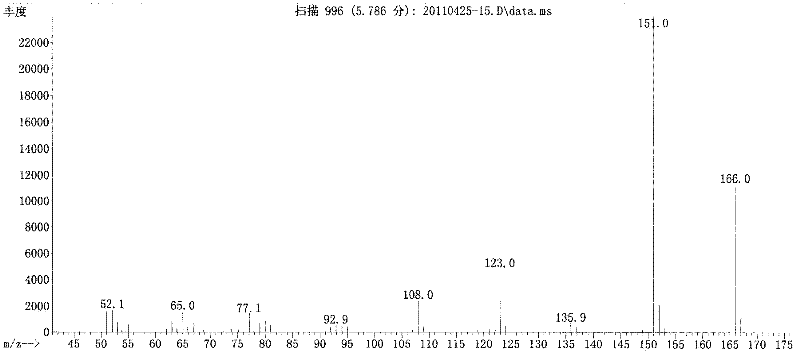
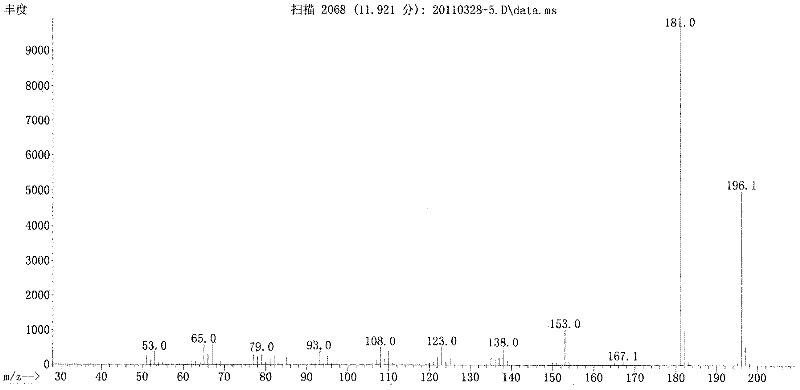
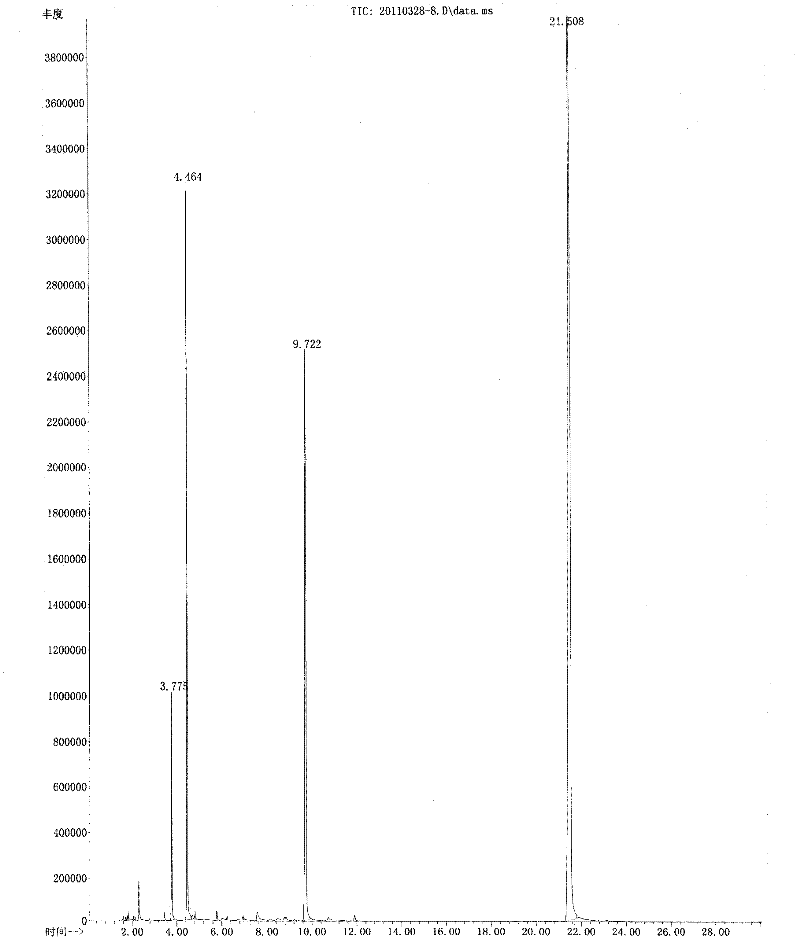
Method for preparing acetosyringone and vanillyl ethyl ketone by oxidizing lignin
A technology of acetosyringone and vanilla ethyl ketone, which is applied in the field of preparation of fine chemicals, can solve the problems of high price, difficulty in preparing vanillyl ethyl ketone and aceto syringone, etc., and achieve the effects of reducing hazards and simplifying reaction steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] 20 mass parts of alkali lignin and 1.2 mass parts of p-nitrobenzoic acid oxidizing agent are stirred uniformly in the sodium hydroxide solution of 2.5mol / L in 200 mL concentration; o C under reaction 5h. The lignin used in addition to alkali lignin can also be one or more of lignin sulfonate, organic solvent lignin or enzymatic lignin. In addition to p-nitrobenzoic acid, the oxidizing agent can also be any of 3,5-dinitrobenzoic acid, 3-nitrosalicylic acid, 5-nitrosalicylic acid or 3,5-dinitrosalicylic acid .
[0032] The reaction liquid was cooled, then acidified to pH 4 with 4 mol / L hydrochloric acid, extracted three times with 250 mL chloroform, combined the extracts and then concentrated by distillation under reduced pressure, the distilled chloroform was recycled, and the concentrate obtained was Crude vanilla ethyl ketone and acetosyringone.
[0033] 10 parts by volume of crude products of vanillyl ethyl ketone and acetosyringone were dissolved in 15 parts by vo...
Embodiment 2
[0035] 20 mass parts of lignosulfonate and 1.2 mass parts of 3,5-dinitrobenzoic acid oxidizing agent are stirred uniformly in the sodium hydroxide solution of 2.5mol / L in 200 mL concentration; o C under reaction 2h. The reaction liquid was cooled, then acidified to pH 4 with 4 mol / L hydrochloric acid, extracted three times with 250 mL chloroform, combined the extracts and then concentrated by distillation under reduced pressure, the distilled chloroform was recycled, and the concentrate obtained was Crude vanilla ethyl ketone and acetosyringone. 10 parts by volume of crude products of vanillyl ethyl ketone and acetosyringone were dissolved in 15 parts by volume of acetone solvent at 40° C., and then separated by three times of recrystallization to obtain vanillyl ethyl ketone and acetosyringone. According to gas chromatography analysis, the separation and purification rates were 96.1% and 97.6%, respectively, and the total yield was 18.1%.
Embodiment 3
[0037]Add 20 parts by mass of lignosulfonate, 0.6 parts by mass of 3,5-dinitrobenzoic acid and 200 mL of 0.5 mol / L sodium hydroxide solution into a 500 mL reactor, stir evenly, at 180 o C for 2 h.
[0038] The reaction liquid was cooled, then acidified to pH 4 with 4 mol / L hydrochloric acid, extracted three times with 250 mL chloroform, combined the extracts and then concentrated by distillation under reduced pressure, the distilled chloroform was recycled, and the concentrate obtained was Crude vanilla ethyl ketone and acetosyringone. 10 parts of the concentrate were dissolved at 60°C with 10 parts of ethanol solvent, and then separated by 4 times of recrystallization to obtain vanillyl ethyl ketone and acetosyringone. According to gas chromatography analysis, the purity was 96.4% and 97.7%, respectively, and the total yield was 18.0 %.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com