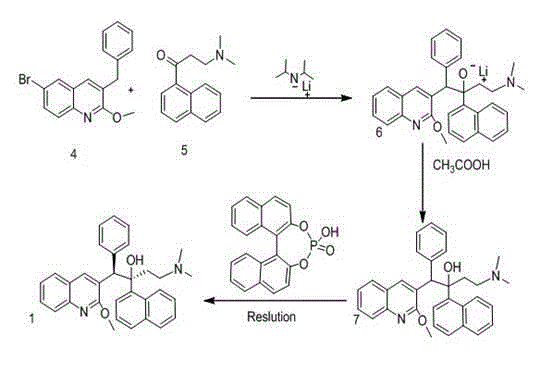
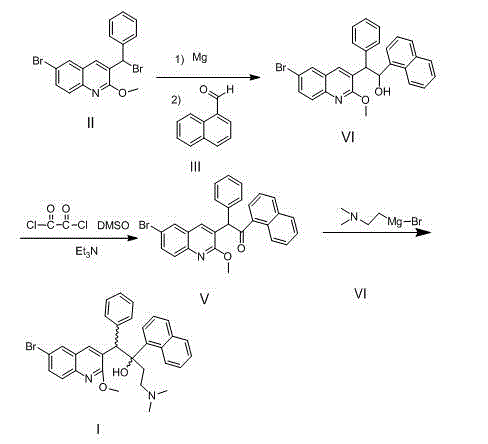
New synthesis route and method of bedaquiline racemate
A bedaquiline racemate and methoxyquinoline technology, which is applied in the field of medicine, can solve the problems of harsh reaction conditions, lengthy synthesis routes, lengthy synthesis steps and the like, and achieves mild and easily controllable reaction conditions, simple and convenient reaction steps, The effect of easy product separation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Preparation of 3-bromobenzyl-6-bromo-2-methoxyquinoline (Ⅱ)
[0034]
[0035] A. Add 30g (0.091mol) of 3-benzyl-6-bromo-2-methoxyquinoline, 16.2g (0.091mol) of NBS, 1g (0.004mol) of BPO and CCl into a 500ml single-necked bottle 4 200ml, stirred and warmed up to 78°C for 2h, filtered while hot to remove insoluble matter, evaporated the solvent under reduced pressure, added 200ml of DCM to dissolve the solid, washed three times with 10% sodium carbonate, dried the organic layer with anhydrous magnesium sulfate, filtered, and spun Dry to obtain white solid 34g, yield 92%, purity 99%.
[0036] [M+H] + :408; 1 HNMR (500MHz, CDCl 3 )δ8.13(d, J =11.6Hz,2H),7.90(d, J =4.5Hz, 2H), 7.41-7.29(m, 4H), 7.28(s, 1H), 6.36(s, 1H), 4.10(s, 3H). B. Add 50g (0.152mol) of 3-benzyl-6-bromo-2-methoxyquinoline, 27g (0.152mol) of NBS, 2.0g (0.008mol) of BPO, and CCl into a 1000ml single-necked bottle 4 400ml, stirred and raised to 78°C and refluxed for 2h, filtered while hot to remov...
Embodiment 2
[0038] Preparation of (6-bromo-2-methoxyquinolin-3-yl)-1-(naphthalene-1-yl)-2-phenylethanol (Ⅳ)
[0039]
[0040]A. Add 2.5g (0.104mol) of magnesium powder to a dry 250ml three-necked bottle, add an appropriate amount of dry ether to cover the magnesium powder, and slowly add 3-bromobenzyl-6-bromo-2 dissolved in 100ml of dry ether -Methoxyquinoline 15g (0.037mol), you can add a few drops of 1,2-dibromoethane to initiate the reaction, keep the reaction system slightly boiling until the addition is complete, keep warm and reflux for 1h; slowly add 1-naphthaldehyde 6.3g (0.040mol), after the dropwise addition, continue to keep warm and reflux for 1h. Cool down to 0°C, add 100ml of saturated ammonium chloride dropwise, adjust pH to 6, filter to remove insoluble matter, extract with dichloromethane, combine organic layers, wash with saturated brine, add anhydrous magnesium sulfate to dry, filter, and reduce pressure The solvent was evaporated to obtain 14.5 g of off-white solid...
Embodiment 3
[0044] Preparation of 2-(6-bromo-2-methoxyquinolin-3-yl)-1-(naphthalene-1-yl)-2-phenylethanone (Ⅴ)
[0045]
[0046] A. Add 13.5ml (0.16mol) oxalyl chloride to a 1000ml dry three-neck flask, cool down to -78°C, slowly add 22ml (0.31mol) dry DMSO dissolved in 100ml DCM into the reaction flask, and keep the temperature to continue After stirring for 30 min, 64.5 g (0.133 mol) of (6-bromo-2-methoxyquinolin-3-yl)-1-(naphthalene-1-yl)-2-phenylethanol dissolved in 30 ml of DMSO was added dropwise, Continue to stir for 1 hour after the dropwise addition, then add 110ml of triethylamine dropwise, and continue to maintain the low temperature reaction for half an hour after the addition. Rise to room temperature and add 400ml of water, let stand to separate the layers, separate the dichloromethane layer, continue to extract the water layer with dichloromethane, combine the dichloromethane layers with 1% hydrochloric acid solution, 5% sodium carbonate solution, water Wash, add anhydr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com