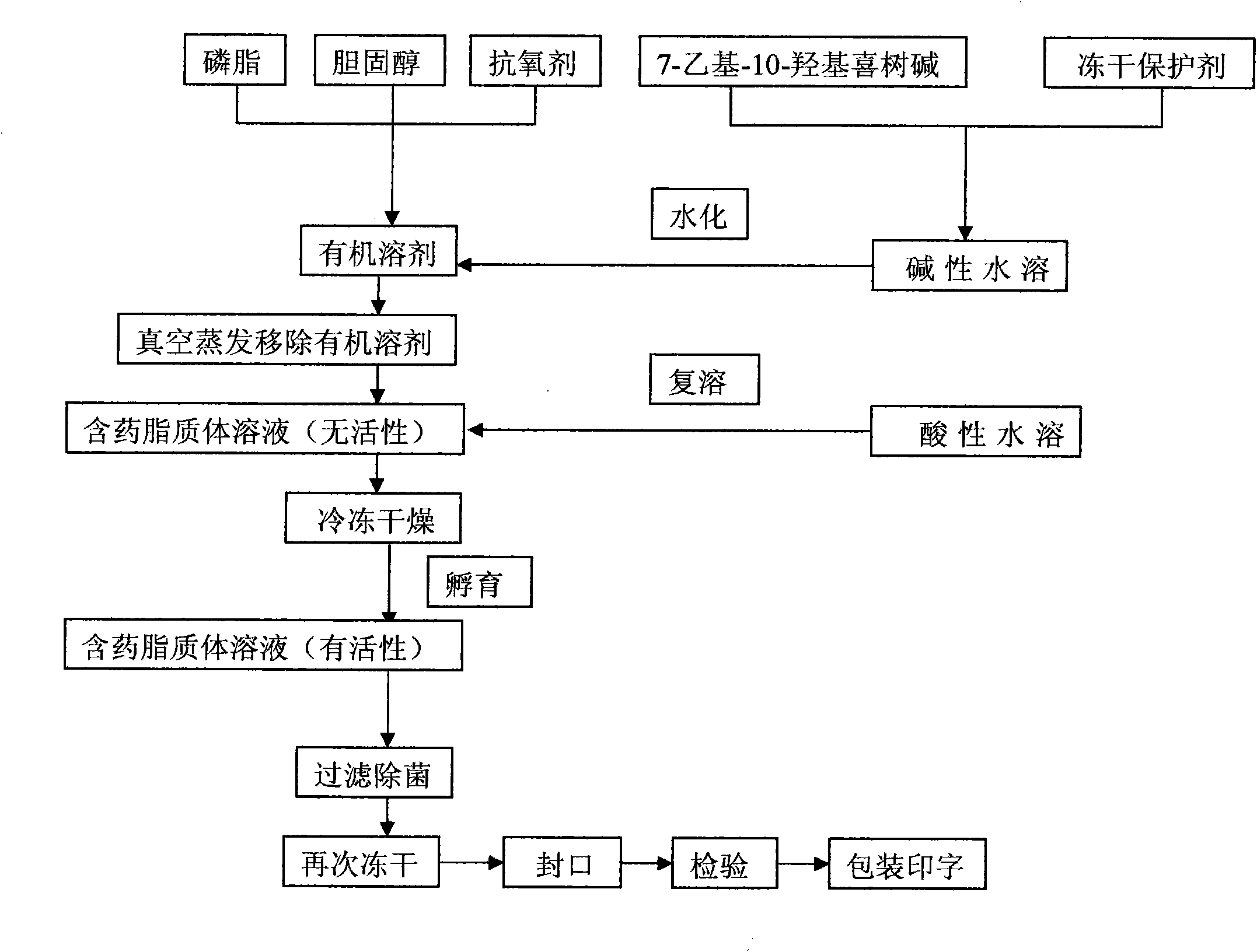
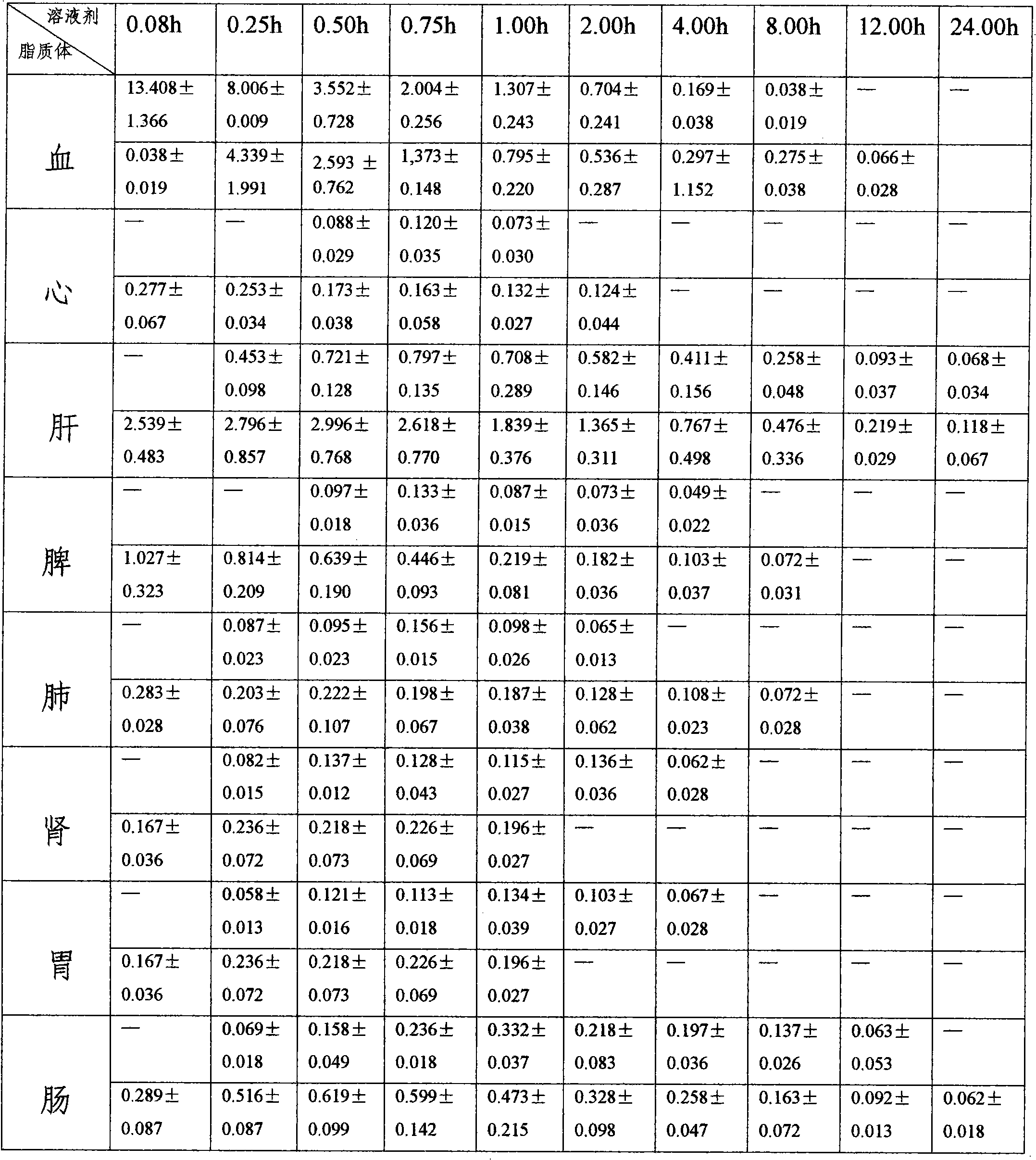
7-ethyl-10-hydroxycamptothecine liposome freeze-dried powder injection and preparation method thereof
A technology of hydroxycamptothecin and hydroxycamptothecin, applied in the field of 7-ethyl-10-hydroxycamptothecin liposome freeze-dried powder injection and its preparation, can solve the problem of low anti-tumor activity, limited application, and high toxicity and other problems to achieve the effect of prolonging the metabolic time, eliminating side effects, and reducing irritation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] (1) Dissolve 30 grams of soybean lecithin (injection grade), 10 grams of cholesterol (injection grade), and 2 grams of α-tocopherol in 2000 ml of ethanol (analytical pure), vacuum rotary transfer organic solvent, and place in a vacuum constant temperature drying oven overnight The residual organic solvent was evaporated to prepare dry lipid film, and the above operations were all completed under sterile conditions.
[0053] (2) Dissolve 1.67 grams of 7-ethyl-10-hydroxycamptothecin and 100 grams of sucrose (injection grade) in 1000 ml of sodium carbonate-sodium bicarbonate buffered saline solution (pH=9.9) at a hydration temperature of 45°C Next, use the above-mentioned alkaline buffered saline solution to hydrate the lipid dry film, and after the hydration is complete, use a milk homogenizer to reduce the particle size to 100-200nm to obtain drug-containing liposomes. All the above operations were completed under sterile conditions.
[0054] (3) Vacuum freeze-dry drug-...
Embodiment 2
[0056] (1) Dissolve 40 grams of sphingomyelin (injection grade), 10 grams of cholesterol (injection grade), and 2 grams of α-tocopherol in 2000 ml of ethanol (analytical pure), vacuum rotary transfer organic solvent, and place in a vacuum constant temperature drying oven The residual organic solvent was evaporated overnight to prepare a dry lipid film. The above operations were all completed under sterile conditions.
[0057] (2) Dissolve 2 grams of 7-ethyl-10-hydroxycamptothecin and 200 grams of trehalose (injection grade) in 2000 ml of potassium dihydrogen phosphate-sodium hydroxide buffer solution (pH=9.9) Hydrate the lipid dry film with the above-mentioned alkaline buffered saline solution at the temperature of 100 °C, and reduce the particle size to 100-200 nm with a homogenizer after the hydration is complete, to obtain the drug-containing liposome. All the above operations were completed under sterile conditions.
[0058] (3) Vacuum freeze-drying of the drug-containing...
Embodiment 3
[0060] (1) Dissolve 30 grams of phosphatidylcholine (injection grade), 10 grams of cholesterol (injection grade), and 2 grams of α-tocopherol in 2000 ml of ethanol (analytical pure), vacuum rotary transfer organic solvent, and vacuum constant temperature drying oven Leave it overnight to evaporate the residual organic solvent to prepare a lipid dry film. The above operations are all completed under sterile conditions.
[0061] (2) Dissolve 1 g of 7-ethyl-10-hydroxycamptothecin and 300 g of lactose (injection grade) in 3000 ml of barbiturate sodium-hydrochloric acid buffer solution (pH=9.9) at a hydration temperature of 45°C The dry lipid film is hydrated with the above-mentioned alkaline buffered saline solution, and after the hydration is complete, the particle size is reduced to 100-200nm with a homogenizer to obtain the drug-containing liposome. The above operations were all done under sterile conditions.
[0062] (3) Vacuum freeze-drying drug-containing liposome solution ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com