Process for synthesizing norfloxacin
A norfloxacin and synthesis method technology, applied in organic chemistry, antibacterial drugs, etc., can solve the problems of long steps, affecting the reaction yield, and difficulty in obtaining it, and achieve mild reaction conditions, simple process methods, and high product purity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
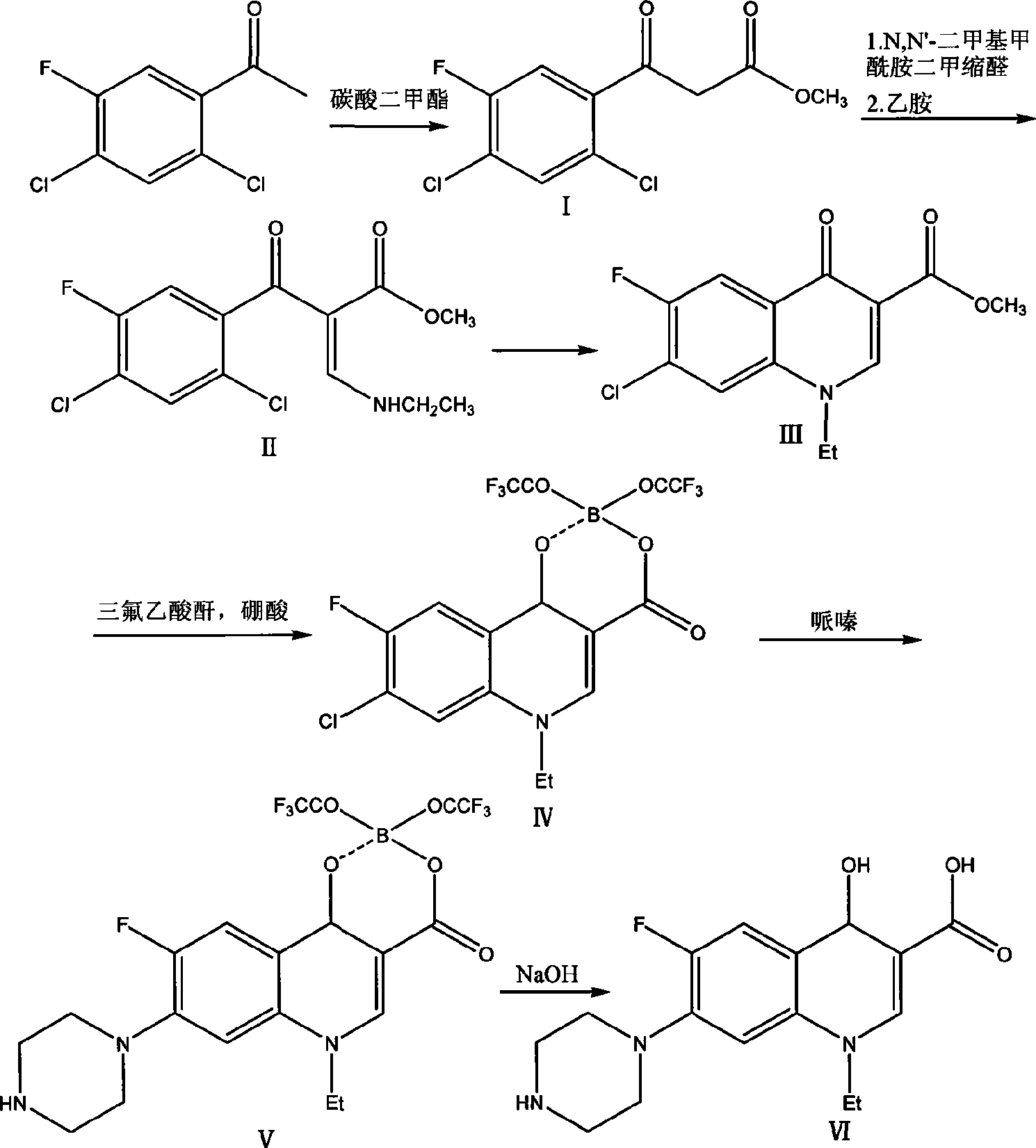
[0016] 1) Add 43.2g of sodium methoxide into a 1L four-neck flask, add 90.0g of dimethyl carbonate, and then add 400ml of toluene. Stir and heat up to 70°C, add dropwise 41.4g of 2,4-dichloro-5-fluoroacetophenone toluene solution (toluene is 100ml), dropwise time is 5 hours, after dropping, raise the temperature to 80°C, and keep it warm for 2 hours After the reaction was completed, 300ml of water was added to adjust the pH to 5. The toluene layer was separated and concentrated to dryness to obtain 47.8g of methyl 2,4-dichloro-5-fluorobenzoylacetate, with a yield of 90.3%.
[0017] 2) Add 64.3g N, N'-dimethylformamide dimethyl acetal, 170ml toluene to 47.8g 2,4-dichloro-5-fluorobenzoic acid methyl ester, reflux for 2 hours, concentrate and reclaim toluene and N,N'-Dimethylformamide dimethyl acetal. At room temperature, 34.2 g of ethylamine-toluene solution (containing 80 ml of toluene) was added dropwise, and the temperature was raised to 40° C. for 2 hours for reaction after...
Embodiment 2
[0022] 1) Add 43.2g of sodium ethoxide into a 1L four-necked flask, add 18.0g of dimethyl carbonate, and then add 400ml of toluene. Stir and heat up to 90°C, add dropwise 41.4g of 2,4-dichloro-5-fluoroacetophenone toluene solution (toluene is 100ml), dropwise time is 5 hours, after dropping, raise the temperature to 70°C, and keep warm for 2 hours After the reaction was completed, 300ml of water was added to adjust the pH to 6. The toluene layer was separated and concentrated to dryness to obtain 39.6g of methyl 2,4-dichloro-5-fluorobenzoylacetate, with a yield of 75.1%.
[0023] 2) Add 17.9g N, N'-dimethylformamide dimethyl acetal, 170ml toluene to 39.6g 2,4-dichloro-5-fluorobenzoic acid methyl ester, reflux for 3 hours, concentrate and reclaim toluene. At room temperature, 34.2 g of ethylamine-toluene solution (containing 80 ml of toluene) was added dropwise, and the temperature was raised to 50° C. for 2 hours for reaction after dropping. Concentrate under reduced pressure...
Embodiment 3
[0028] 1) Add 43.2 g of potassium tert-butoxide into a 1 L four-necked flask, add 54.0 g of dimethyl carbonate, and then add 400 ml of toluene. Stir and heat up to 80°C, add dropwise 41.4g of 2,4-dichloro-5-fluoroacetophenone toluene solution (100ml of toluene), dropwise for 5 hours, raise the temperature to 90°C after dropping, and keep warm for 2 hours After the reaction was completed, 300ml of water was added to adjust the pH to 5. The toluene layer was separated and concentrated to dryness to obtain 47.3g of methyl 2,4-dichloro-5-fluorobenzoylacetate, with a yield of 90.0%.
[0029] 2) Add 47.6g N, N'-dimethylformamide dimethyl acetal, 170ml toluene to 47.3g 2,4-dichloro-5-fluorobenzoic acid methyl ester, reflux for 3 hours, concentrate and reclaim toluene and N,N'-Dimethylformamide dimethyl acetal. At room temperature, 34.2 g of ethylamine-toluene solution (containing 80 ml of toluene) was added dropwise, and the temperature was raised to 40° C. for 2 hours for reaction ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com