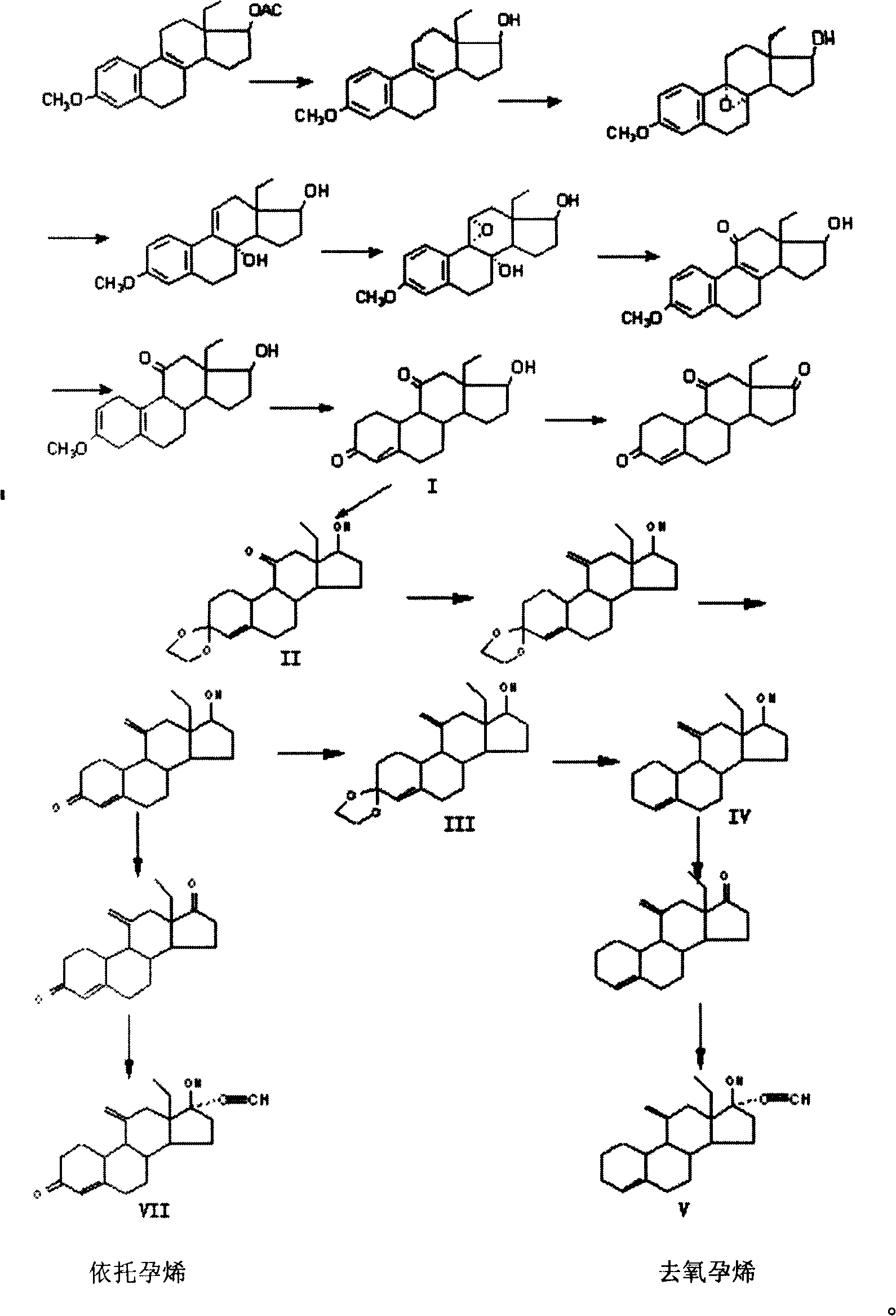
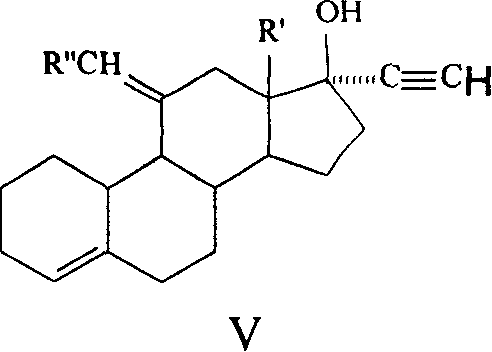
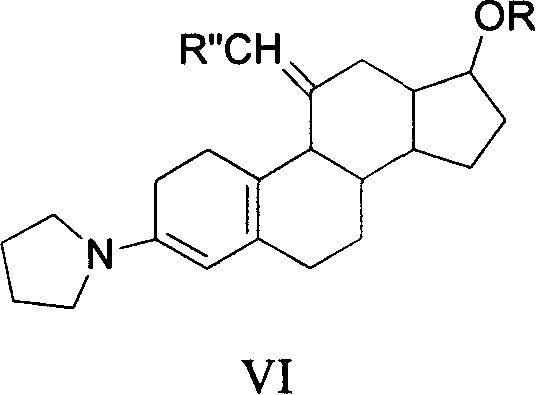
Method for synthesizing steroid progestogen
A progesterone and steroid technology, applied in the field of medicinal chemistry, can solve the problems of low yield and achieve high yield, low cost, and large-scale industrial production value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Example 1, 13-Ethyl-3-methoxy-estro-1,3,5(10),8(9)-tetraen-17β-alcohol
[0040] Dissolve 50 grams of 13-ethyl-3-methoxy-estro-1,3,5(10), 8(9)-tetraen-17β-alcohol-acetate in 500 ml of methanol, add 20 g Potassium hydroxide was heated, and potassium hydroxide was dissolved. After 2 hours of reaction, TLC detected that the reaction was complete. The solution was transferred to a single-necked flask, and part of the solvent was evaporated under reduced pressure. A solid appeared, which was analyzed with 3000 ml of water, filtered, and dried to obtain 40 grams of solids.
Embodiment 2
[0041] Example 2, 13-ethyl-3-methoxy-estro-1,3,5(10)-triene-9,11-epoxy-8α, 17β-diol
[0042] 40 grams of 13-ethyl-3-methoxy-estro-1,3,5(10),8(9)-tetraen-17β-alcohol was dissolved in 400 milliliters of benzene, 60 grams of peracid was added, 500 1 ml of diethyl ether, continue to react for 2 hours, TLC detects that the reaction is complete, add potassium carbonate solution to stop the reaction, separate the organic layer, extract the water layer with ethyl acetate, combine the organic layers, wash with water to medium size, wash with saturated saline, and anhydrous sulfuric acid The sodium was dried and distilled under reduced pressure to obtain a solid, which was filtered to obtain a white solid weighing 32 grams.
Embodiment 3
[0043] Example 3, 13-Ethyl-3-methoxy-estro-1,3,5(10),8(9)-tetraen-11-one 17β-alcohol
[0044] Dissolve 10 g of epoxide 13-ethyl-3-methoxy-estro-1,3,5(10)-triene-9,11-epoxy-8α,17β-diol in 50 mL of methanol , add dropwise 55 milliliters of 18% hydrochloric acid under stirring, stir for 1 hour after dripping, add 200 milliliters of water to make the product precipitate as much as possible, filter to obtain a white solid, wash with water until neutral, dry at room temperature, weighing: 9.25 grams.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com