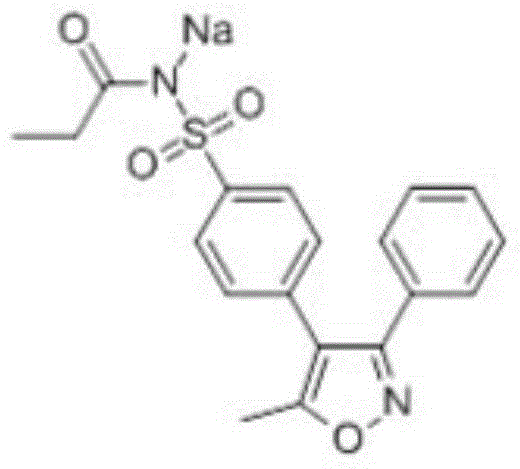
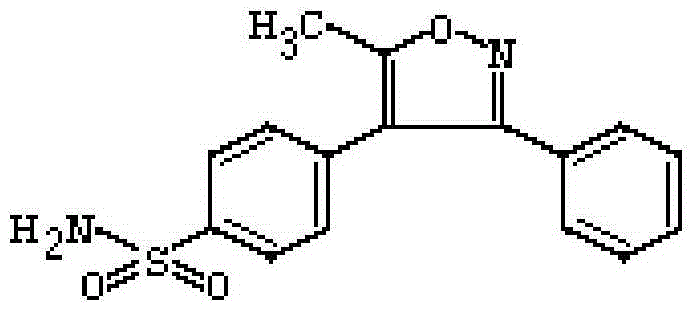
Preparation method of anhydrous and non-solvation A crystallization parecoxib sodium
A technology of parecoxib sodium and parecoxib, which is applied in the field of preparation of anhydrous, non-solvated A crystal form parecoxib sodium, and can solve high equipment requirements, high safety requirements, and long reaction time And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
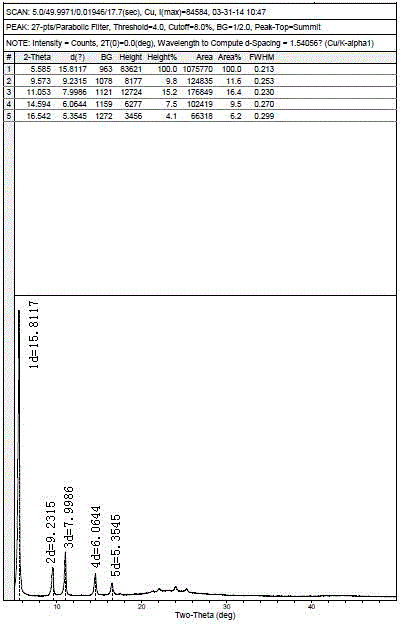
Image
Examples
Embodiment 1
[0068] (1) Preparation of 1-(1,2-diphenylvinyl)pyrrolidine (DPVP)
[0069] Add 304.0g of 1,2-diphenylethanone, 335.2g of pyrrolidine, 9.3g of glacial acetic acid, and 808.6g of cyclohexane to a 3L three-necked round-bottomed flask equipped with a Dean / Stark water separator and a reflux condenser, and stir Control the temperature of the feed liquid to rise to 80±5°C, and stir the reaction at this temperature, and track and detect the reaction process according to the water separation of the Dean / Stark water separator. After the reaction was completed, the reaction solution was cooled to 30±5°C, concentrated under reduced pressure at 70±5°C until no organic solvent was distilled off, and 430.6 g of a brown viscous liquid product was obtained.
[0070] (2) Preparation of 4,5-dihydro-5-methyl-3,4-diphenyl-5-isoxazolol (MPHI)
[0071]The viscous liquid obtained in the above steps was placed in a round bottom flask, and 391.4 g of acetonitrile was added, and stirred to make it diss...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com