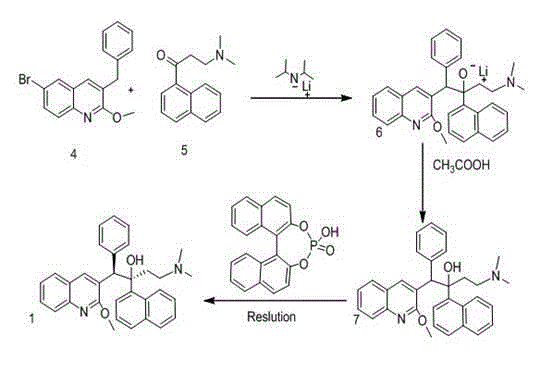
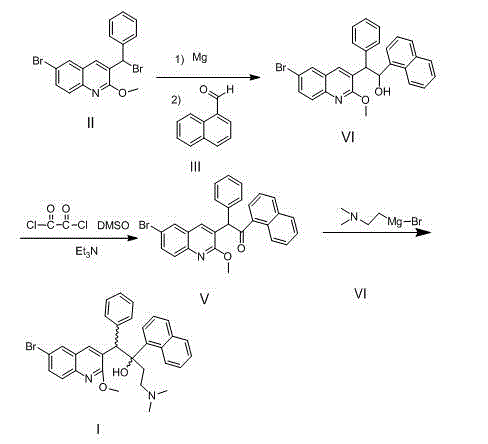
New synthesis route and method of bedaquiline racemate
The invention relates to a new synthesis route and method of a bedaquiline racemate, and belongs to the medical technical field. The method comprises the steps: (1) carrying out a reaction of a starting material 3-bromobenzyl-6-bromo-2-methoxyquinoline (II) with metal magnesium in THF to prepare a Grignard reagent, then adding 1-naphthaldehyde (III) in the prepared Grignard reagent, carrying out a reflux reaction to obtain a compound (IV) 2-(6-bromo-2-methoxyquinoline-3-yl)-1-(naphthalen-1-yl)-2-phenylethanol; (2) undergoing swern oxidation of the compound (IV) to obtain a carbonyl compound 2-(6-bromo-2-methoxyquinoline-3-yl)-1-(naphthalen-1-yl)-2-phenyl ethyl ketone (a compound V); and (3) carrying out a Grignard reaction of the compound (V) with the Grignard reagent (VI) to obtain the bedaquiline racemate (I). The method is simple in route, is mild in reaction conditions, low in cost, high in yield, and suitable for industrialized production application.
Owner:CHONGQING SHENGHUAXI PHARMA CO LTD +1
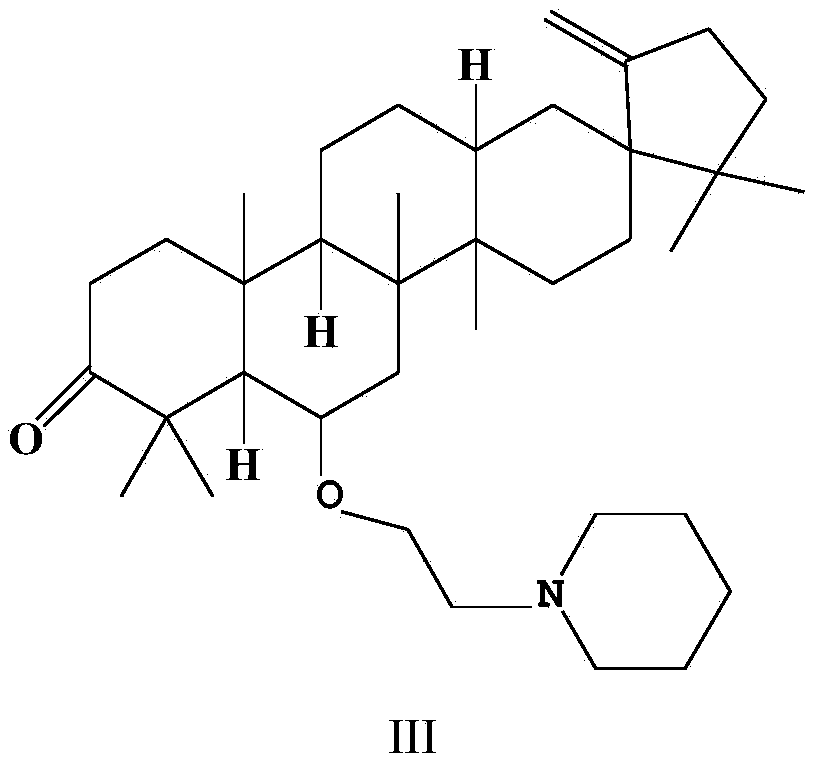
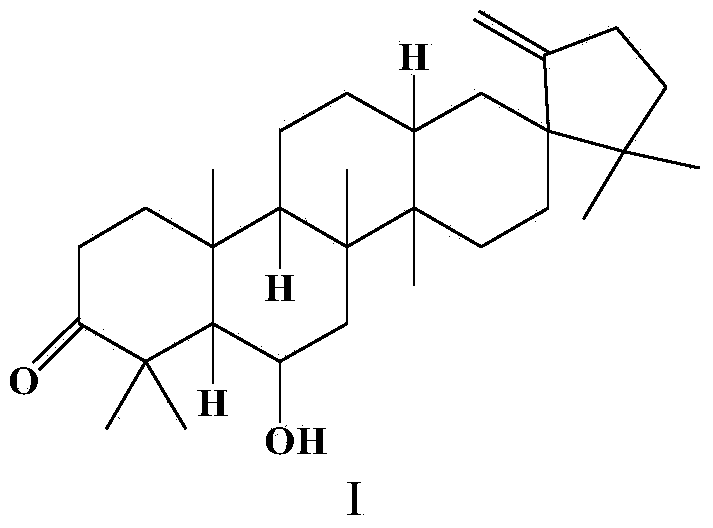
Application of O-(piperidinyl) ethyl derivative of Cleistanone in preparing drugs for resisting liver fibrosis
ActiveCN104095859AStrong anti-fibroblast proliferative activityStrong proliferative activityOrganic active ingredientsDigestive systemCleistanoneLiver fibrosis
The invention relates to the field of organic synthesis and medicinal chemistry and particularly to an O-(piperidinyl) ethyl derivative of Cleistanone, a preparation method for the O-(piperidinyl) ethyl derivative of the Cleistanone and the application of the O-(piperidinyl) ethyl derivative of the Cleistanone in preparing drugs for resisting liver fibrosis. The invention synthesizes a novel O-(piperidinyl) ethyl derivative of the Cleistanone and discloses the preparation method thereof. Pharmacological experiment results show that the O-(piperidinyl) ethyl derivative of the Cleistanone has the function of resisting liver fibrosis and has the value of developing drugs for resisting liver fibrosis.
Owner:徐州乐源牧业有限公司
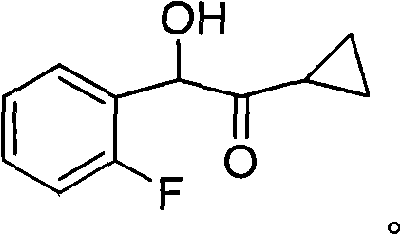
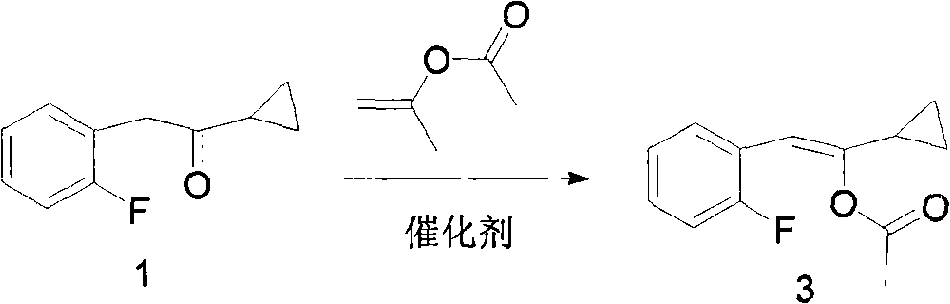
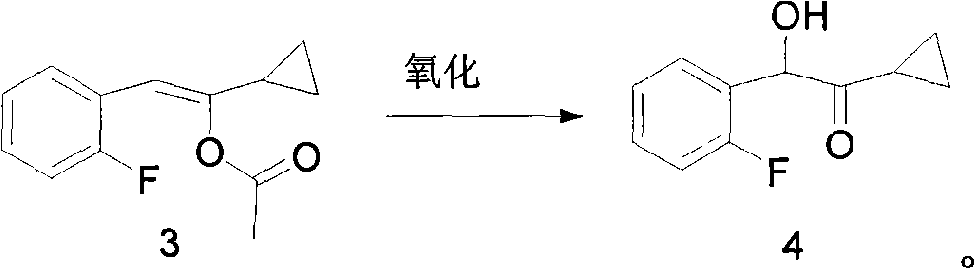
New compound 1-cyclopropyl-2-(2-fluorine phenyl)-2-hydroxyl ethanone, preparation method and application thereof
ActiveCN101402556AThree wastes lessEasy to operateOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsAntithrombotic AgentPharmaceutical drug
The invention relates to the technical field of an intermediate of an antithrombotic Prasugrel and a preparation method thereof. The intermediate is a compound of 1-cyclopropyl-2-(2- fluobenzene radical)-2-hydroxy butanone. Compared with the prior art, the method for preparing the Prasugrel by the intermediate has the advantages that the method adopts raw materials and agents which are easily obtained with low toxicity and price, and generates less three wastes; moreover, the operation is simple and the yield is higher. The method is suitable for industrialized production.
Owner:SHANGHAI SHYNDEC PHARMA CO LTD +1
Method for preparing acetosyringone and vanillyl ethyl ketone by oxidizing lignin
InactiveCN102295547AEfficient use ofHarm reductionCarbonyl compound preparation by oxidationGas liquid chromatographicNitrobenzene
The invention discloses a method for preparing acetovanillone and acetosyringone (AS) through oxidation of lignin by using an oxidizing agent. According to the method, an oxidizing agent reacts with the lignin in an alkaline solution; the resulting reaction solution is subjected to acidification, extraction and concentration to obtain the crude product after completing the reaction; the treatments of recrystallization and rectification are performed to obtain the acetovanillone and the AS. The oxidizing agent is the one selected from p-nitrobenzoic acid, 3,5-dinitrobenzoic acid, 3-nitrosalicylic acid, 5-nitrosalicylic acid or 3,5-dinitrosalicylic acid. According to the present invention, the oxidizing agent has low toxicity, such that the harm to the environment can be reduced; the post-treatment steps are simplified, and the uses of the organic solvents are reduced, such that the secondary pollution to the environment is reduced; the yield is relatively high, the purities of the products are respectively 97.3% and 98.2% through the gas chromatography analysis; the two important chemical raw materials of guaiacol and lilac alcohol can be synchronously obtained when preparing the acetovanillone and the AS.
Owner:INST OF CHEM IND OF FOREST PROD CHINESE ACAD OF FORESTRY
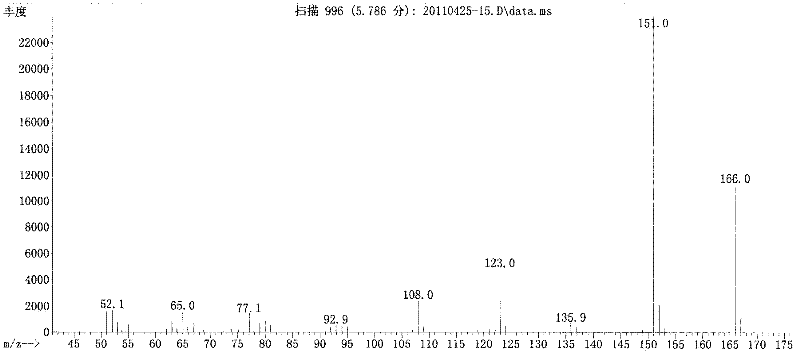
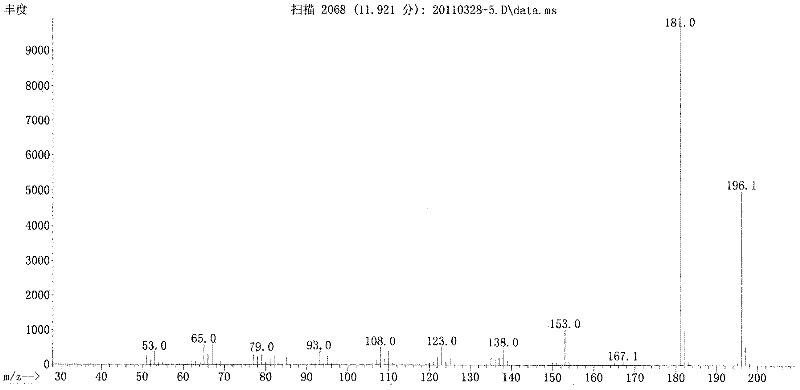
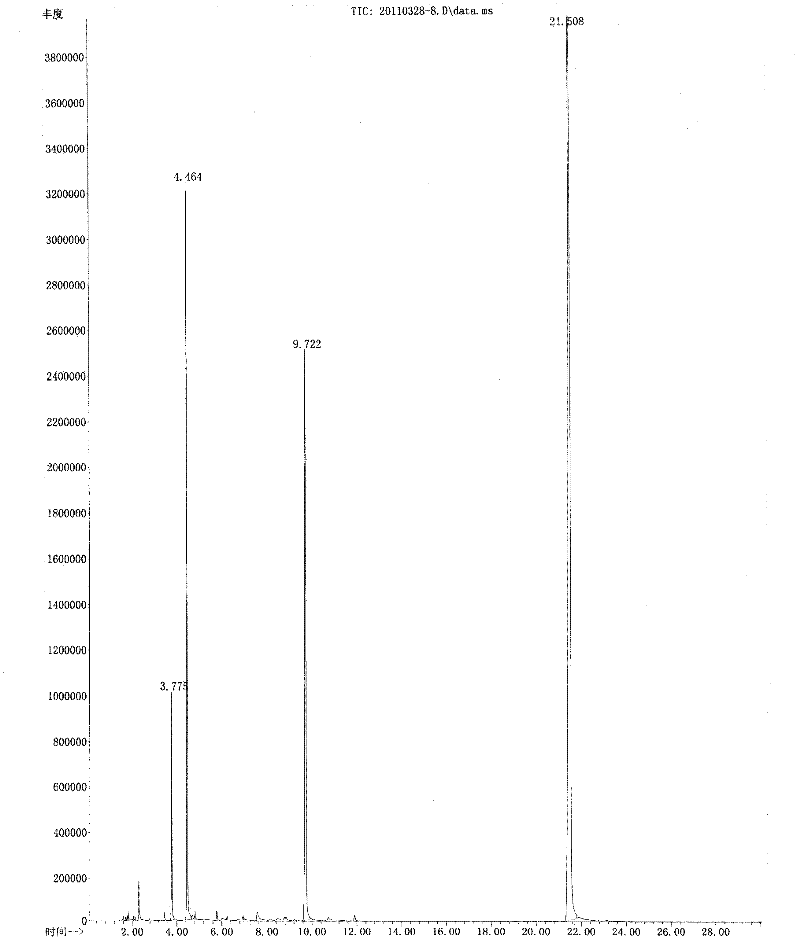
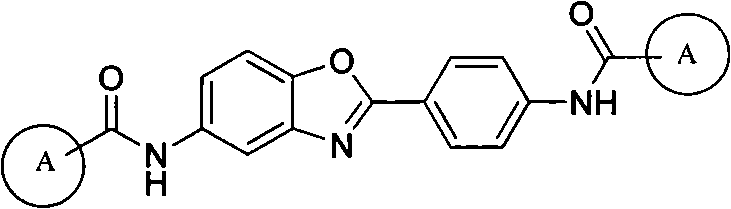
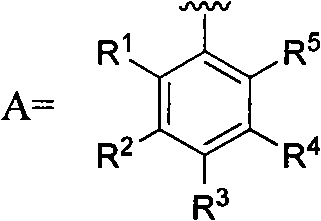
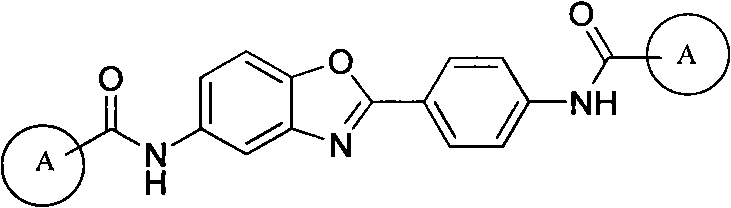
5-substituted-2-(4-substituted phenyl)benzoxazole derivatives and preparation method and application thereof
The invention provides 5-substituted-2-(4-substituted phenyl)benzoxazole derivatives. A general structural formula thereof is as follows: when A is Ar, wherein R1 is H, F, Cl, Br, I, NO2, OMe or Me; R2 is H, F, Cl, Br, I, NO2, OMe or Me; R3 is H, F, Cl, Br, I, NO2, NH2, Me, Et, i-Pr, t-But or OMe; R4 is H, F, Cl, Br, I, NO2, OMe or Me; R5 is H, F, Cl, Br, I, NO2, OMe or Me; and R6 is H, F, Cl, Br, I, NO2, OMe or Me. When A is R, A is methyl, ethyl, propyl, chloromethyl, bromomethyl, 2-chloroethyl or 2-bromoethyl. The invention also relates to a method for preparing the compounds and application of the compounds when taken as an influenza virus inhibitor.
Owner:SHANDONG UNIV
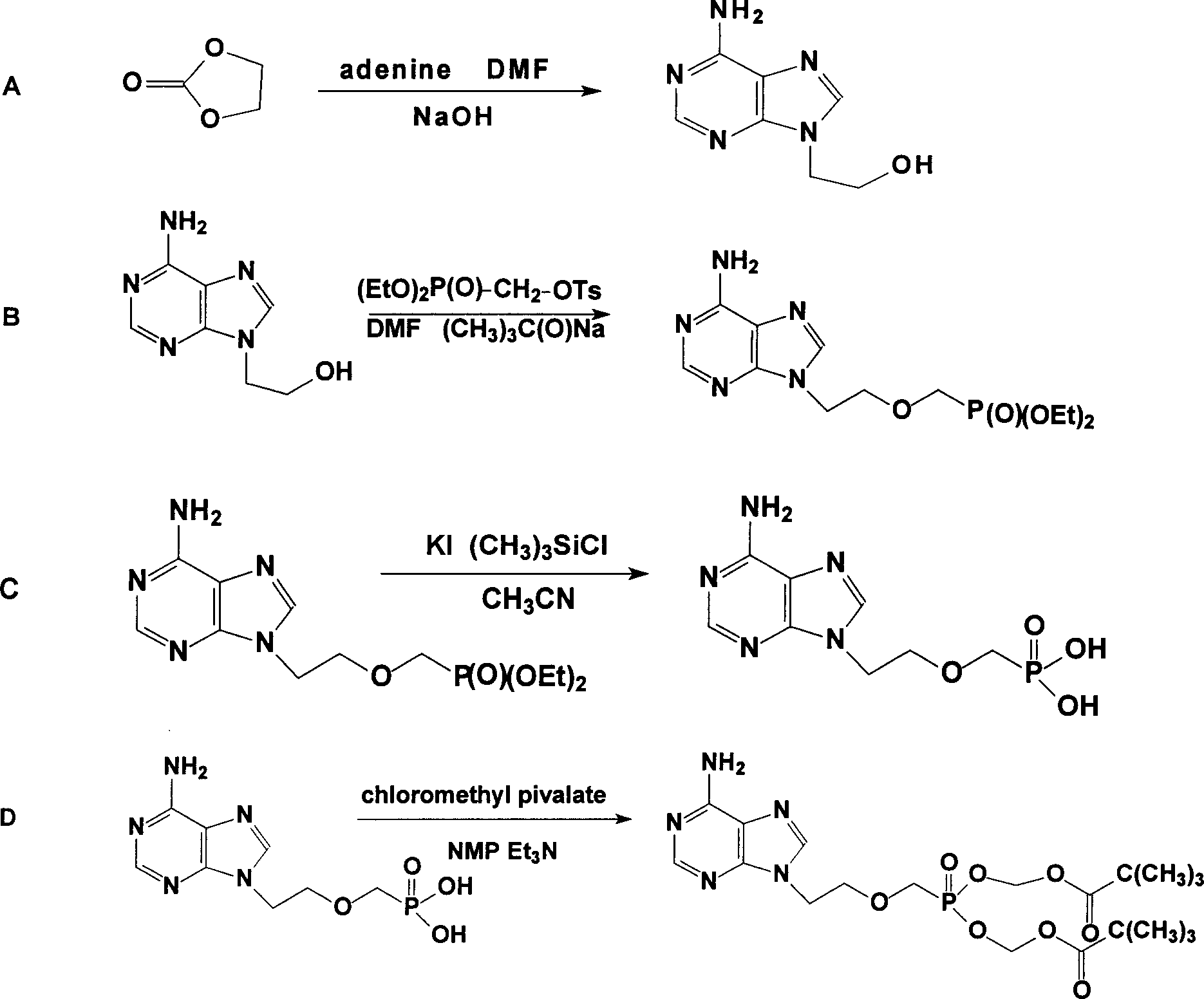
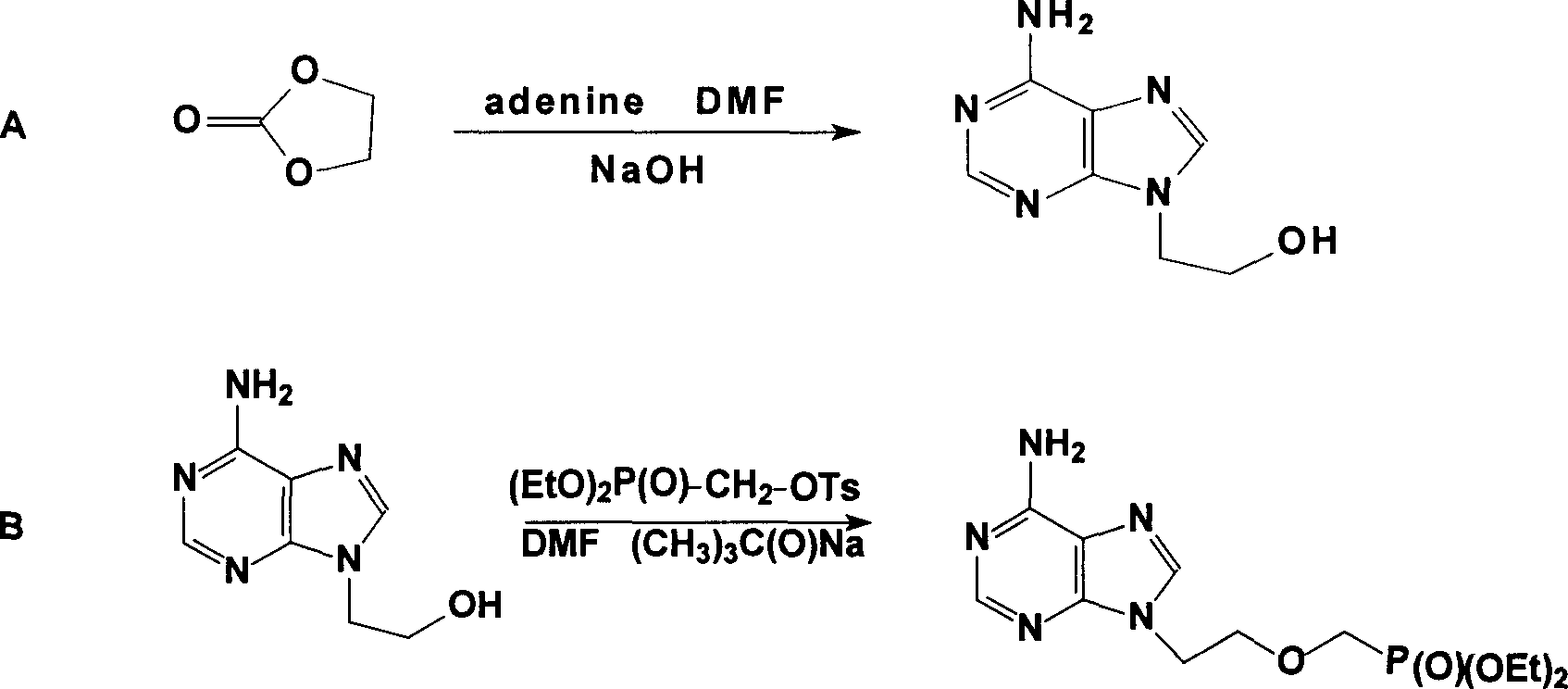
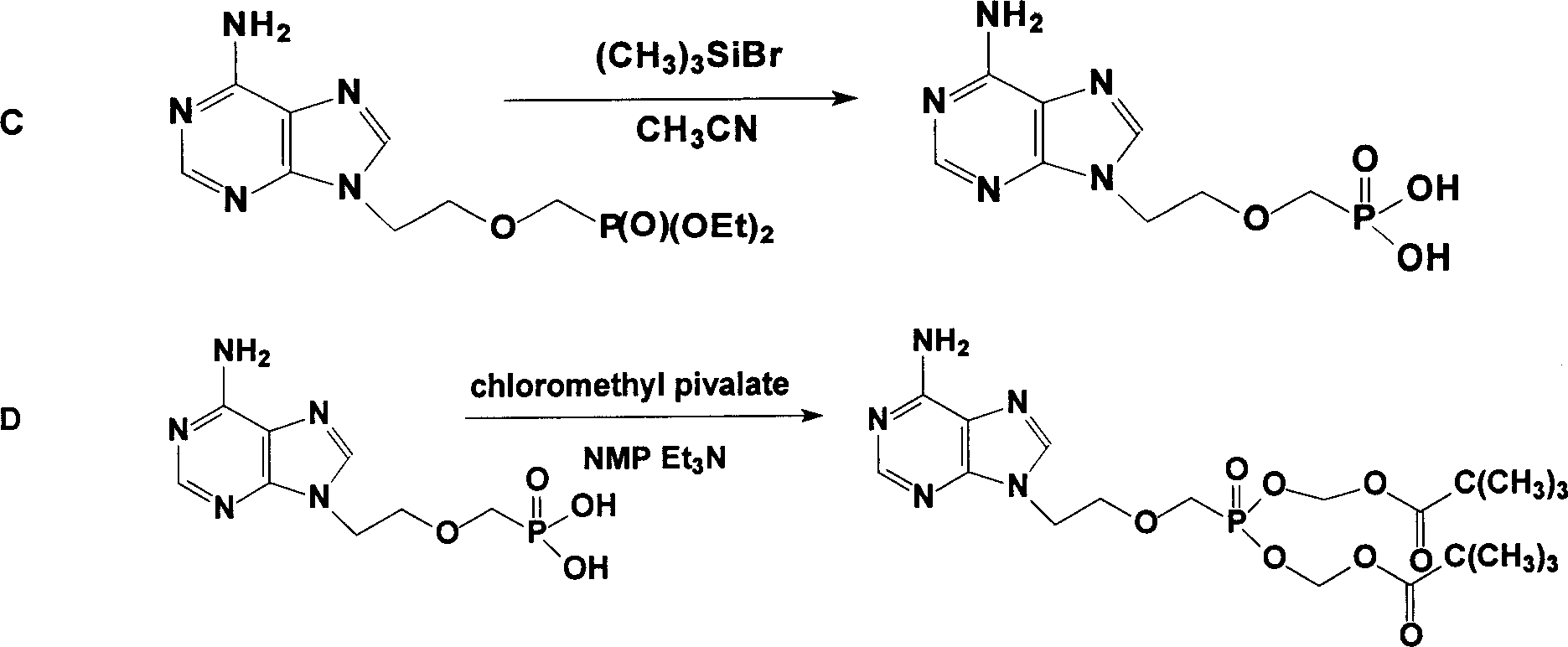
Prepn process of Adefovir dipivalate
InactiveCN1506370ASuitable for industrializationReduce manufacturing costGroup 5/15 element organic compoundsAntiviralsTrimethylsilyl chloridePurine
The present invention discloses one improved preparation process of Adefovir dipivalate. In the synthesis of the mother compound 9-[2-(phosphinocarboxyl methoxy) ethyl adenine, trimethyl chlorsilane, rather than expensive trimethyl bromosilane, as material and potassium iodide as nucleophilic reagentare used to reduce cost while the product yield and quality is maintained. The production process of the present invention may be used in industrial production.
Owner:HANGZHOU HUADONG MEDICINE GRP PHARMA RES INST
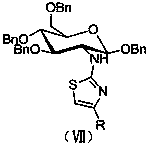
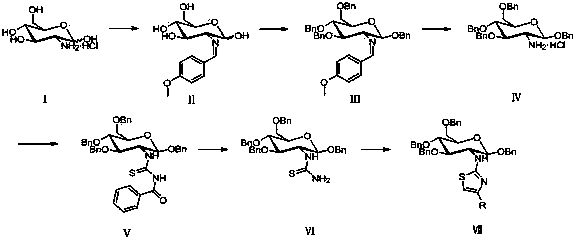
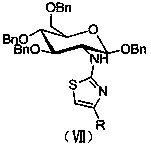
Amino sugar thiazole derivative as well as synthetic method and application thereof
ActiveCN104292280AImprove biological activityHigh activityNervous disorderSugar derivativesThioureaKetone
The invention discloses an amino sugar thiazole derivative as well as a synthetic method thereof. When synthesis is carried out, glucosamine hydrochloride is taken as a raw material, and benzyl ether protection is carried out on hydroxy, so that the selective reaction of amino is realized, a novel intermediate glycosyl thiourea-N-(1,3,4,6-tetra-0-benzyl-2-deoxygenation- beta-D-glucopyranose-2-group) thiocarbamide is synthesized, and glycosyl thiazole-N-(1,3,4,6-tetra-0-benzyl-beta-D-glucopyranose-2-group)-2-amido-4-substituted thiazole is synthesized by cyclizing the novel intermediate glycosyl thiourea-N-(1,3,4,6-tetra-0-benzyl-2-deoxygenation beta-D-glucopyranose-2-group) thiocarbamide with 1-bromine-2-substituted ethyl ketone. The synthetic method disclosed by the invention has the advantages of easiness and safety for operation, wide application scope, low cost of the raw material, easiness for raw material obtaining, easiness and convenience for post-processing and high yield, is a fast high-efficiency synthetic method and has a wide application prospect on the aspect of preparing an acetylcholinesterase resistant drug because the synthesized compound has high inhibiting effect on acetylcholinesterase.
Owner:HUAIHAI INST OF TECH
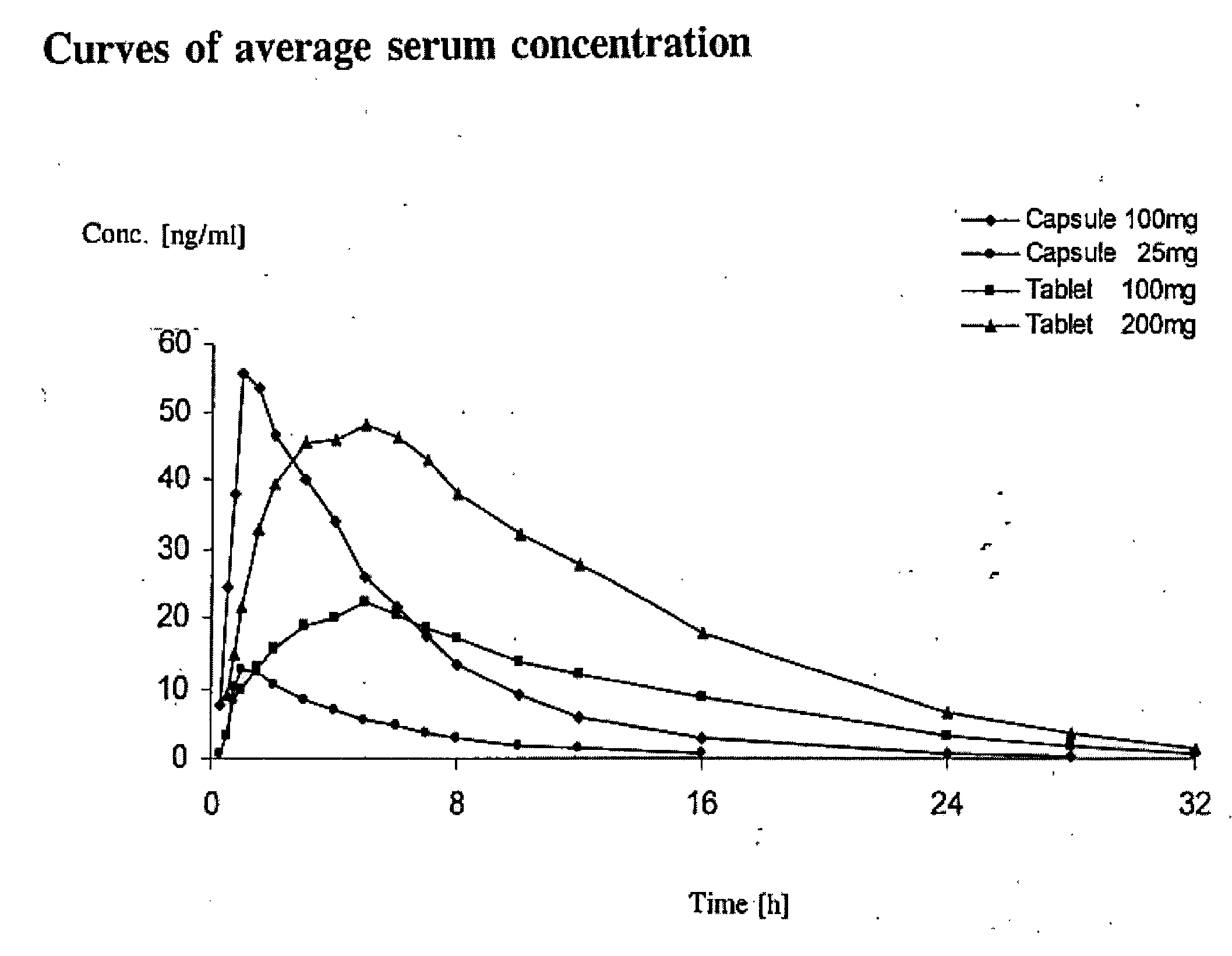
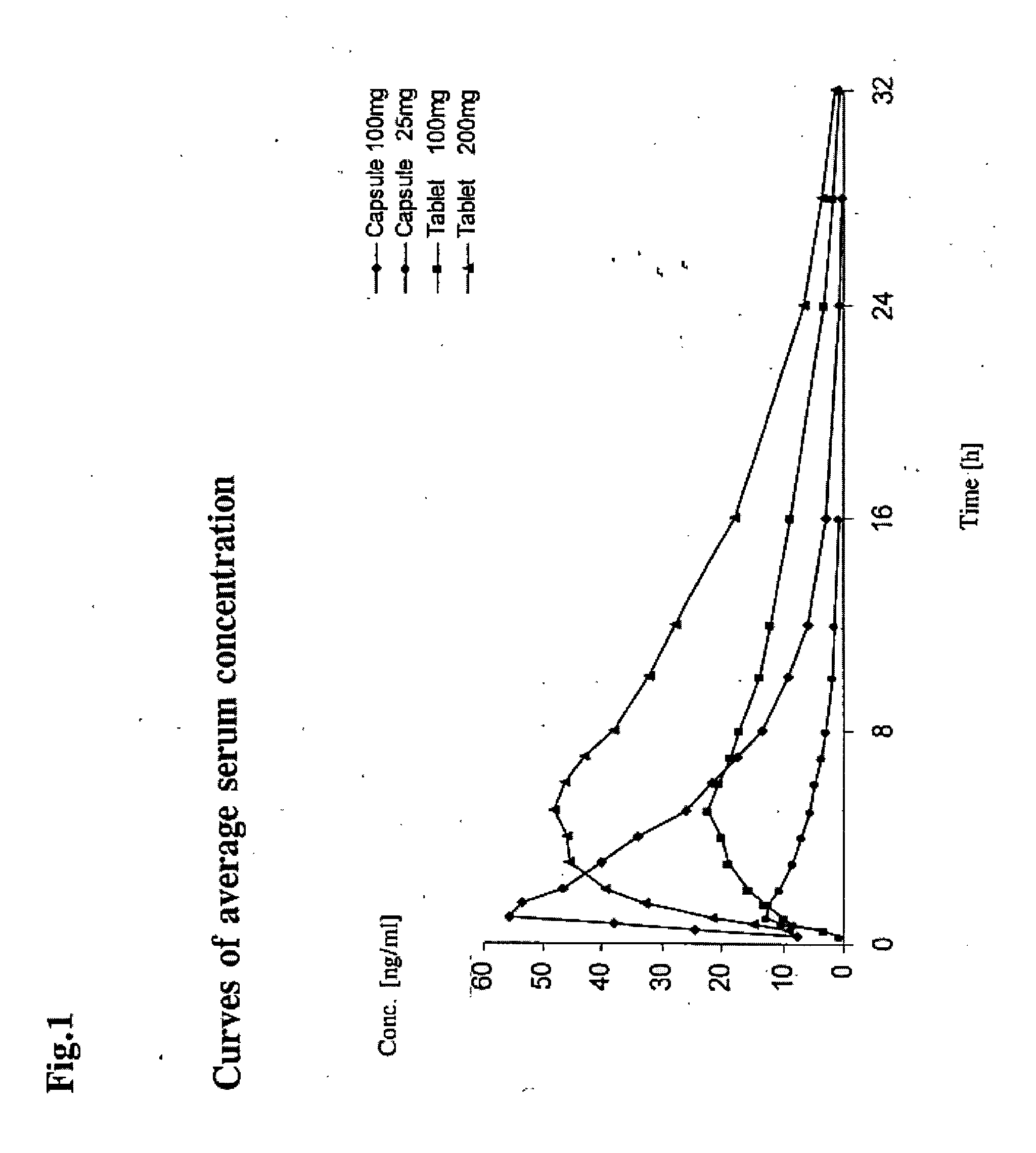
Sustained-release preparation containing 5-acetyl-4,6-dimethyl-2[2-[4-(2-methoxyphenyl) piperazinyl]ethylamino] pyrimidine trihydrochloride as active ingredient
InactiveUS7041317B2Not cause periodical deterioration and coloringOrganic active ingredientsUrinary disorderWater insolubleBULK ACTIVE INGREDIENT
A sustained-release formulation of 5-acetyl-4,6-dimethyl-2-[2-[4-(2-methoxyphenyl)piperazinyl]ethylamino]pyrimidine trihydrochloride coated with a release-controlling film comprising a water-insoluble polymer film having no hydrophilic group. The formulation of the present invention has such a release pattern that the drug release lasts for 20 hours or more, so that can be appropriately administered for treatment. Furthermore, the formulation itself is so stable that its release pattern does not change with pH and the formulation does not suffer from deterioration, coloration and the like with the lapse of time.
Owner:ONO PHARMA CO LTD
Preparation of venlafaxine intermediate 1-[2-amino-1-(4-methoxy phenyl)ethyl] cyclohexanol
InactiveCN101503365ASuitable for industrial productionMild reaction conditionsNervous disorderMetabolism disorderPotassium borohydrideVenlafaxine
The invention discloses a method for preparing venlafaxine intermediate 1, (2- amino-1-(4- methoxyphenyl) ethide cyclohexanol. The method is characterized in that 1- cyano-((4- methoxyphenyl) methyl) cyclohexanol is deacidized by sodium borohydride or potassium borohydride under the catalysis of elemental iodine to obtain the 1, (2- amino-1-(4- methoxyphenyl) ethide cyclohexanol. The method has the advantages of mild reaction condition, simple post treatment, high yield, good product purity and low cost, and is suitable for commercial process.
Owner:CHENGDU QIAOFENG TECH DEV
Prolonged Release Pharmaceutical Composition Containing 3-(3-Dimethylamino-1-Ethyl-2-Methyl-Propyl)Phenol
InactiveUS20120034304A1Reduce releaseRelieve painOrganic active ingredientsBiocideHydrophobic polymerProlonged release
A pharmaceutical formulation for prolonged release of the active ingredient 3-(3-dimethylamino-1-ethyl-2-methylpropyl)phenol or a pharmaceutically acceptable salt thereof in a matrix containing between 1 and 80 wt. % of at least one pharmaceutically acceptable hydrophilic or hydrophobic polymer as a matrix forming agent and exhibiting in vivo the following release rate: 3 to 35% by weight (based on 100% by weight active ingredient) 3-(3-dimethylamino-1-ethyl-2-methyl-propyl)phenol released after 0.5 hours; 5 to 50% by weight 3-(3-dimethylamino-1-ethyl-2-methyl-propyl)phenol released after 1 hour; 10 to 75% by weight 3-(3-dimethylamino-1-ethyl-2-methyl-propyl)phenol released after 2 hours; 15 to 82% by weight 3-(3-dimethylamino-1-ethyl-2-methyl-propyl)phenol released after 3 hours; 30 to 97% by weight 3-(3-dimethylamino-1-ethyl-2-methyl-propyl)phenol released after 6 hours; more than 50% by weight 3-(3-dimethylamino-1-ethyl-2-methyl-propyl)phenol released after 12 hours; more than 70% by weight 3-(3-dimethylamino-1-ethyl-2-methyl-propyl)phenol released after 18 hours, and more than 80% by weight 3-(3-dimethylamino-1-ethyl-2-methyl-propyl)phenol released after 24 hours.
Owner:GRUNENTHAL GMBH
Preparation method of 3,4,5-trisubstituted 1,2,4-triazole compound
ActiveCN113105402AStrong designabilityWide range of toleranceOrganic chemistryAgainst vector-borne diseasesArylPtru catalyst
The invention discloses a preparation method of a 3,4,5-trisubstituted 1,2,4-triazole compound. The preparation method comprises the following steps: adding aryl ethanone and iodine into dimethyl sulfoxide, conducting heating to 90-110 DEG C, performing reacting for 4-6 hours, adding iodine, sodium dihydrogen phosphate, pyridine and trifluoroethyl imine hydrazide into the organic solution, carrying out heating to 110-130 DEG C, performing reacting for 12-20 hours, and after the reaction is completed, carrying out post-treatment to obtain the 3,4,5-trisubstituted 1,2,4-triazole compound. The preparation method is simple to operate, the initial raw materials are cheap and easy to obtain, the reaction does not need to be carried out under anhydrous and anaerobic conditions, heavy metal does not need to be used as a catalyst, the reaction can be easily expanded to gram level, operation is convenient, and the applicability of the method is widened.
Owner:ZHEJIANG SCI-TECH UNIV
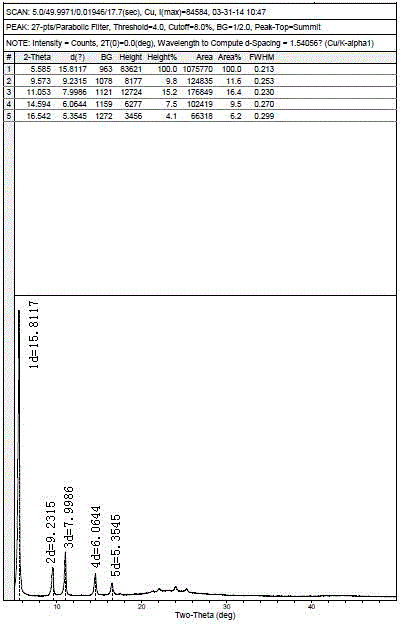
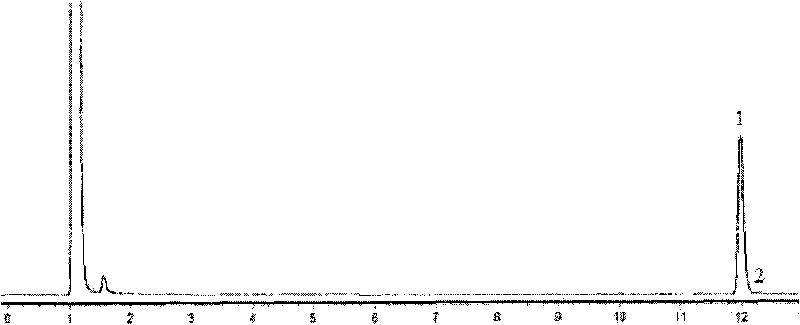
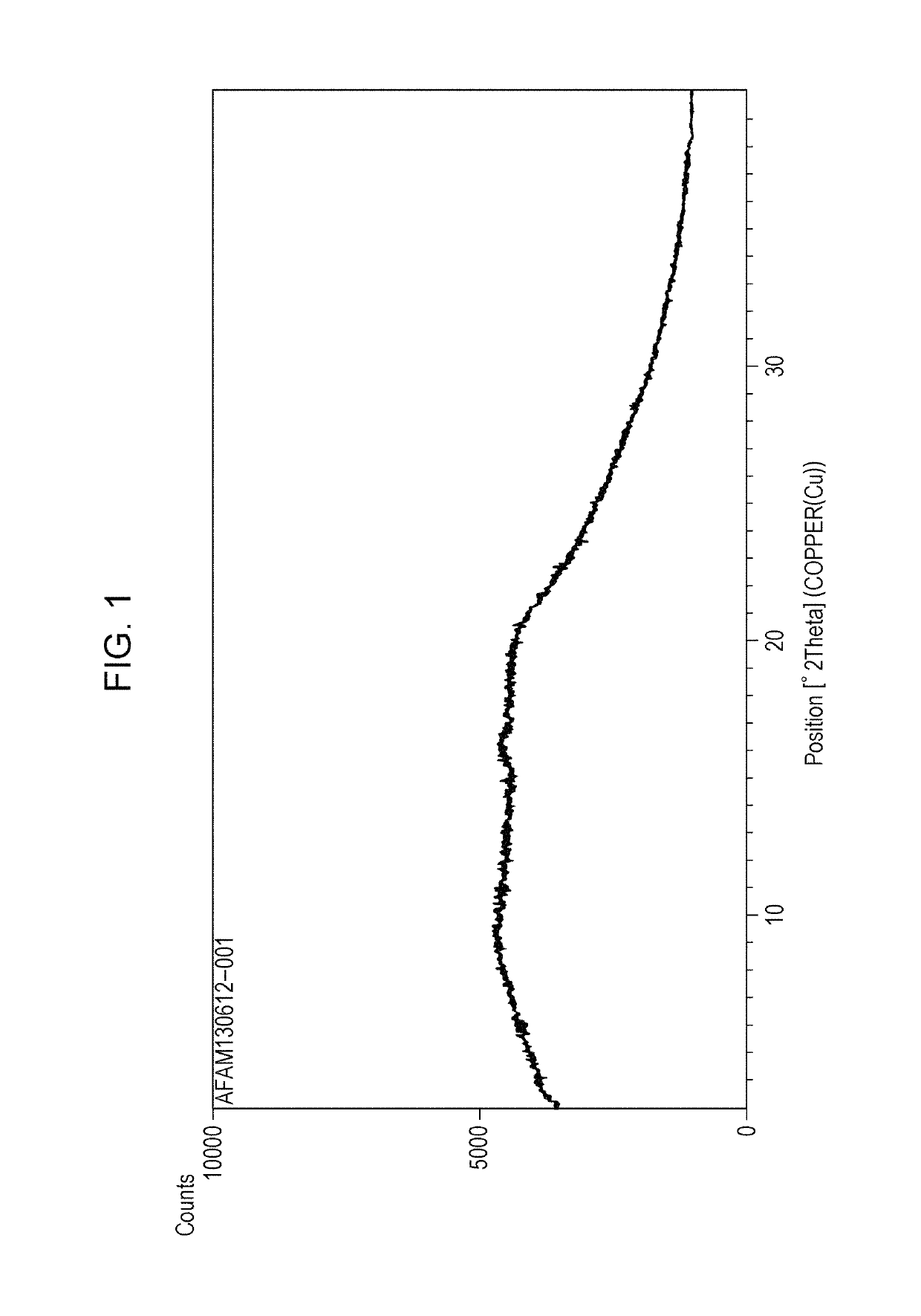
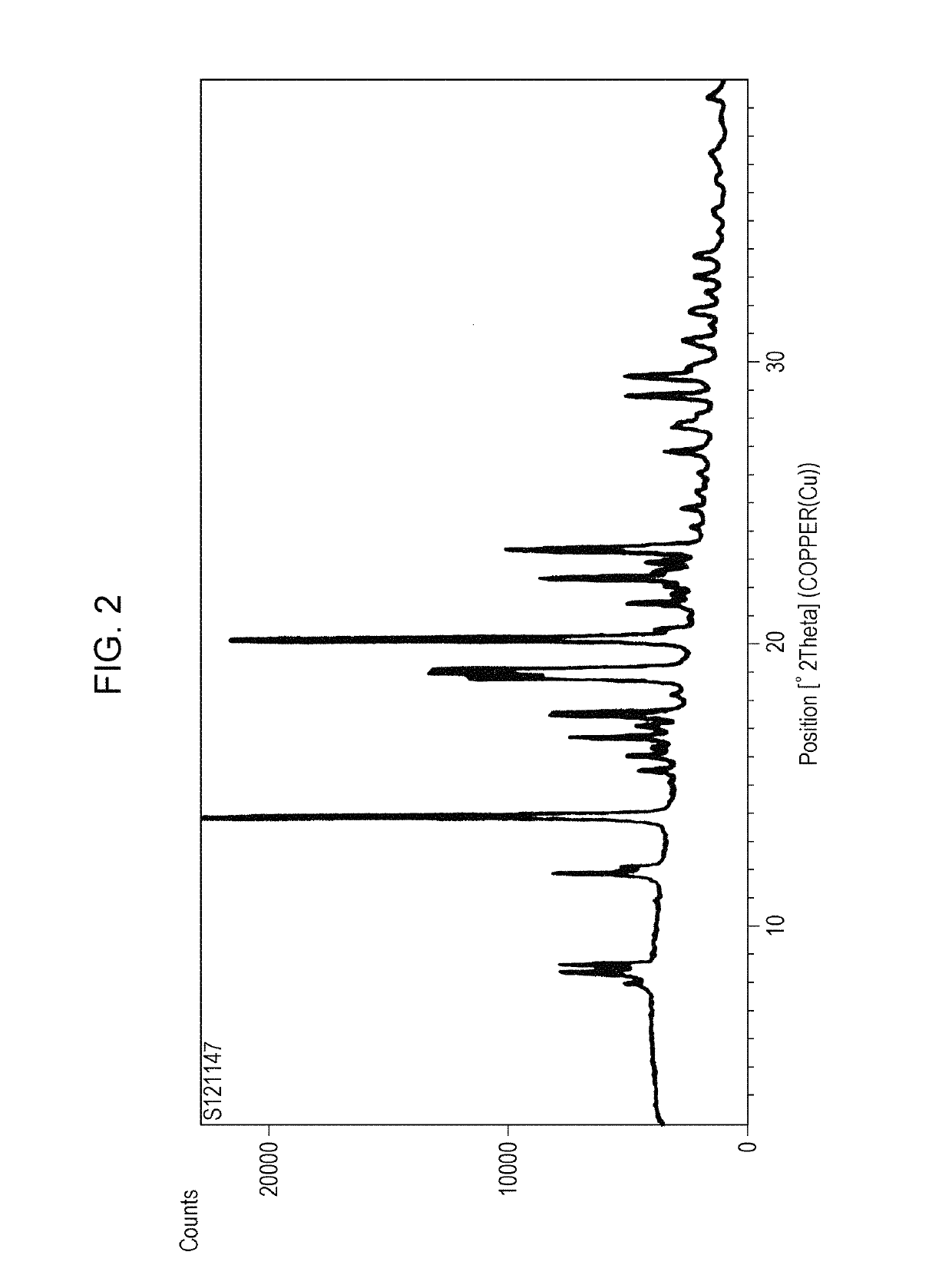
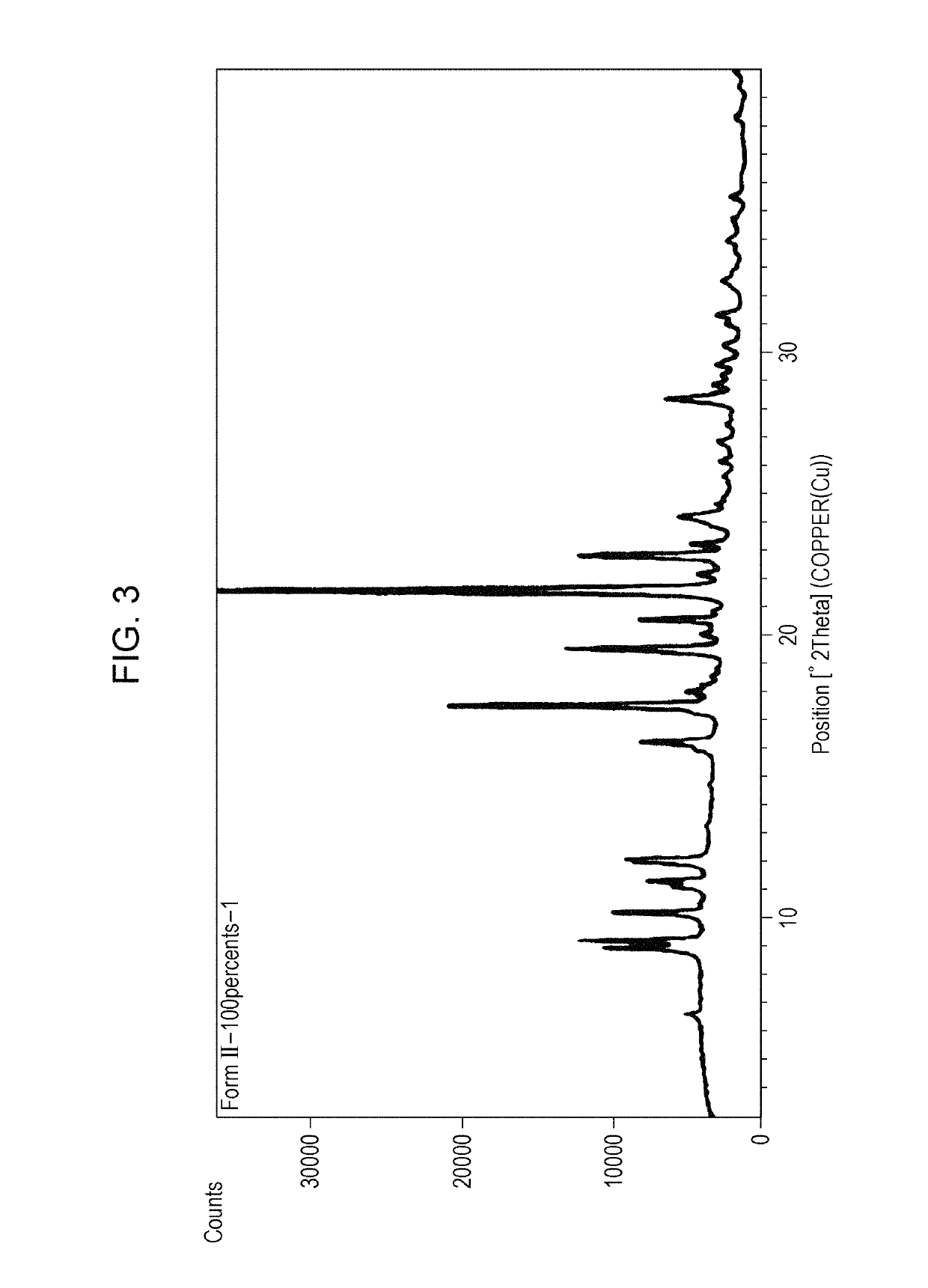
Polymorph of acid 4-[2-[4-[1-2-ethoxyethyl)-1h-benzimidazole-2-il]-1-piper idinyl]ethyl]-dollar G (A), dollar G (A)-dimethyl-benzeneacetic
The invention relates to polymorph 1 of acid 4-[2-[4-[1-(2-ethoxyethyl)-1H-benzimidazole-2-il]-1-piperidinyl]ethyl-alpha, alpha-dimethyl-benzeneacetic having formula (I), preparation methods thereof, pharmaceutical formulations containing polymoph 1 and the use of polymorph 1 for the treatment of allergic reactions and pathological processes mediated by histamine in mammals such as humans.
Owner:FAES FARMA SA
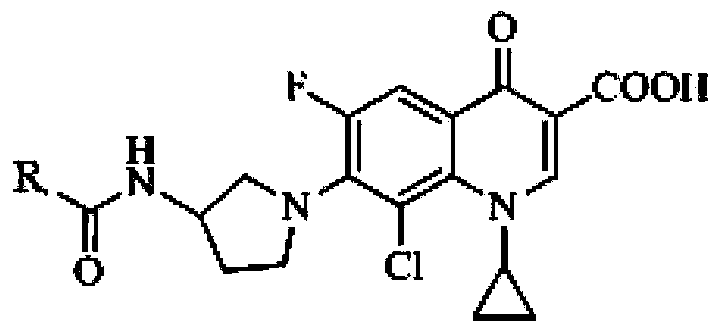
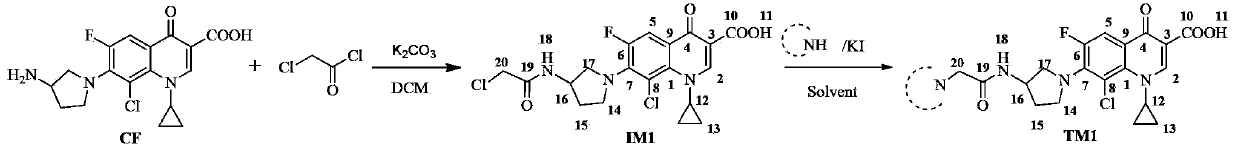
Application of clinafloxacin amino derivatives and medicinal salts thereof in preparing antitubercular medicaments
ActiveCN103405435AHas inhibitory effectStrong anti-tuberculosis activityAntibacterial agentsOrganic active ingredientsThioureaMoxifloxacin
The invention discloses an application of clinafloxacin amino derivatives and medicinal salts thereof in preparing antitubercular medicaments. In the structural general formula of the clinafloxacin amino derivatives, R is -(CH2)nNR<1>R<2>, -CH(CH2CH2XCH3)NH2, 2-pyrrolidyl or -OR3; n is 0 or 1; R1 and R2 are separately hydrogen, methyl, 2-hydroxyethyl, (R)-1-ethyl-2-hydroxyethyl, 2-aminoethyl, 3-(dimethylamino)propyl, hydroxyl, amino, methylamino, methoxyl or thiourea group; X is S or SO2; R3 is methyl or isobutyl; the compounds have certain inhibitory effect on standard sensitive strains, clinically isolated sensitive strains and clinically isolated drug-resistant strains of mycobacterium tuberculosis, the antitubercular activity of a part of compounds is stronger than that of ofloxacin and clinafloxacin and weaker than that of moxifloxacin, thus providing a new research direction for the antitubercular medicaments, and being beneficial to clinical treatment of tuberculosis.
Owner:SOUTHWEST UNIVERSITY
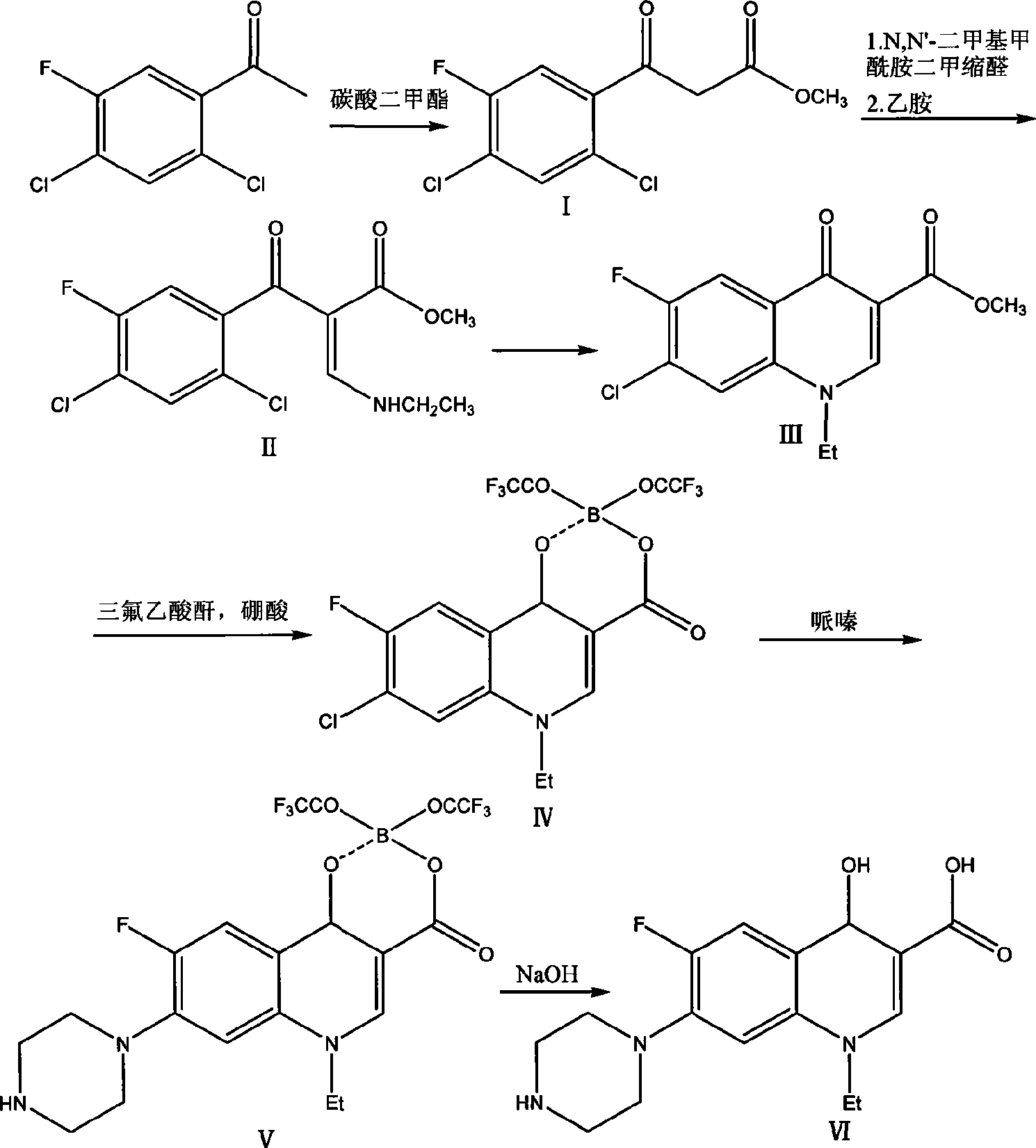
Process for synthesizing norfloxacin
ActiveCN101481350ASimple processing methodMild reaction conditionsAntibacterial agentsOrganic chemistryN dimethylformamideDimethyl acetal
The invention discloses a synthetic method of norfloxacin. The method comprises the following steps: taking toluene as a solvent, causing methyl carbonate to react with 2,4-dichloroo-5-fluoroacetophenone in the presence of a catalyst at the temperature of 70-90 DEG C to obtain 2,4-dichloro-5-fluorobenzenepropionic acid methyl ester, reflux reacting with N,N-dimethylformamide dimethyl acetal in toluene solution for 2-3h to obtain 3-ethylamino-2-(2,4-dichloro-5-fluorobenzoyl) methyl acrylate, cyclizing to obtain 1-ethyl-7-chloro-6-fluo-1,4-dihydro-4-oxoquinoline-3-carboxylic acid methyl ester, reacting with a chelating agent at the temperature of 80-110 DEG C for 2-3h to obtain 1-ethyl-7-chloro-6-fluo-1,4-dihydro-4-oxoquinoline-3-carboxylic acid methyl ester trifluoracetic acid anhydride bononized chelate, reacting with piperazine at the temperature of 20-40 DEG C for 10-24h to obtain the norfloxacin. The method has the advantages of simple process and mild reaction condition, avoids production of reverse ring in the traditional process, provides high-purity product, and is applicable to industrialized production.
Owner:ZHEJIANG LEPU PHARMA CO LTD
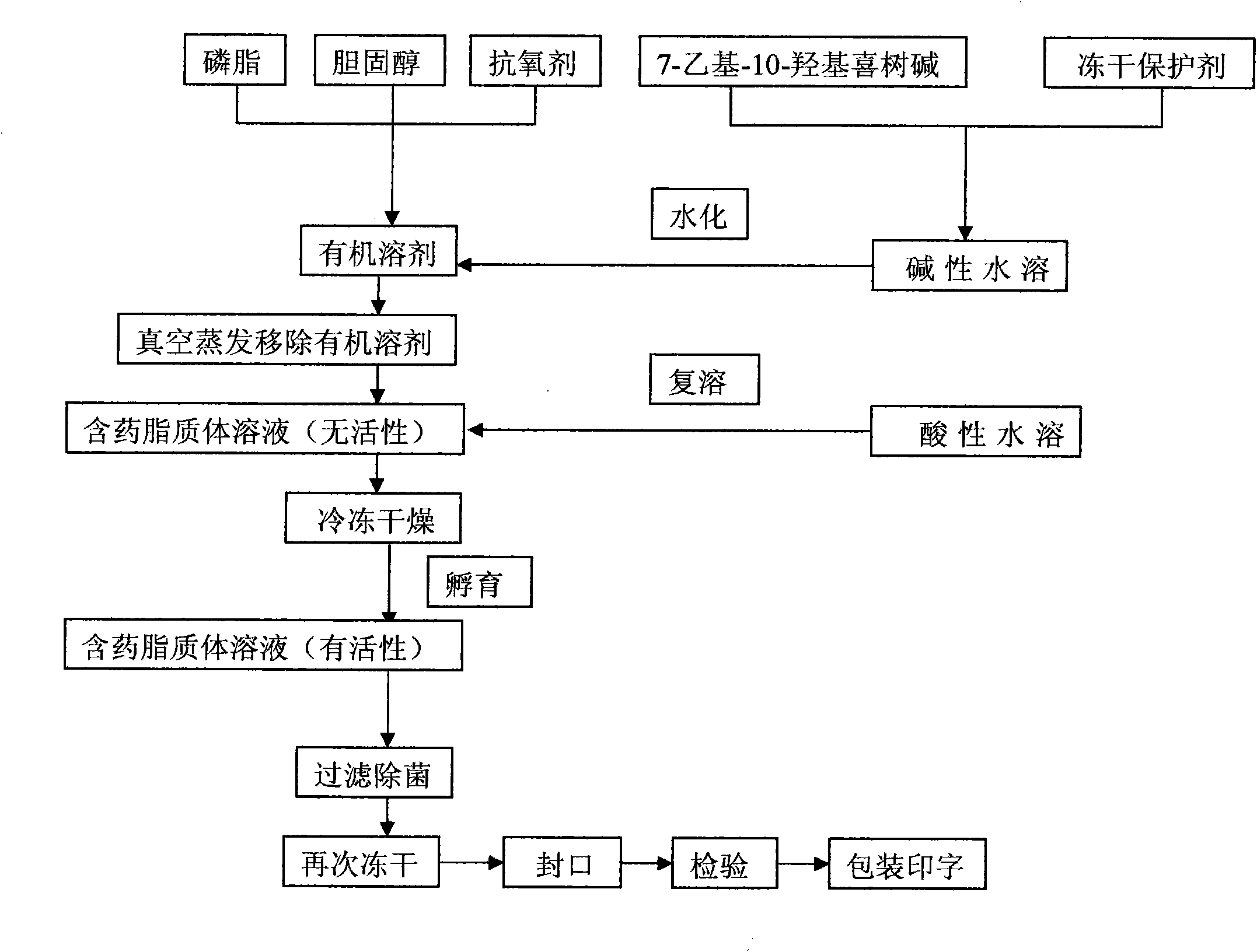
7-ethyl-10-hydroxycamptothecine liposome freeze-dried powder injection and preparation method thereof
InactiveCN101874788AImprove stabilityImprove in vivo stabilityPowder deliveryOrganic active ingredientsSolubilityFreeze-drying
The invention belongs to the medical technical field, and discloses 7-ethyl-10-hydroxycamptothecine liposome freeze-dried powder injection and a preparation method thereof. The 7-ethyl-10-hydroxycamptothecine liposome freeze-dried powder injection comprises the following components: 1-10g of 7-ethyl-10-hydroxycamptothecine, 30-60g of phospholipids, 10-40g of cholesterol, 2-8g of VE, 100-300g of a freeze drying protectant, 2000-8000ml of an organic solvent, 1000-4000ml of alkaline buffer salt solution and 1000-4000ml of acid buffer salt solution. The preparation method comprises the following steps: dissolving liposoluble components in the organic solvent and water-soluble components in the alkaline buffer salt; transferring the organic solvent, and then adding the alkaline buffer salt for hydration; and carrying freeze drying in vacuum, re-dissolving with the acid buffer salt, incubating, filtering, sterilizing, and carrying out freeze drying again to obtain the 7-ethyl-10-hydroxycamptothecine liposome freeze-dried powder injection for injection. The invention solves the problems of low solubility and fast in-vivo metabolism of the 7-ethyl-10-hydroxycamptothecine, thus lowering toxic reaction, eliminating side reaction, having higher target distribution characteristics, prolonging metabolism time and improving solubility and bioavailability.
Owner:SHENYANG PHARMA UNIVERSITY
Deuterated N-ethyl-N-phenyl-1,2-dihydro-4-hydroxy-5-chloro-1-methyl-2-oxoquinoline-3-carboxamide, salts and uses thereof
The subject invention provides deuterated N-ethyl-N-phenyl-1,2-dihydro-4-hydroxy-5-chloro-1-methyl-2-oxoquinoline-3-carboxamide, its salts and uses.
Owner:TEVA PHARMA IND LTD
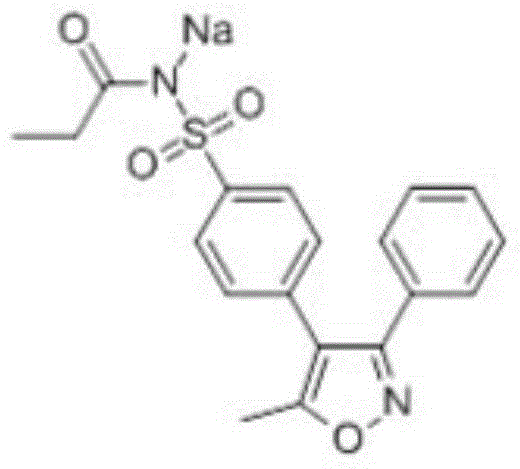
Preparation method of anhydrous and non-solvation A crystallization parecoxib sodium
InactiveCN104910091AHigh crystallinityImprove stabilityOrganic chemistrySodium acetateChlorosulfuric acid
The invention relates to a preparation method of anhydrous and non-solvation A crystallization parecoxib sodium. The method comprises the following steps: 1,2-phenylacetophenone and pyrrolidine are condensed to generate 1-(1,2-diphenylvinyl)pyrrolidine, then acetylation is carried out, and 4,5-dihydro-5-methyl-3,4-dibenzyl-5-isoxzzole alcohol are subjected to cyclization under sodium acetate and hydroxylamine hydrochloride. The compound is directly reacted to chlorosulfonic acid, dehydration and chlorine sulfonation reaction are carried out, and then valdecoxib can be obtained through ammonification, valdecoxib is refined, then is subjected to acetylation to obtain parecoxib, and salt forming is performed to obtain the target compound parecoxib sodium.
Owner:北京华睿鼎信科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
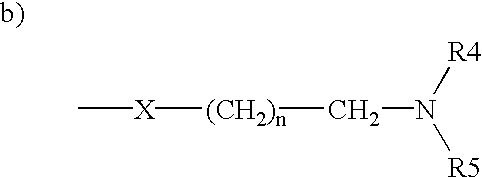

![5-ethyl-2-{4-[4-(4-tetrazol-1-yl-phenoxymethyl)-thiazol-2-yl]-piperidin-1-yl}-pyrimidine 5-ethyl-2-{4-[4-(4-tetrazol-1-yl-phenoxymethyl)-thiazol-2-yl]-piperidin-1-yl}-pyrimidine](https://images-eureka.patsnap.com/patent_img/3ae644fc-9a10-45d9-a30b-421eab0a7beb/US07638541-20091229-D00001.png)
![5-ethyl-2-{4-[4-(4-tetrazol-1-yl-phenoxymethyl)-thiazol-2-yl]-piperidin-1-yl}-pyrimidine 5-ethyl-2-{4-[4-(4-tetrazol-1-yl-phenoxymethyl)-thiazol-2-yl]-piperidin-1-yl}-pyrimidine](https://images-eureka.patsnap.com/patent_img/3ae644fc-9a10-45d9-a30b-421eab0a7beb/US07638541-20091229-D00002.png)
![5-ethyl-2-{4-[4-(4-tetrazol-1-yl-phenoxymethyl)-thiazol-2-yl]-piperidin-1-yl}-pyrimidine 5-ethyl-2-{4-[4-(4-tetrazol-1-yl-phenoxymethyl)-thiazol-2-yl]-piperidin-1-yl}-pyrimidine](https://images-eureka.patsnap.com/patent_img/3ae644fc-9a10-45d9-a30b-421eab0a7beb/US07638541-20091229-D00003.png)
![Preparing method of N-[2-(7- anisyl-1- naphthyl) ethide] acetamide Preparing method of N-[2-(7- anisyl-1- naphthyl) ethide] acetamide](https://images-eureka.patsnap.com/patent_img/304a2a1f-93bf-4b9b-8f00-84c91fb25cf8/G2009101403386D0000012.PNG)
![Preparing method of N-[2-(7- anisyl-1- naphthyl) ethide] acetamide Preparing method of N-[2-(7- anisyl-1- naphthyl) ethide] acetamide](https://images-eureka.patsnap.com/patent_img/304a2a1f-93bf-4b9b-8f00-84c91fb25cf8/G2009101403386D0000031.PNG)
![Preparing method of N-[2-(7- anisyl-1- naphthyl) ethide] acetamide Preparing method of N-[2-(7- anisyl-1- naphthyl) ethide] acetamide](https://images-eureka.patsnap.com/patent_img/304a2a1f-93bf-4b9b-8f00-84c91fb25cf8/G2009101403386D0000051.PNG)

![Sustained-release preparation containing 5-acetyl-4,6-dimethyl-2[2-[4-(2-methoxyphenyl) piperazinyl]ethylamino] pyrimidine trihydrochloride as active ingredient Sustained-release preparation containing 5-acetyl-4,6-dimethyl-2[2-[4-(2-methoxyphenyl) piperazinyl]ethylamino] pyrimidine trihydrochloride as active ingredient](https://images-eureka.patsnap.com/patent_img/181e47a5-361c-46e2-bba8-4891943a3203/US07041317-20060509-D00001.png)
![Sustained-release preparation containing 5-acetyl-4,6-dimethyl-2[2-[4-(2-methoxyphenyl) piperazinyl]ethylamino] pyrimidine trihydrochloride as active ingredient Sustained-release preparation containing 5-acetyl-4,6-dimethyl-2[2-[4-(2-methoxyphenyl) piperazinyl]ethylamino] pyrimidine trihydrochloride as active ingredient](https://images-eureka.patsnap.com/patent_img/181e47a5-361c-46e2-bba8-4891943a3203/US07041317-20060509-D00002.png)
![Sustained-release preparation containing 5-acetyl-4,6-dimethyl-2[2-[4-(2-methoxyphenyl) piperazinyl]ethylamino] pyrimidine trihydrochloride as active ingredient Sustained-release preparation containing 5-acetyl-4,6-dimethyl-2[2-[4-(2-methoxyphenyl) piperazinyl]ethylamino] pyrimidine trihydrochloride as active ingredient](https://images-eureka.patsnap.com/patent_img/181e47a5-361c-46e2-bba8-4891943a3203/US07041317-20060509-D00003.png)
![Preparation of venlafaxine intermediate 1-[2-amino-1-(4-methoxy phenyl)ethyl] cyclohexanol Preparation of venlafaxine intermediate 1-[2-amino-1-(4-methoxy phenyl)ethyl] cyclohexanol](https://images-eureka.patsnap.com/patent_img/28783494-7032-4a6b-8f4d-92fe3861cd01/a200910058269d00031.PNG)
![Preparation of venlafaxine intermediate 1-[2-amino-1-(4-methoxy phenyl)ethyl] cyclohexanol Preparation of venlafaxine intermediate 1-[2-amino-1-(4-methoxy phenyl)ethyl] cyclohexanol](https://images-eureka.patsnap.com/patent_img/28783494-7032-4a6b-8f4d-92fe3861cd01/a200910058269d00041.PNG)



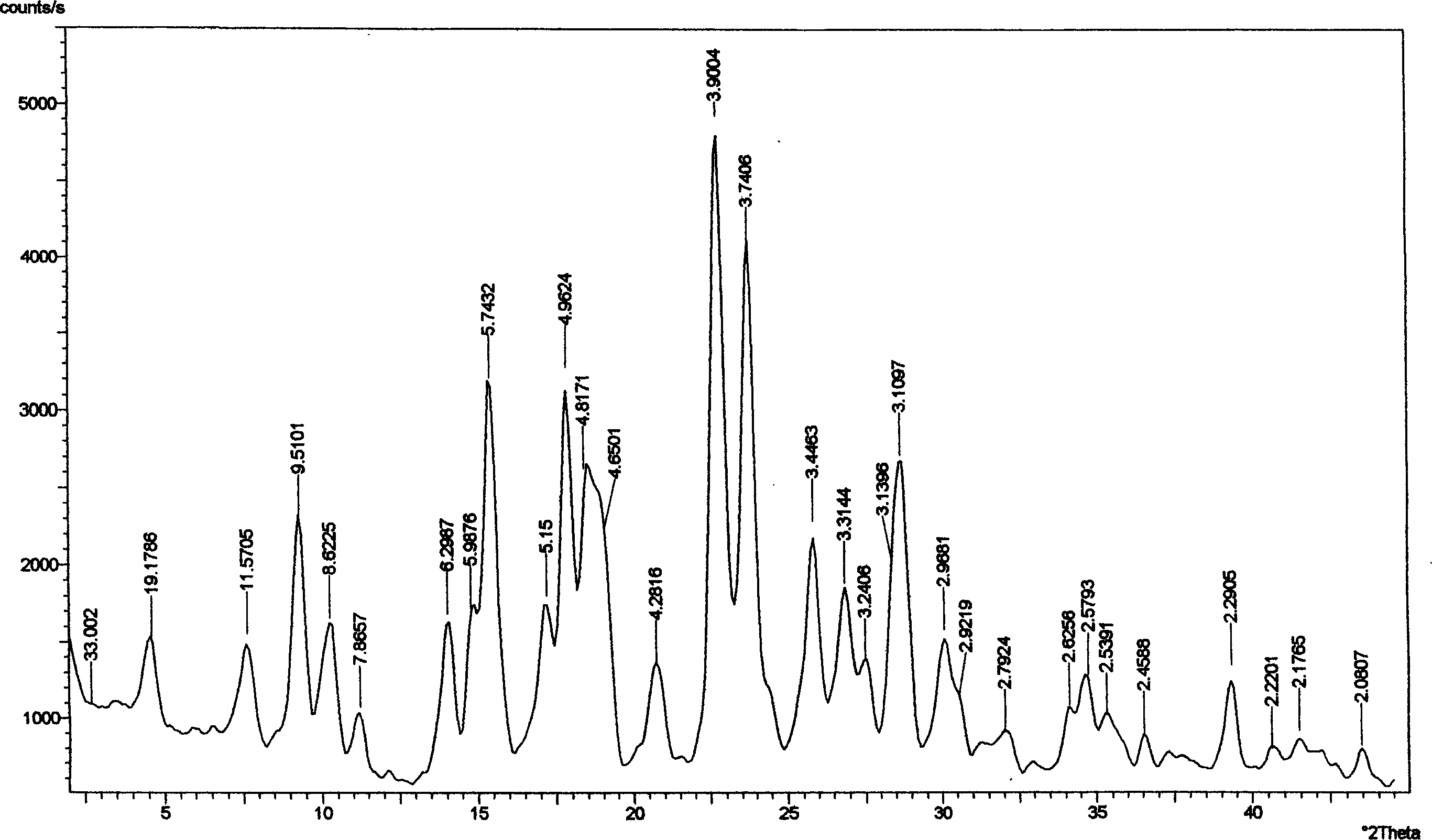
![Polymorph of acid 4-[2-[4-[1-2-ethoxyethyl)-1h-benzimidazole-2-il]-1-piper idinyl]ethyl]-dollar G (A), dollar G (A)-dimethyl-benzeneacetic Polymorph of acid 4-[2-[4-[1-2-ethoxyethyl)-1h-benzimidazole-2-il]-1-piper idinyl]ethyl]-dollar G (A), dollar G (A)-dimethyl-benzeneacetic](https://images-eureka.patsnap.com/patent_img/fa3f2371-d99d-4c66-82f0-fb9a7e006a59/A0282898700121.PNG)
![Polymorph of acid 4-[2-[4-[1-2-ethoxyethyl)-1h-benzimidazole-2-il]-1-piper idinyl]ethyl]-dollar G (A), dollar G (A)-dimethyl-benzeneacetic Polymorph of acid 4-[2-[4-[1-2-ethoxyethyl)-1h-benzimidazole-2-il]-1-piper idinyl]ethyl]-dollar G (A), dollar G (A)-dimethyl-benzeneacetic](https://images-eureka.patsnap.com/patent_img/fa3f2371-d99d-4c66-82f0-fb9a7e006a59/A0282898700131.PNG)
![Polymorph of acid 4-[2-[4-[1-2-ethoxyethyl)-1h-benzimidazole-2-il]-1-piper idinyl]ethyl]-dollar G (A), dollar G (A)-dimethyl-benzeneacetic Polymorph of acid 4-[2-[4-[1-2-ethoxyethyl)-1h-benzimidazole-2-il]-1-piper idinyl]ethyl]-dollar G (A), dollar G (A)-dimethyl-benzeneacetic](https://images-eureka.patsnap.com/patent_img/fa3f2371-d99d-4c66-82f0-fb9a7e006a59/A0282898700141.PNG)