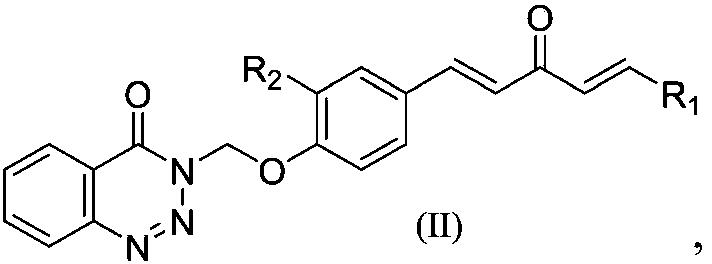
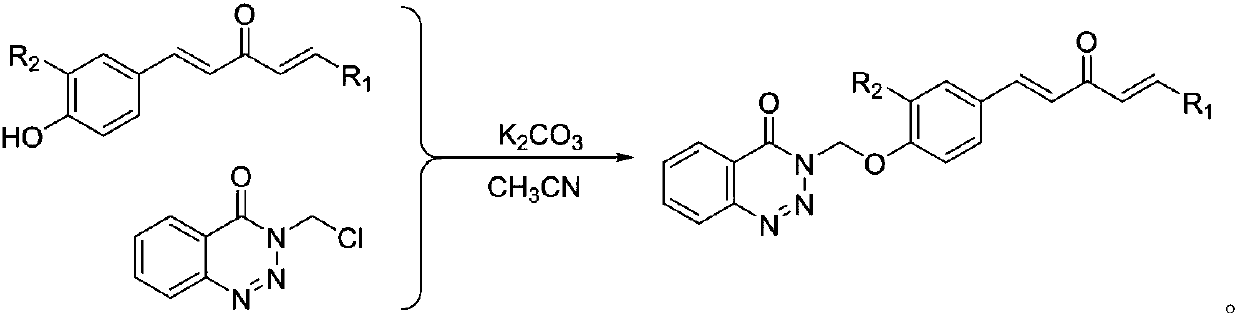
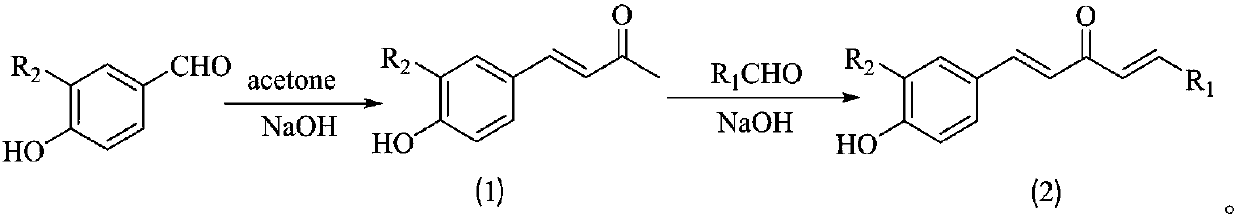
1,4-pentadiene-3-ketone derivative containing benzotriazinone as well as preparation method and application of 1,4-pentadiene-3-ketone derivative
A technology of benzotriazinone and pentadiene, which is applied in the fields of botanical equipment and methods, chemicals for biological control, biocides, etc., can solve the problems of human harm, long residual time and high residual amount, achieve good inhibitory effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039]Synthesis of 1-(4-(3-chloromethylbenzotriazin-4-one)phenyl)-5-(2-pyridyl)-1,4-pentadien-3-one (Compound No. for II-1), including the following steps:
[0040] (1) Synthesis of 4-(hydroxyphenyl)-3-buten-2-one: 4-hydroxybenzaldehyde (6.1g) was added to 60mL of acetone, after stirring for about 15min, the reaction system was ice-bathed for about After 30 min, add about 100 mL of 5% NaOH solution to the system, after the dropwise addition is complete, remove the ice bath, and stir at room temperature for about 24 h. After the reaction is over, transfer the system to a 500mL beaker and add an appropriate amount of ice water, and then use 5% dilute hydrochloric acid solution to adjust the pH of the system to about 5-6. After a large amount of yellow solids precipitate, extract the solids, and finally use ethanol to / water system recrystallized to obtain a yellow solid with a yield of 65%.
[0041] (2) Synthesis of 1-(2-thienyl)-5-(4-hydroxyphenyl)-1,4-pentadien-3-one: 4-(hyd...
Embodiment 2
[0047] Synthesis of 1-(4-(3-chloromethylbenzotriazin-4-one)phenyl)-5-(4-methylphenyl)-1,4-pentadien-3-one( Compound number is II-2), comprises the following steps:
[0048] (1) Synthesis of 4-(hydroxyphenyl)-3-buten-2-one: as in step (1) of Example 1.
[0049] (2) Synthesis of 1-(4-methylphenyl)-5-(4-hydroxyphenyl)-1,4-pentadien-3-one: as in step (2) of Example 1, the difference is With 4-methylbenzaldehyde as raw material.
[0050] (3) Synthesis of 3-chloromethylbenzotriazin-4-one: as in step (5) of Example 1.
[0051] (4) Synthesis of 1-(4-(3-methylbenzotriazin-4-one)phenyl)-5-(4-methylphenyl)-1,4-pentadien-3-one : As in Example 1 (6) step, the difference is that 1-(4-methylphenyl)-5-(4-hydroxyphenyl)-1,4-pentadien-3-one is used as raw material.
Embodiment 3
[0053] Synthesis of 1-(4-(3-methylbenzotriazin-4-one)phenyl)-5-(3-pyridyl)-1,4-pentadien-3-one (compound number is II -3), including the following steps:
[0054] (1) Synthesis of 4-(hydroxyphenyl)-3-buten-2-one: as in step (1) of Example 1.
[0055] (2) Synthesis of 1-(3-pyridyl)-5-(4-hydroxyphenyl)-1,4-pentadien-3-one: as in step (2) of Example 1, the difference is that pyridine -3-formaldehyde as raw material.
[0056] (3) Synthesis of 3-chloromethylbenzotriazin-4-one: as in step (5) of Example 1.
[0057] (4) Synthesis of 1-(4-(3-methylbenzotriazin-4-one) phenyl)-5-(3-pyridyl)-1,4-pentadien-3-one: as Step (6) of Example 1, the difference is that 1-(3-pyridyl)-5-(4-hydroxyphenyl)-1,4-pentadien-3-one is used as raw material.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com