Amino sugar thiazole derivative as well as synthetic method and application thereof
A synthesis method and sugar thiazole technology, applied in the field of drug preparation, can solve the problems such as the inability to prevent the degeneration and death of central cholinergic neurons, the decrease in the efficacy of acetylcholinesterase inhibitors, the large side effects of gastrointestinal irritation, and the like. Scope of application, strong inhibitory activity, effect of reducing production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
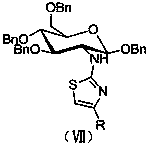
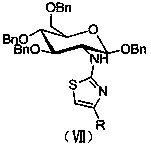
[0029] Embodiment 1, an aminoglucothiazole derivative, its structural formula is as follows formula VII:
[0030]
[0031] Wherein, the R is selected from CH 3 -, n-C 4 h 9 -, C 6 h 5 -, 4-CH 3 C 6 h 4 -, 3-CH 3 C 6 h 4 -, 2-CH 3 C 6 h 4 -, 4-CH 3 OC 6 h 4 -, 3-CH 3 OC 6 h 4 -, 2,3-di-CH 3 OC 6 h 3 -, 4-FC 6 h 4 -, 2-FC 6 h 4 -, 4-ClC 6 h 4 -, 3-ClC 6 h 4 -, 4-BrC 6 h 4 -, 3-BrC 6 h 4 -, 4-NO 2 C 6 h 4 -, 3-NO 2 C 6 h 4 -, 4-OHC 6 h 4 -, 4-NH 2 C 6 h 4 -, 4-PhC 6 h 4 -, 3-C 6 h 4 N-, 2-C 5 h 3 S-.
Embodiment 2
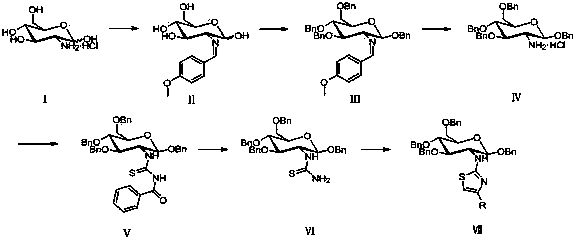
[0032] Embodiment 2, a kind of synthetic method of aminoglucothiazole derivative as described in embodiment 1, its steps are as follows:
[0033] (1) N-(1,3,4,6-tetra-O-benzyl-2-deoxy- β -D-glucopyranose-2-yl)thiourea preparation: first 2-deoxy-2-amino-1,3,4,6-tetra-O-benzyl- β -D-pyranose hydrochloride reacts with benzoyl isothiocyanate, and the product reacts with alkali to obtain glycosylthiourea; 1-benzoyl-3-(1,3,4,6-tetra-O -Benzyl- β The molar ratio of -D-glucopyranose-2-yl)thiourea to base is 1:1, the reaction temperature is 50°C, and the reaction time is 0.5 hours;
[0034] (2) N-(1,3,4,6-tetra-O-benzyl- β -D-glucopyranose-2-yl)-2-amino-4-substituted thiazole: 1-benzoyl-3-(1,3,4,6-tetra-O-benzyl- β -D-glucopyranose-2-yl)thiourea reacts with 1-bromo-2-substituted ethanone to give N-(1,3,4,6-tetra-O-benzyl- β -D-glucopyranose-2-yl)-2-amino-4-substituted thiazole; reaction with ethanol as solvent, N-(1,3,4,6-tetra-O-benzyl-2-deoxy- β The molar ratio of -D-glucopyran...
Embodiment 3
[0035] Embodiment 3, a kind of synthetic method of aminoglucothiazole derivative as described in embodiment 1, its steps are as follows:
[0036] (1) N-(1,3,4,6-tetra-O-benzyl-2-deoxy- β -D-glucopyranose-2-yl)thiourea preparation: first 2-deoxy-2-amino-1,3,4,6-tetra-O-benzyl- β -D-pyranose hydrochloride reacts with benzoyl isothiocyanate, and the product reacts with alkali to obtain glycosylthiourea; 1-benzoyl-3-(1,3,4,6-tetra-O -Benzyl- β The molar ratio of -D-glucopyranose-2-yl)thiourea to base is 1: 2, the reaction temperature is 70°C, and the reaction time is 1.5 hours;
[0037] (2) N-(1,3,4,6-tetra-O-benzyl- β -D-glucopyranose-2-yl)-2-amino-4-substituted thiazole: 1-benzoyl-3-(1,3,4,6-tetra-O-benzyl- β -D-glucopyranose-2-yl)thiourea reacts with 1-bromo-2-substituted ethanone to give N-(1,3,4,6-tetra-O-benzyl- β -D-glucopyranose-2-yl)-2-amino-4-substituted thiazole; reaction with ethanol as solvent, N-(1,3,4,6-tetra-O-benzyl-2-deoxy- β The molar ratio of -D-glucopyra...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com