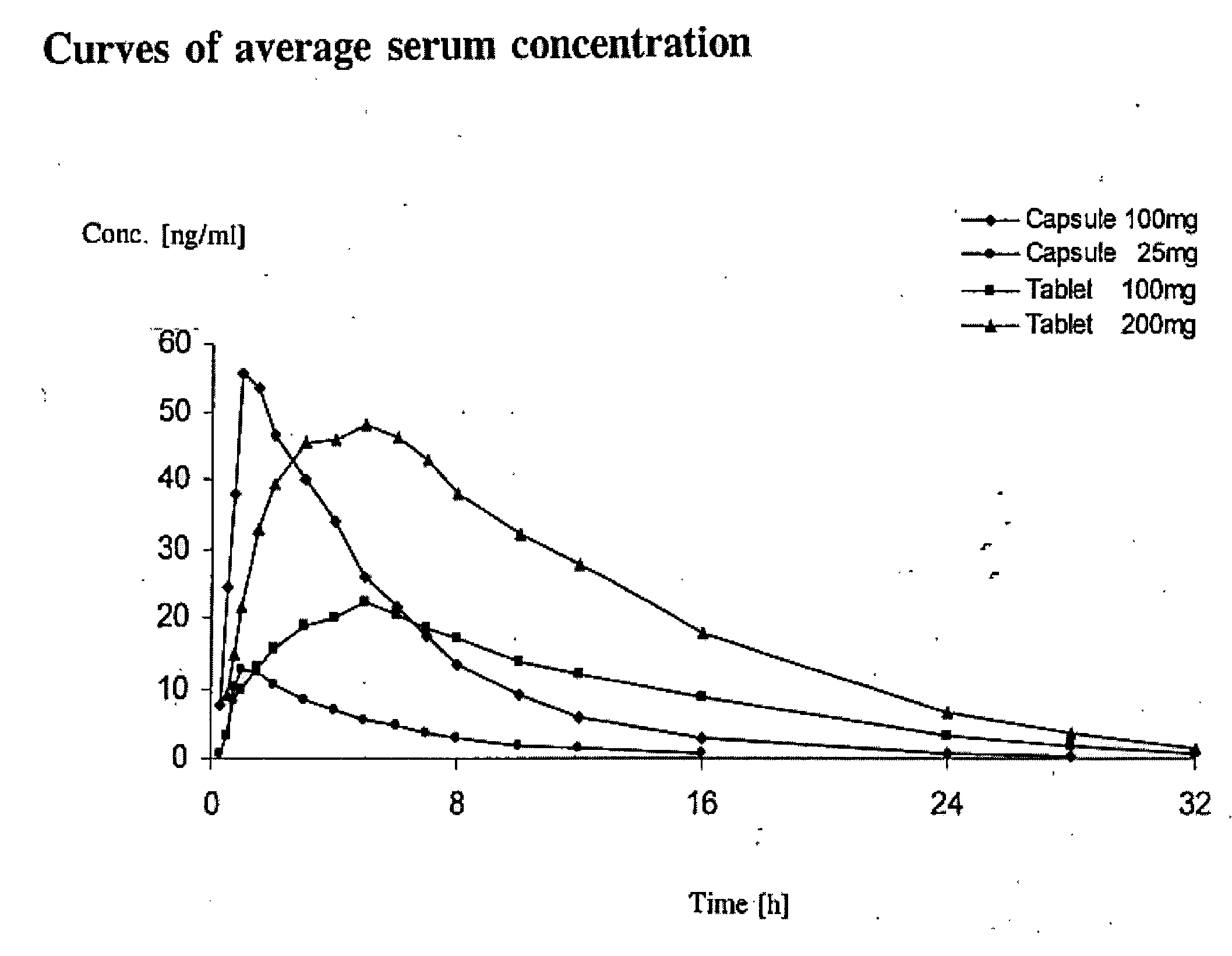
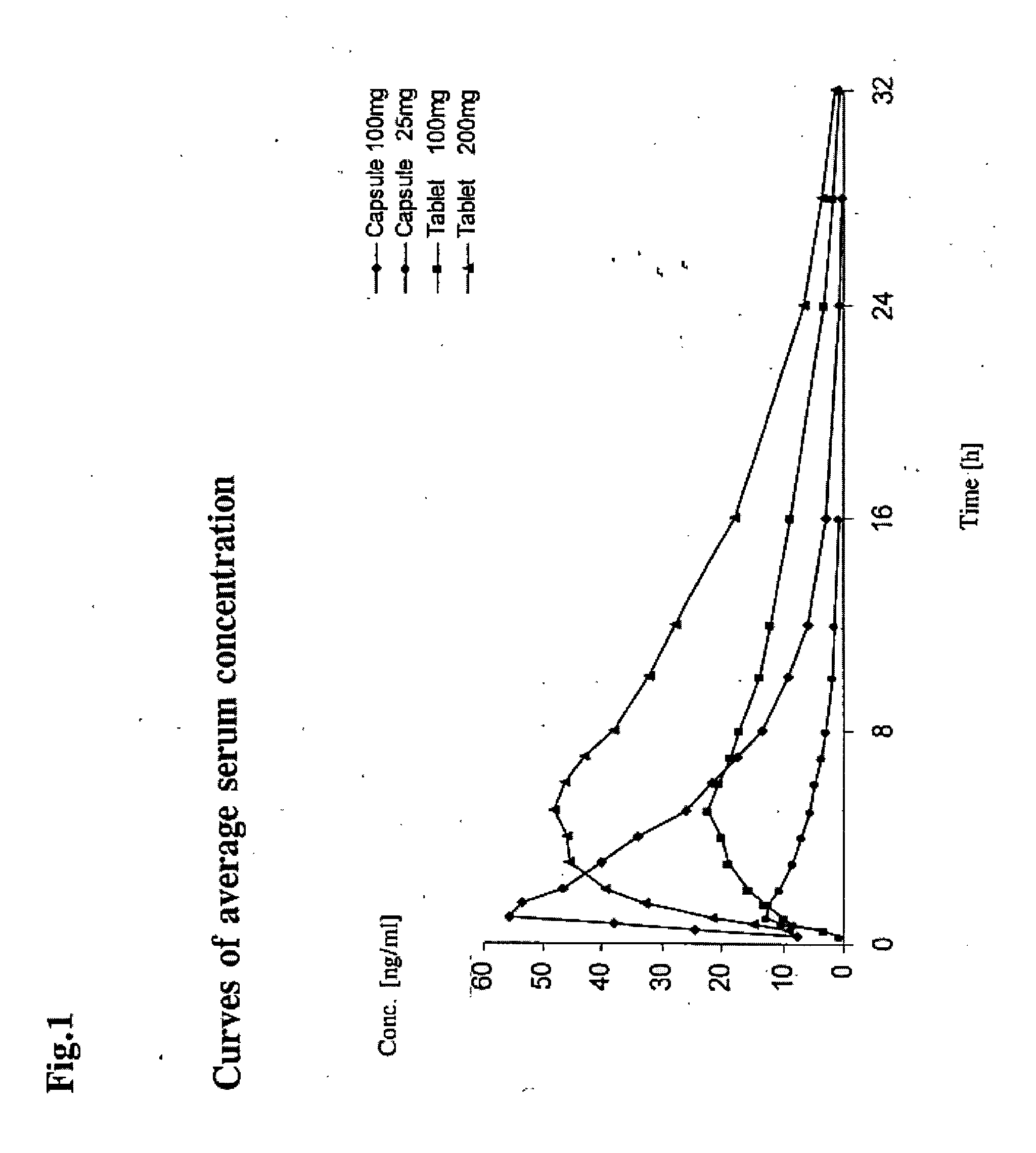
Prolonged Release Pharmaceutical Composition Containing 3-(3-Dimethylamino-1-Ethyl-2-Methyl-Propyl)Phenol
a technology of dimethylamino-1-ethyl-2-methylpropyl and pharmaceutical composition, which is applied in the direction of drug compositions, biocide, microcapsules, etc., can solve the problems of undesirable concentration variation and errors in administration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0046]A batch of 1,000 matrix tablets was produced as described below having with the following composition per tablet:
(−)-(1R,2R)3-(3-dimethylamino-1-ethyl-2-methyl-propyl)phenol100 mghydrochlorideHydroxypropylmethyl cellulose (Metolose 90 SH 100,000 80 mgfrom Shinetsu, 100,000 mPa · sMicrocrystalline cellulose (Avicel PH 102 from FMC)123 mgHighly dispersed silicon dioxide 4 mgMagnesium stearate 3 mgTotal amount310 mg
All components were weighed in and screened on a Quadro Comil U10 screening machine using a screen size of 0.813 mm, mixed in a container mixer (Bohle LM 40) for 15 minutes±15 seconds at a speed of 20±1 rpm and pressed on a Korsch EKO eccentric press to tablets curved in the manner of dragees with a diameter of 10 mm, a radius of curvature of 8 mm and an average tablet weight of 310 mg. The in vitro release was determined by the Ph. Eur. Paddle Method at 75 rpm in 900 ml pH 6.8 buffer according to Ph. Eur. at 37° C. and with detection using a UV spectrometer, and is re...
example 2
[0047]Using a process similar to that described in Example 1, 3,000 matrix tablets were produced having the following composition per tablet:
(−)-(1R,2R)3-(3-dimethylamino-1-ethyl-2-methyl-propyl)phenol200 mghydrochlorideHydroxypropylmethyl cellulose (Metolose 90 SH 100,000 80 mgfrom Shinetsu, 100,000 mPa · sMicrocrystalline cellulose (Avicel PH 102 from FMC) 23 mgHighly dispersed silicon dioxide 4 mgMagnesium stearate 3 mgTotal amount310 mg
[0048]The in vitro release was determined as in Example 1.
Time (min)Total amount of active ingredient released [%]0030196030120461805824068360844809372099
example 3
[0049]Using a process similar to that described in Example 1, a batch of 3,000 matrix tablets were produced having the following composition per tablet:
(−)-(1R,2R)3-(3-dimethylamino-1-ethyl-2-methyl-propyl)phenol100 mghydrochlorideHydroxypropylmethyl cellulose (Metolose 90 SH 100,000 40 mgfrom Shinetsu, 100,000 mPa · sMicrocrystalline cellulose (Avicel PH 102 from FMC)163 mgHighly dispersed silicon dioxide 4 mgMagnesium stearate 3 mgTotal amount310 mg
The in vitro release was determined as in Example 1. In addition, the release was determined under otherwise identical conditions at stirring speeds of 50 and 100 rpm.
Total amountTotal amountTotal amount of activeof activeof activeTimeingredient released [%]ingredient releasedingredient released(min)at 50 rpm[%] at 75 rpm[%] at 100 rpm00003020202160353335120545153180676366240767376360898789480979597600100100100
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| viscosity | aaaaa | aaaaa |
| viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com