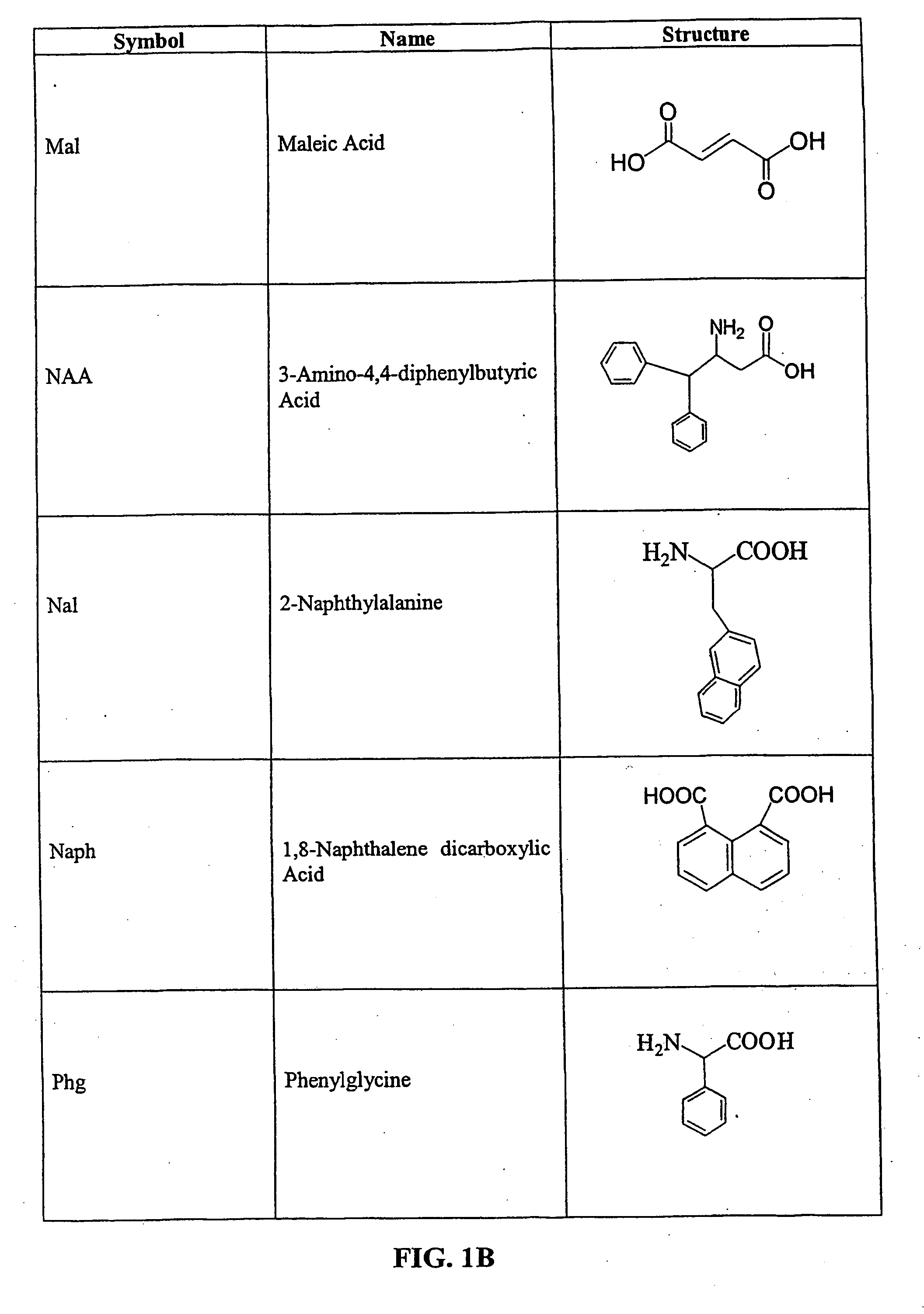
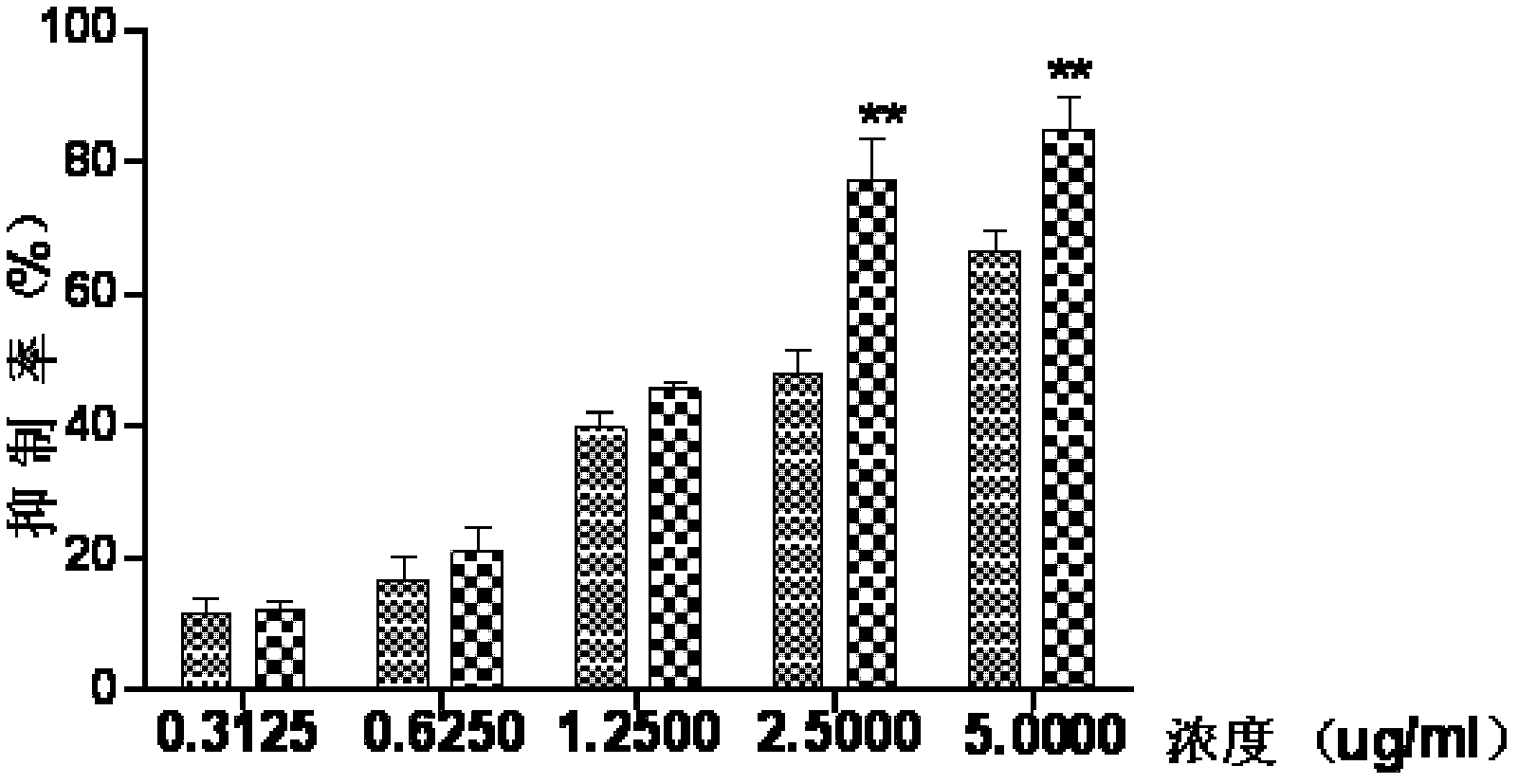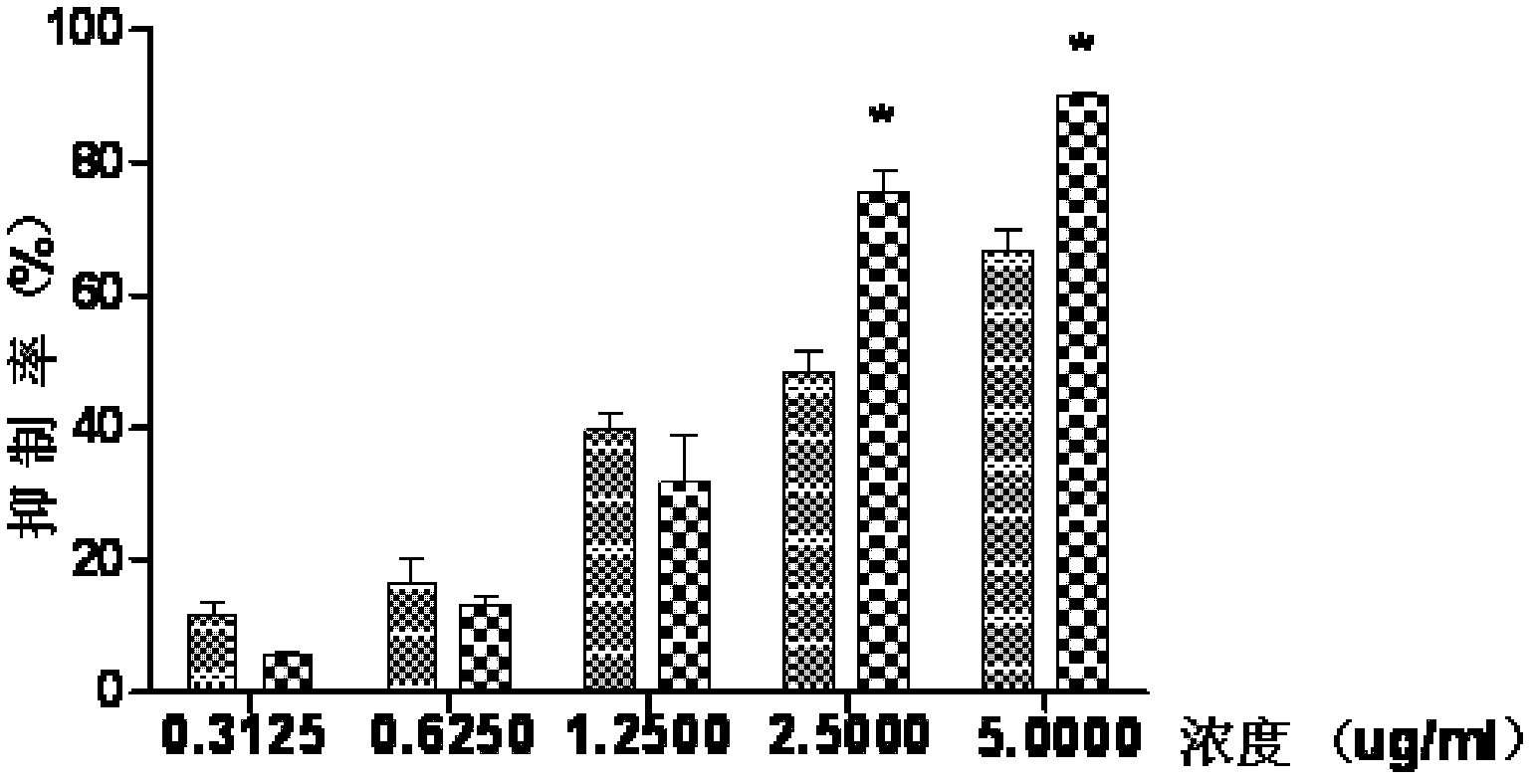Patents
Literature
115results about How to "Reduce toxicity" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
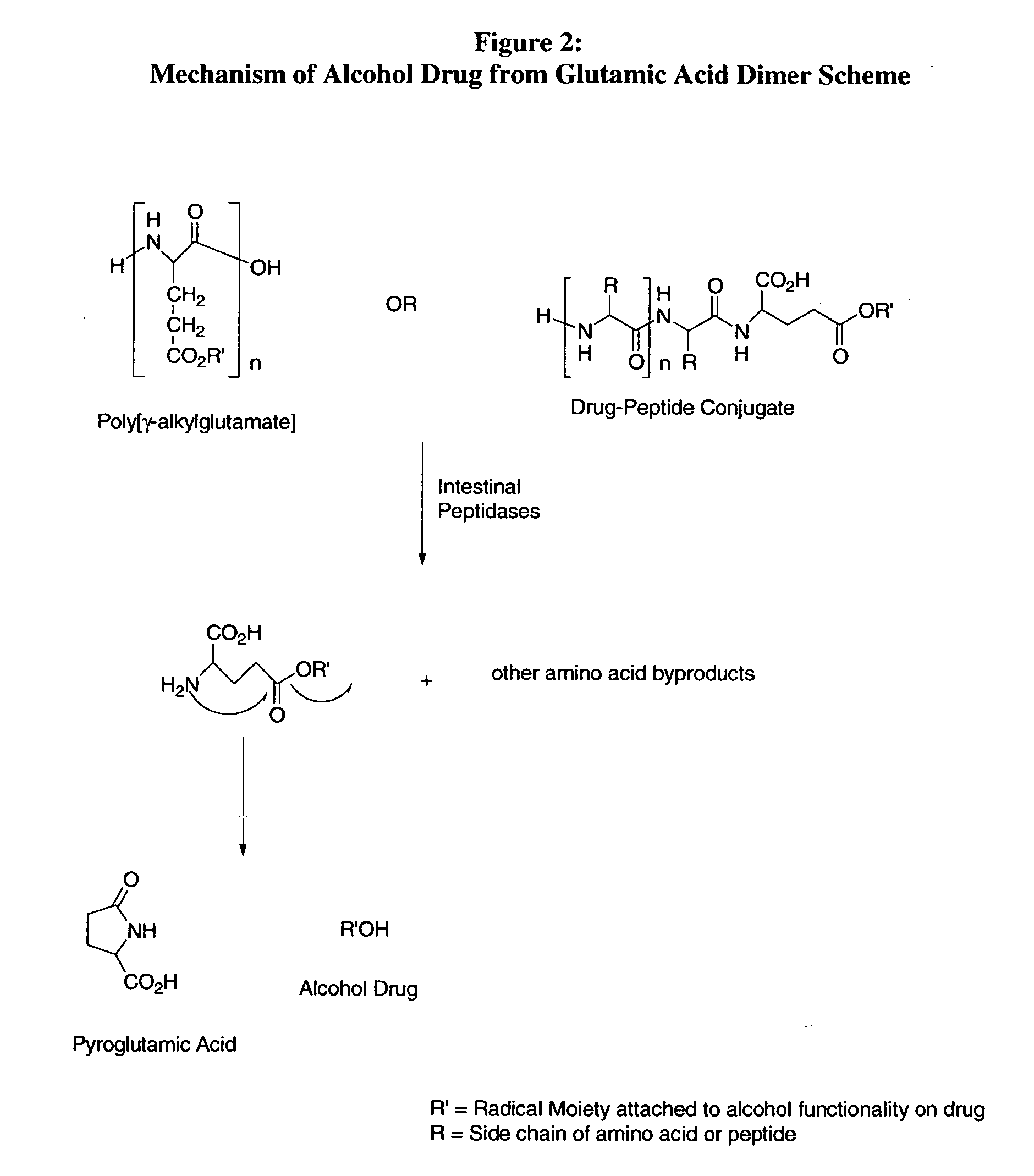
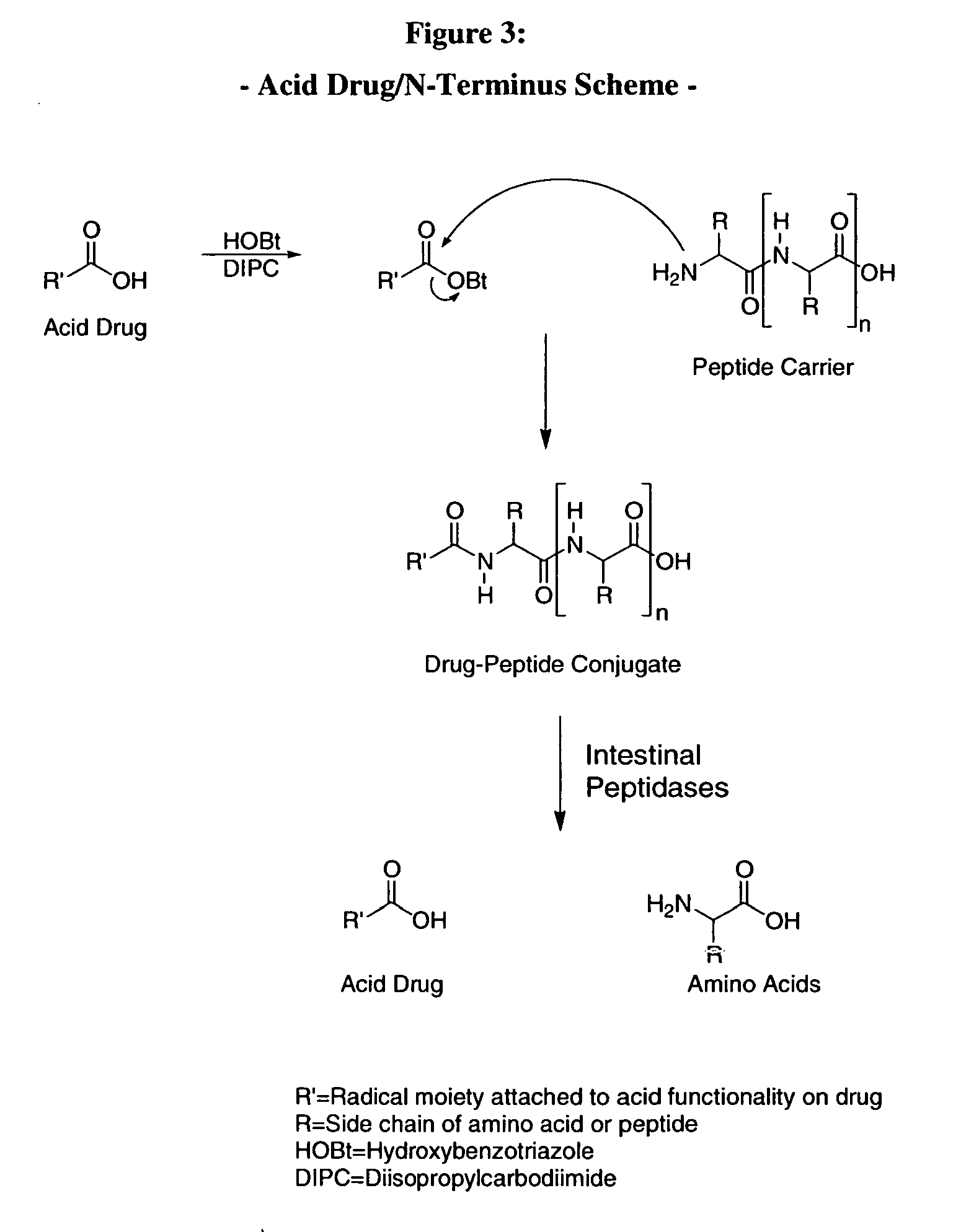
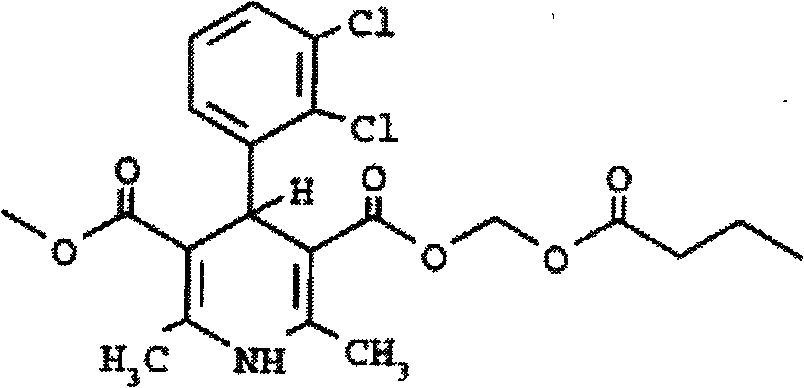
Prodrug compounds with isoleucine
InactiveUS20070020694A1High specificity of actionReduce toxicityAntipyreticAnalgesicsEnzymeIsoleucine
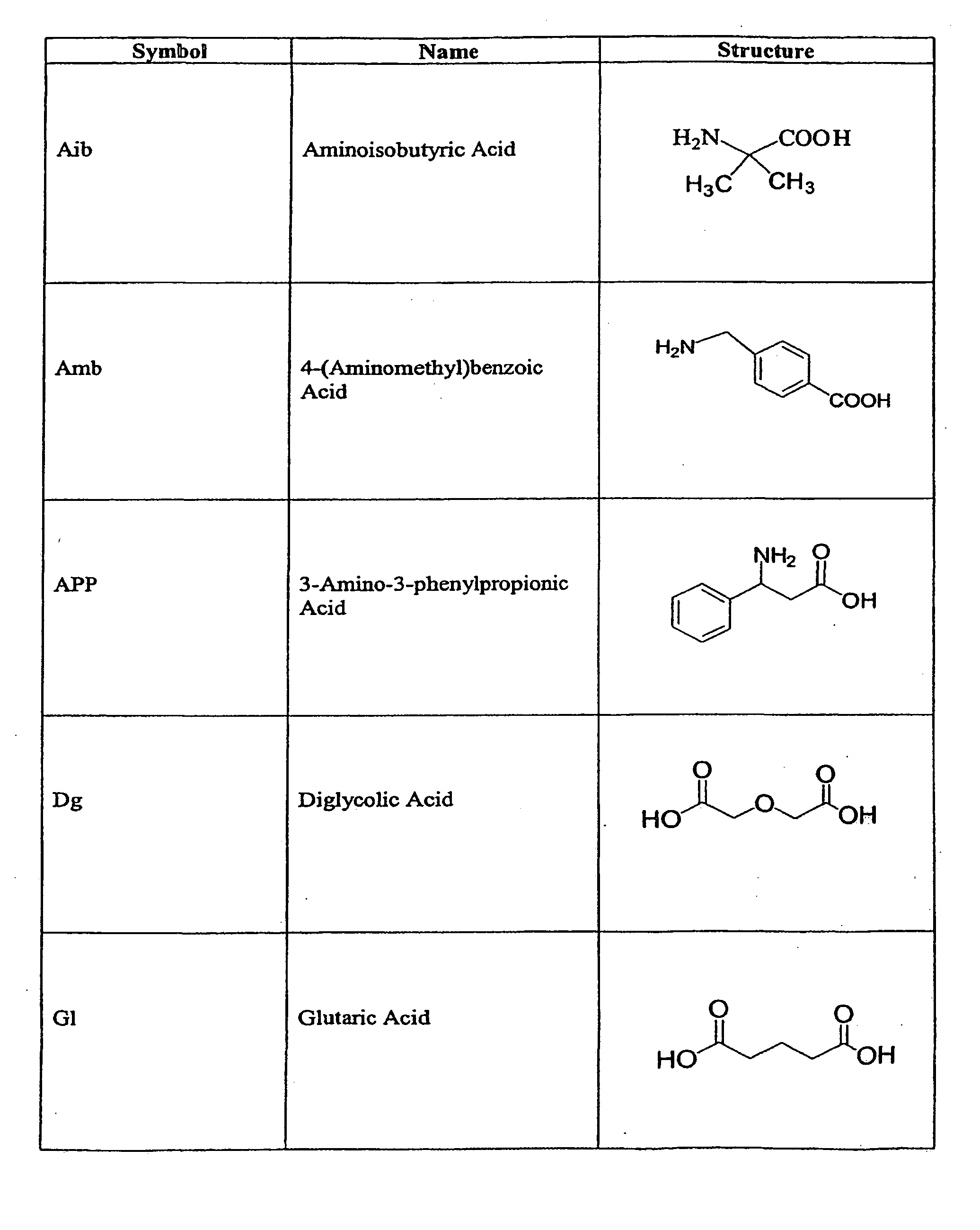
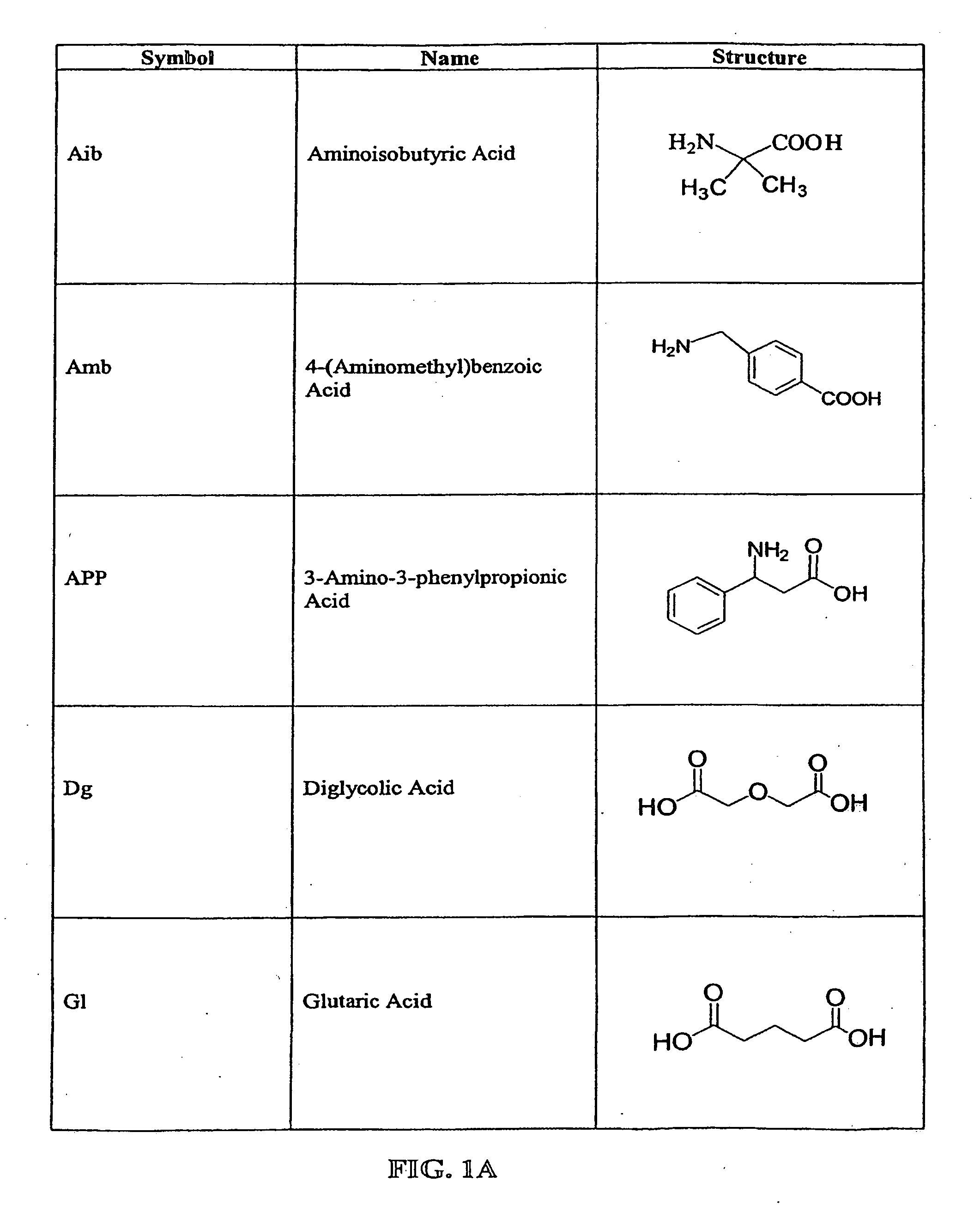
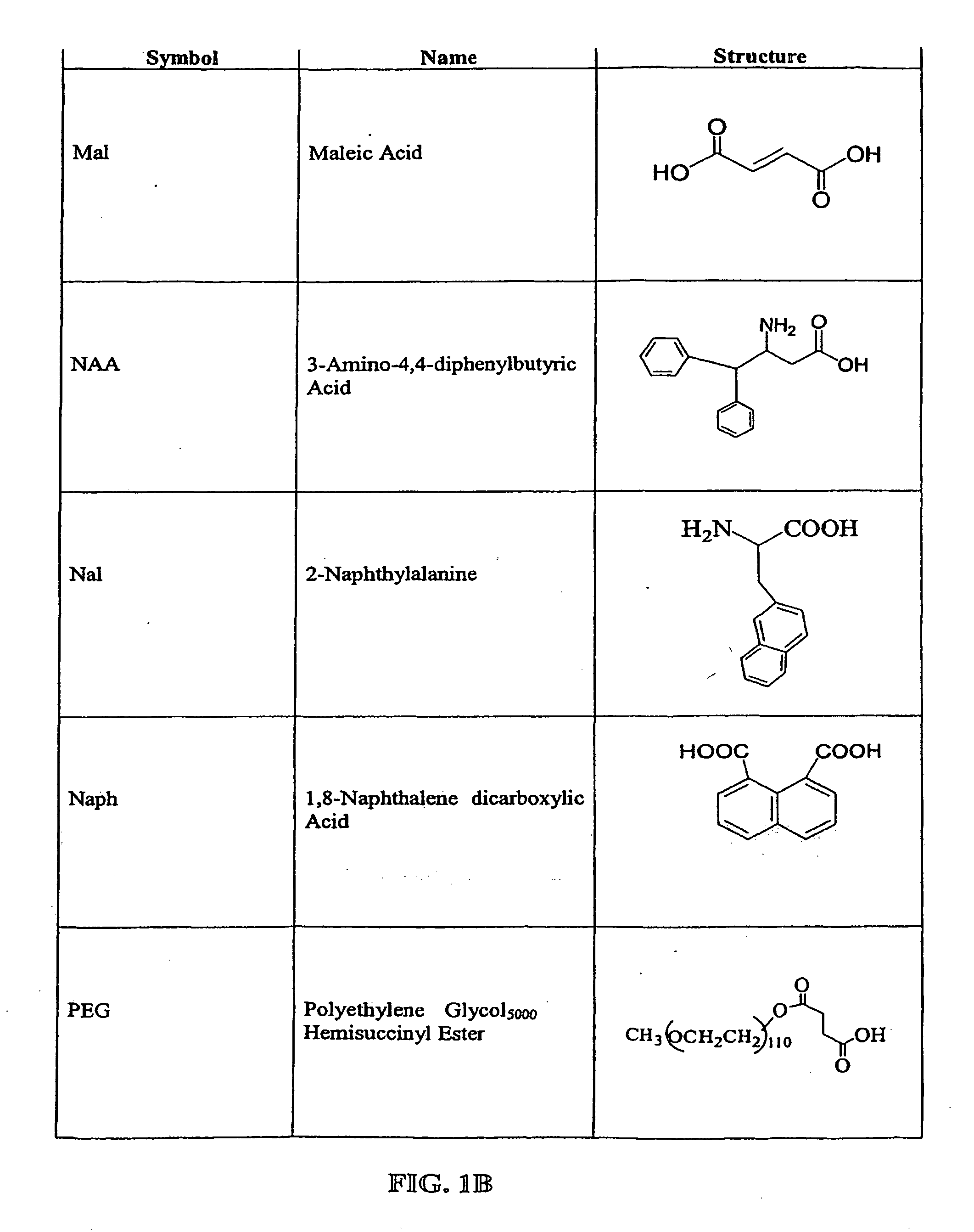
The compounds of the invention are modified forms of therapeutic agents. A typical prodrug compound of the invention comprises a therapeutic agent, an oligopeptide having an isoleucine residue, a stabilizing group and, optionally, a linker group. The prodrug is cleavable by an enzyme associated with the target cell. Methods of making and using the compounds are also disclosed.
Owner:MEDAREX LLC
Active agent delivery systems and methods for protecting and administering active agents
The present invention relates to active agent delivery systems and more specifically to compositions that comprise amino acids, as single amino acids or peptides, covalently attached to active agents and methods for administering conjugated active agent compositions.
Owner:TAKEDA PHARMACEUTICA CO LTD
Low VOC ultra high solids thermosetting coating composition and method of its preparation
The present invention is directed to a low VOC ultra high solids thermosetting coating composition. A polymeric component of the composition includes a low molecular weight polymer polymerized from a monomer mixture containing less than 45 percent by weight of at least one active hydrogen containing monomer. The polymerization takes place in the presence of a reactive diluent. By including the reactive diluent in the polymeric component of the composition, the amount of solvent used in the composition can be substantially reduced, while still providing a pot mix of the composition with a viscosity sufficient to permit efficient coating application. The coatings from the composition are durable; glossy; impact and solvent resistant; and adherent to a wide variety of substrates, such as automobile bumpers, road surfaces and office equipment. The surface of these coatings when subjected to scuffing or surface marring action can be substantially restored to its original condition by simply buffing or polishing the marred or scuffed surface. The invention is also directed to include a combination of chemicals in the thermosetting composition containing an isocyanate cross linking component. Such a combination simultaneously increases the pot life of the pot mix while decreasing the drying time of a coating from the pot mix at ambient temperature over a wide variety of substrate surfaces.
Owner:ROHM & HAAS CO
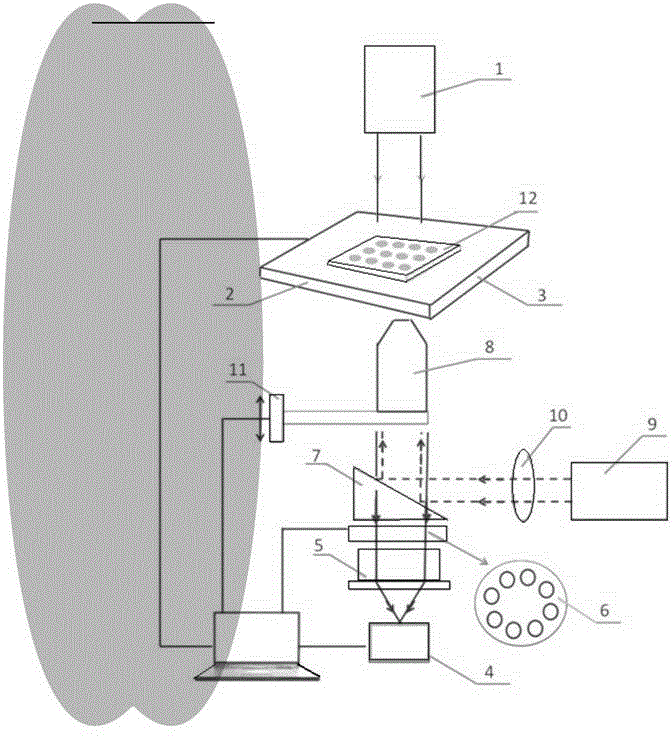
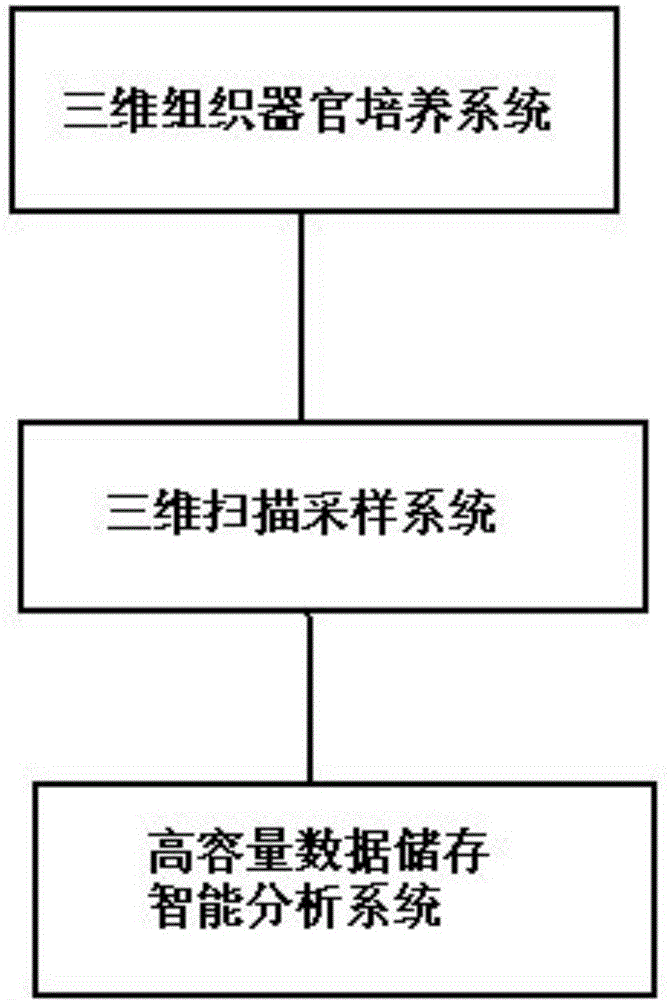
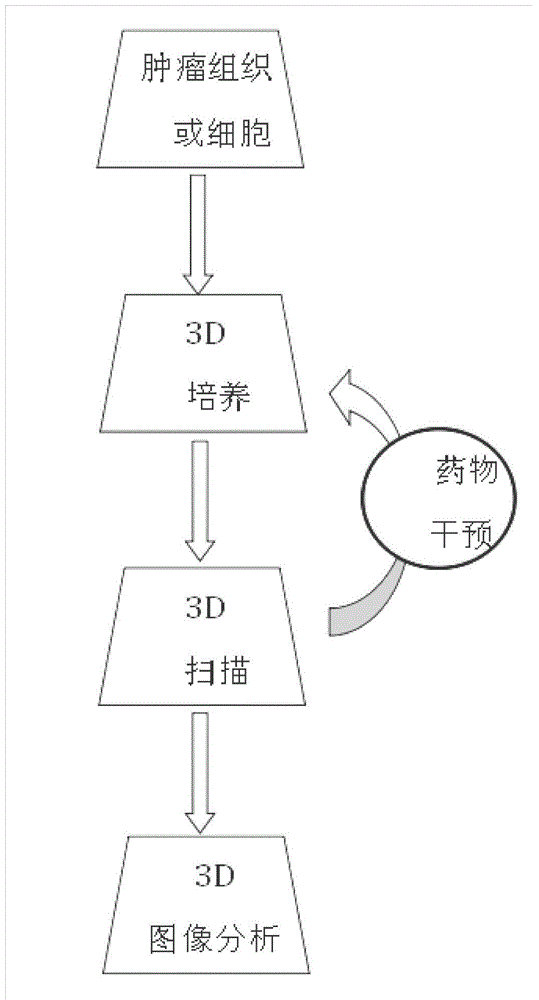
Three dimensional tissue and organ culture model, high throughput automatic stereo image analyzing platform and applications thereof
ActiveCN104403923APrecise screeningHigh speedBioreactor/fermenter combinationsBiological substance pretreatmentsBiological macromoleculeComputer analysis
The invention discloses a three dimensional tissue and organ culture model, a high throughput automatic stereo image analyzing platform thereof, and a method utilizing the provided platform to screen antitumor drugs. The platform can be applied to the culture system of three dimensional tissue and organ / organ-like substance, three dimensional scanning and sampling system, and high-volume data storage and computer analysis system. The provided platform can simultaneously process a large amount of clinical samples and screen a plurality of antitumor drugs, is capable of greatly reducing the cost and maximally reducing the detection time, thus is widely applied to the drug selection schemes and dosage optimization in clinic, novel drug development, and basic researches on interactions among tissue, organ, biological macromolecules, and other micromolecules.
Owner:NANJING KDRB BIOTECH INC LTD
Liquid crystals with reduced toxicity and applications thereof
InactiveUS20050079487A1Reduce toxicityLow toxicityLiquid crystal compositionsMaterial nanotechnologyCell culture mediaLiquid crystal
Liquid crystal compositions that exhibit little or no toxicity with respect to cells include liquid crystals with chemical functional groups such as fluorine atoms, fluorophenyl groups, or difluorophenyl groups. Liquid crystals with little or no toxicity to cell lines may be added to cell culture media or added to components used in cell culture media. Cells may be grown in cell culture media that includes liquid crystals that exhibit little or no toxicity to cells.
Owner:WISCONSIN ALUMNI RES FOUND
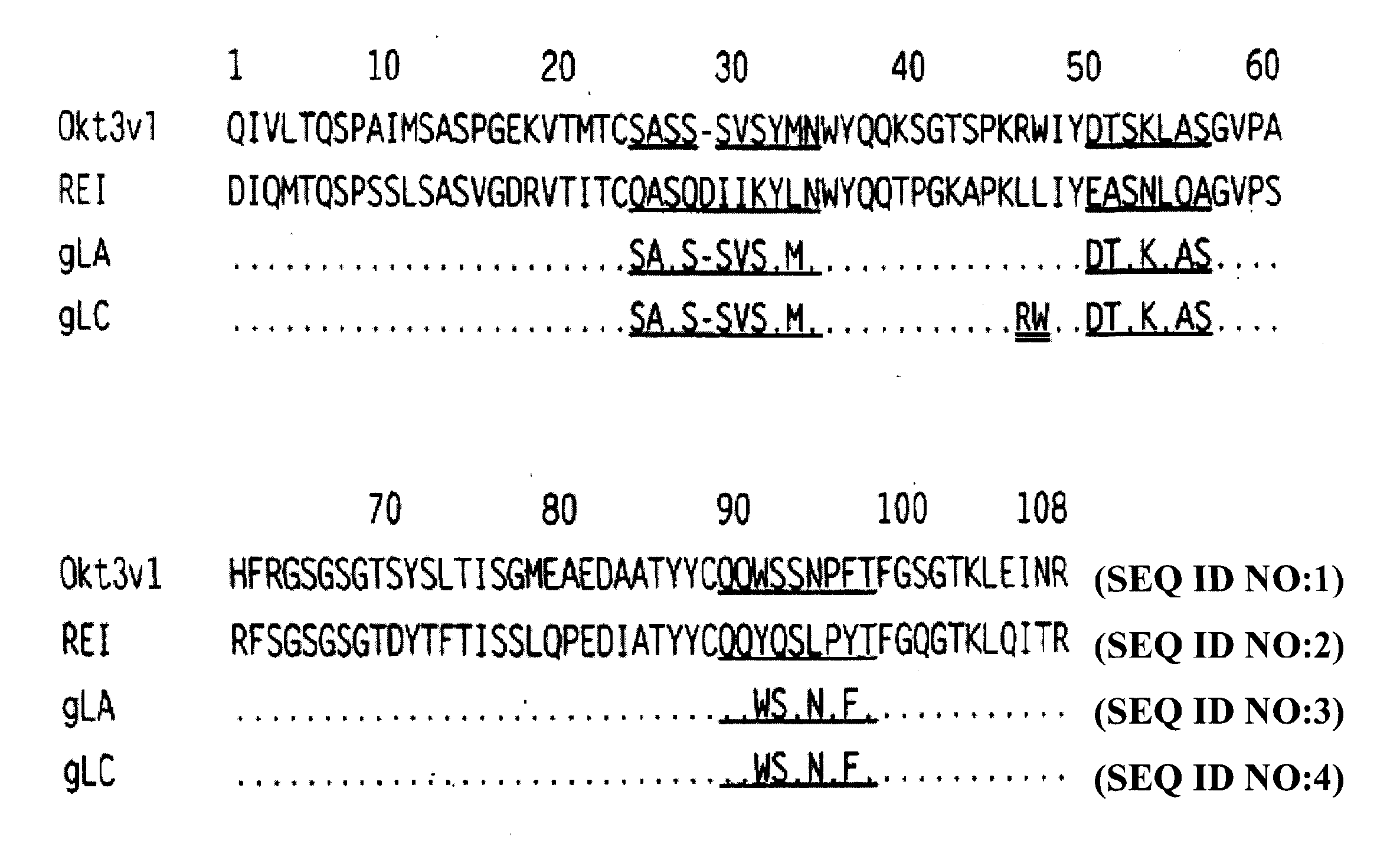
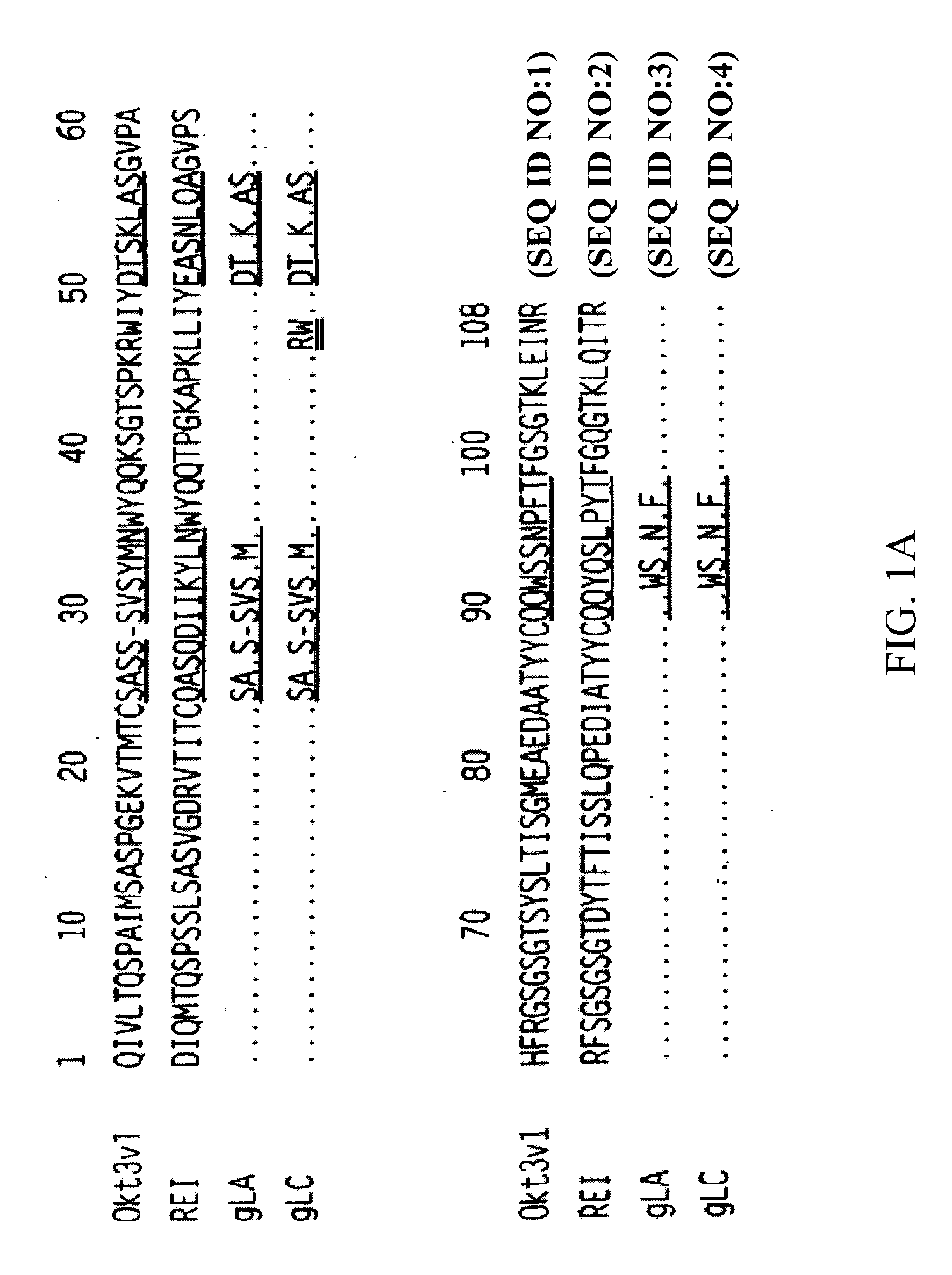
Methods for the treatment of lada and other adult- onset autoimmune using immunosuppressive monoclonal antibodies with reduced toxicity
InactiveUS20100015142A1Reduce toxicityHigh level of functionMetabolism disorderAntibody ingredientsCD3 AntibodyImmunosuppression
The present invention provides methods of treating, preventing or ameliorating the symptoms of Latent Autoimmune Diabetes in Adults (LADA) and adult-onset type 1 diabetes through the use of anti-human CD3 antibodies. In particular, in invention provides methods of preventing or delaying insulin requirement in patients diagnosed with LADA. The methods of the invention provide for administration of antibodies that specifically bind the epsilon subunit within the human CD3 complex. Such antibodies modulate the T cell receptor / alloantigen interaction and, thus, regulate the T cell mediated cytotoxicity associated with autoimmune disorders. Additionally, the invention provides for modification of the anti-human CD3 antibodies such that they exhibit reduced or eliminated effector function and T cell activation as compared to non-modified anti-human CD3 antibodies.
Owner:MACROGENICS INC
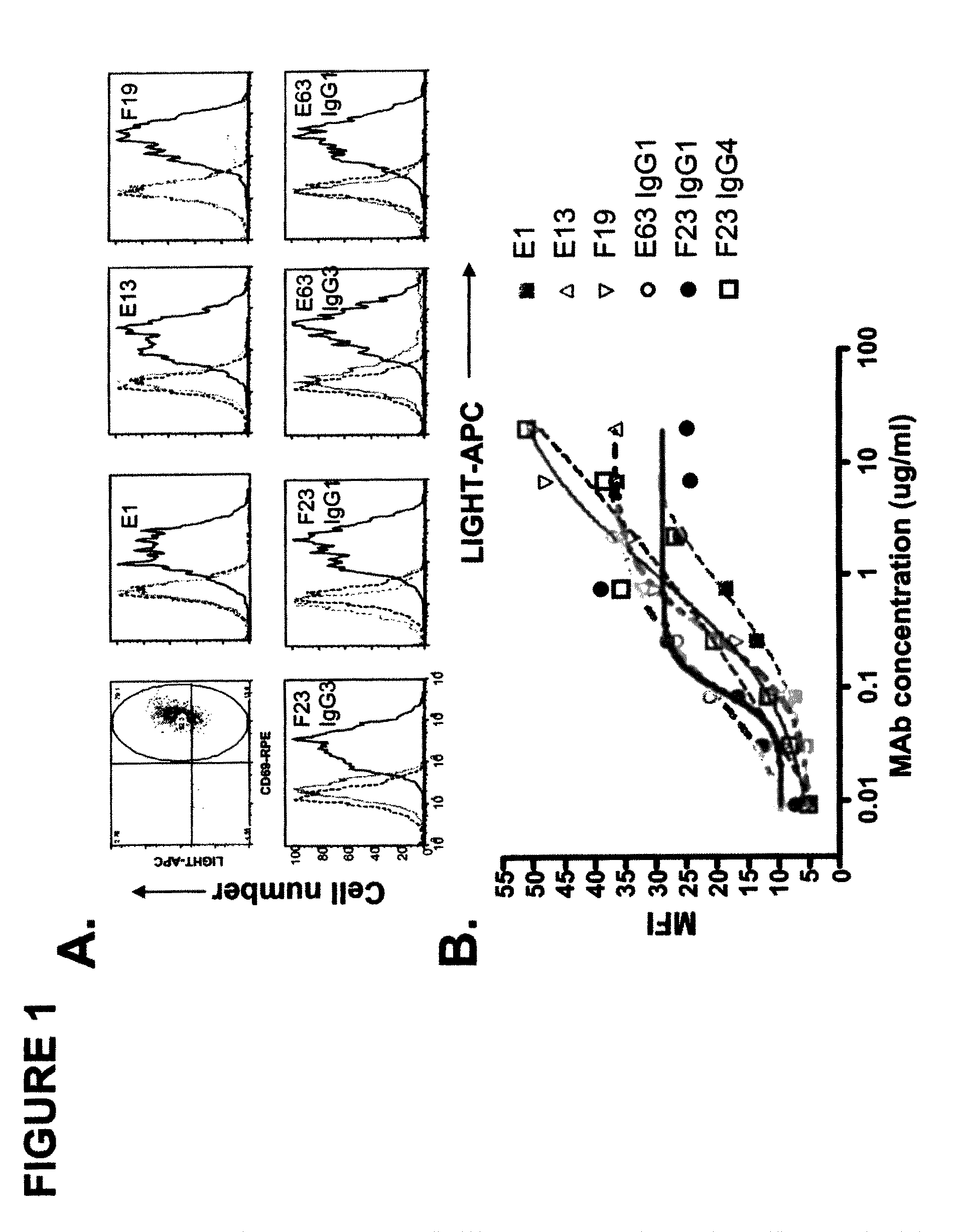
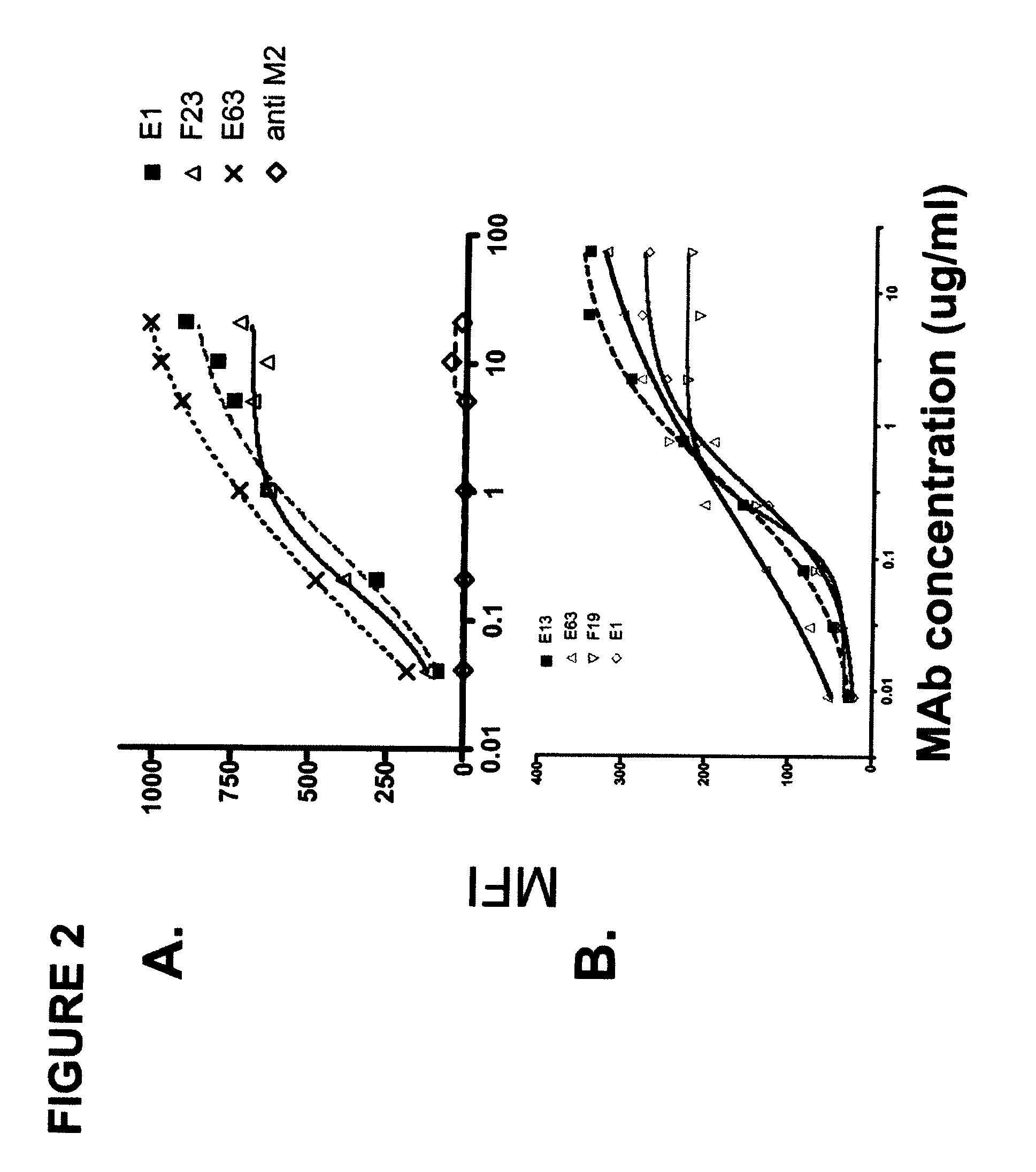
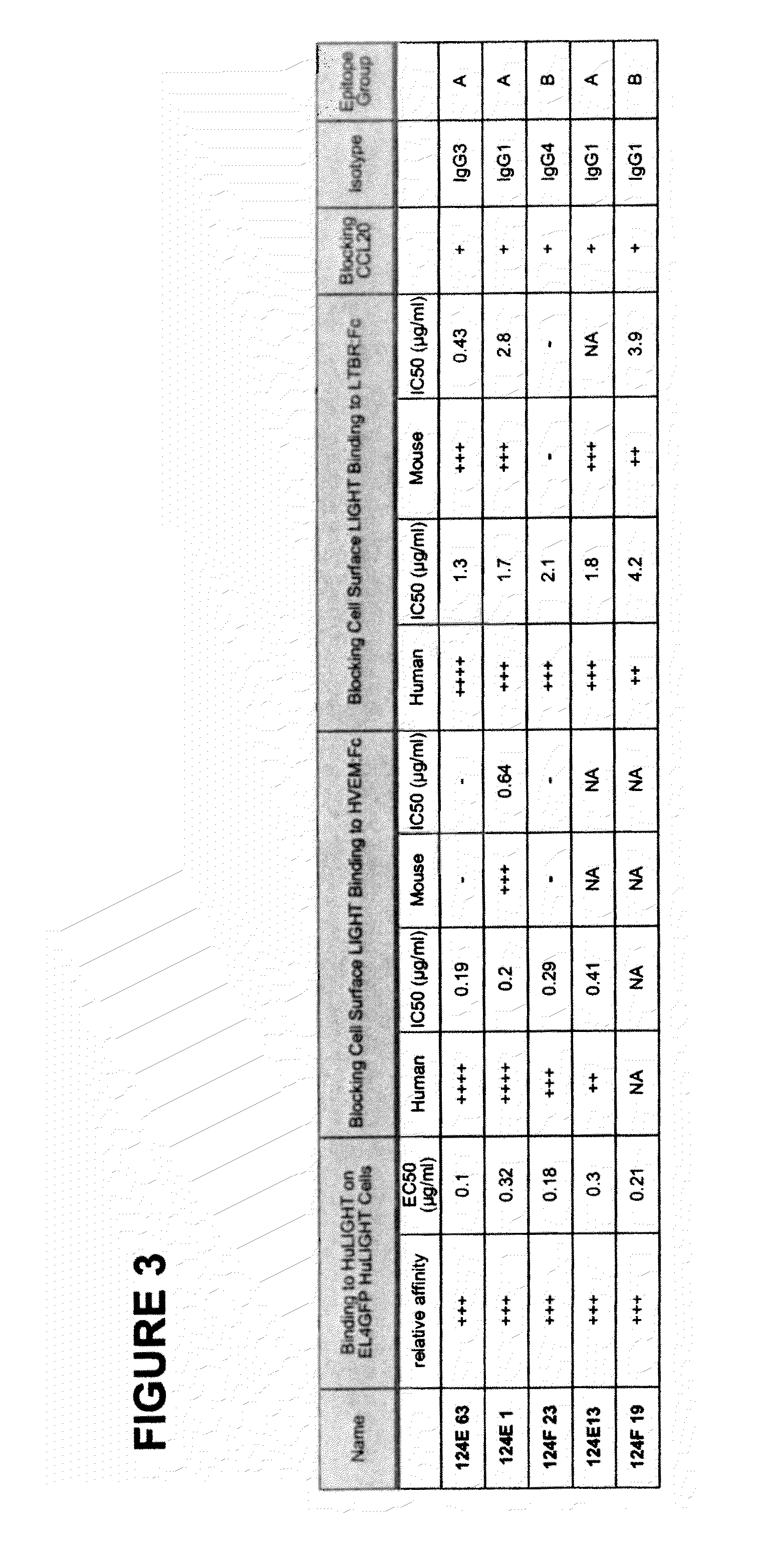
Antagonistic human LIGHT-specific human monoclonal antibodies
ActiveUS8058402B2Reduce toxicityReduce or avoid effectAntibacterial agentsAntipyreticImmunologic specificityDisease
Provided herein are antibodies, such as fully human antibodies that immunospecifically bind to an hLIGHT polypeptide. Also provided are isolated nucleic acids encoding antibodies, such as fully human antibodies, that immunospecifically bind to a hLIGHT polypeptide. Further provided are vectors and host cells comprising nucleic acids encoding antibodies, such as fully human antibodies, that immunospecifically bind to a hLIGHT polypeptide. Also provided are methods of making antibodies, such as fully human antibodies, that immunospecifically bind to a hLIGHT polypeptide. Also provided herein is a method of treating a hLIGHT-mediated disease in a subject comprising administering to the subject an antibody, such as a fully human antibody, that immunospecifically binds to a hLIGHT polypeptide. In preferred embodiments, that anti-hLIGHT antibodies provided herein will ameliorate, neutralize or otherwise inhibit hLIGHT biological activity in vivo (e.g., the hLIGHT-mediated production or secretion of CCL20, IL-8 or RANTES from a cell expressing a hLIGHT receptor). Also provided herein is a method for the detection of hLIGHT in a sample as well as a method for ameliorating, neutralizing or otherwise inhibiting hLIGHT activity, e.g., in a human subject suffering from a disorder in which hLIGHT activity is detrimental.
Owner:KYOWA HAKKO KIRIN CO LTD +1
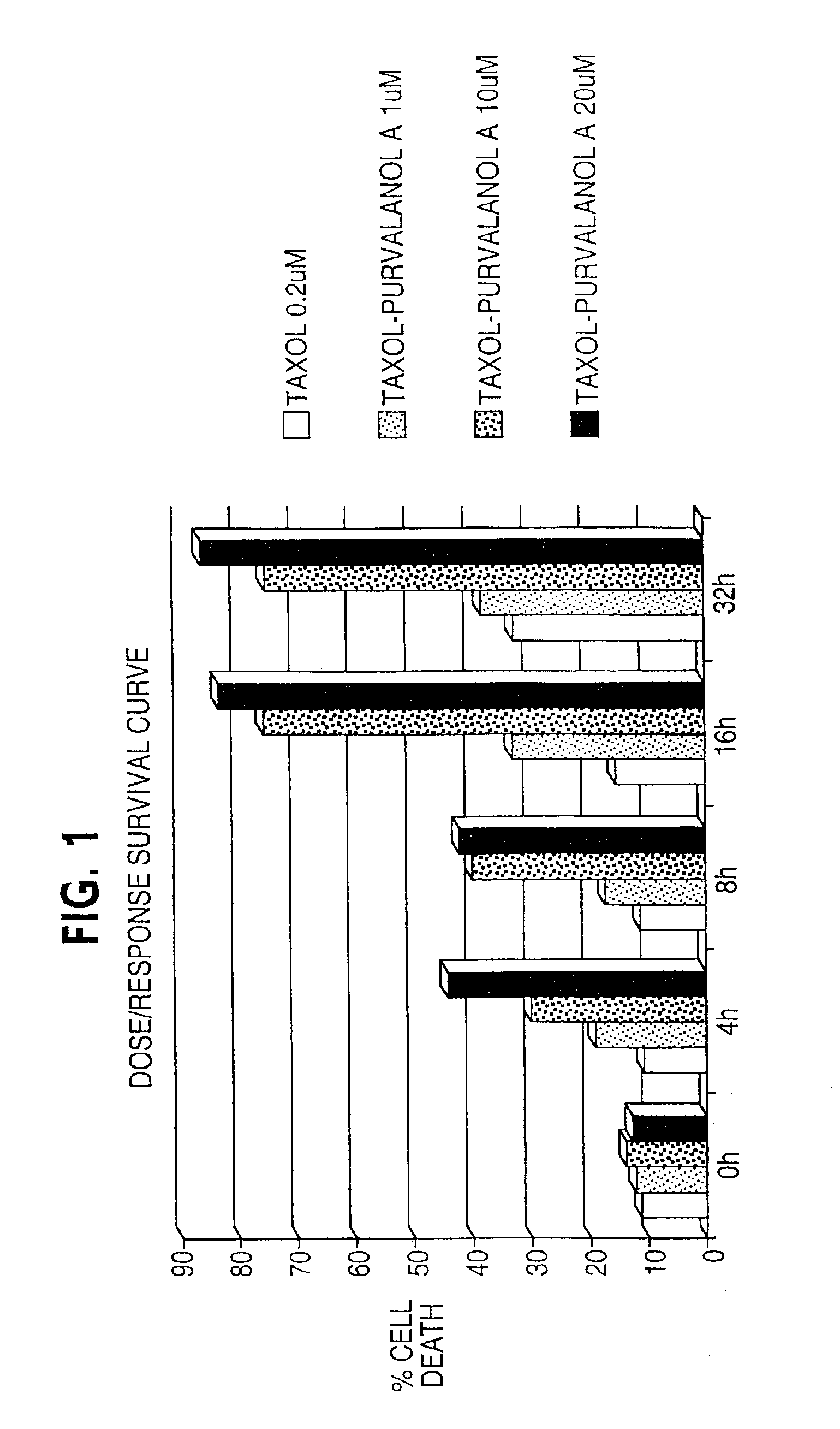
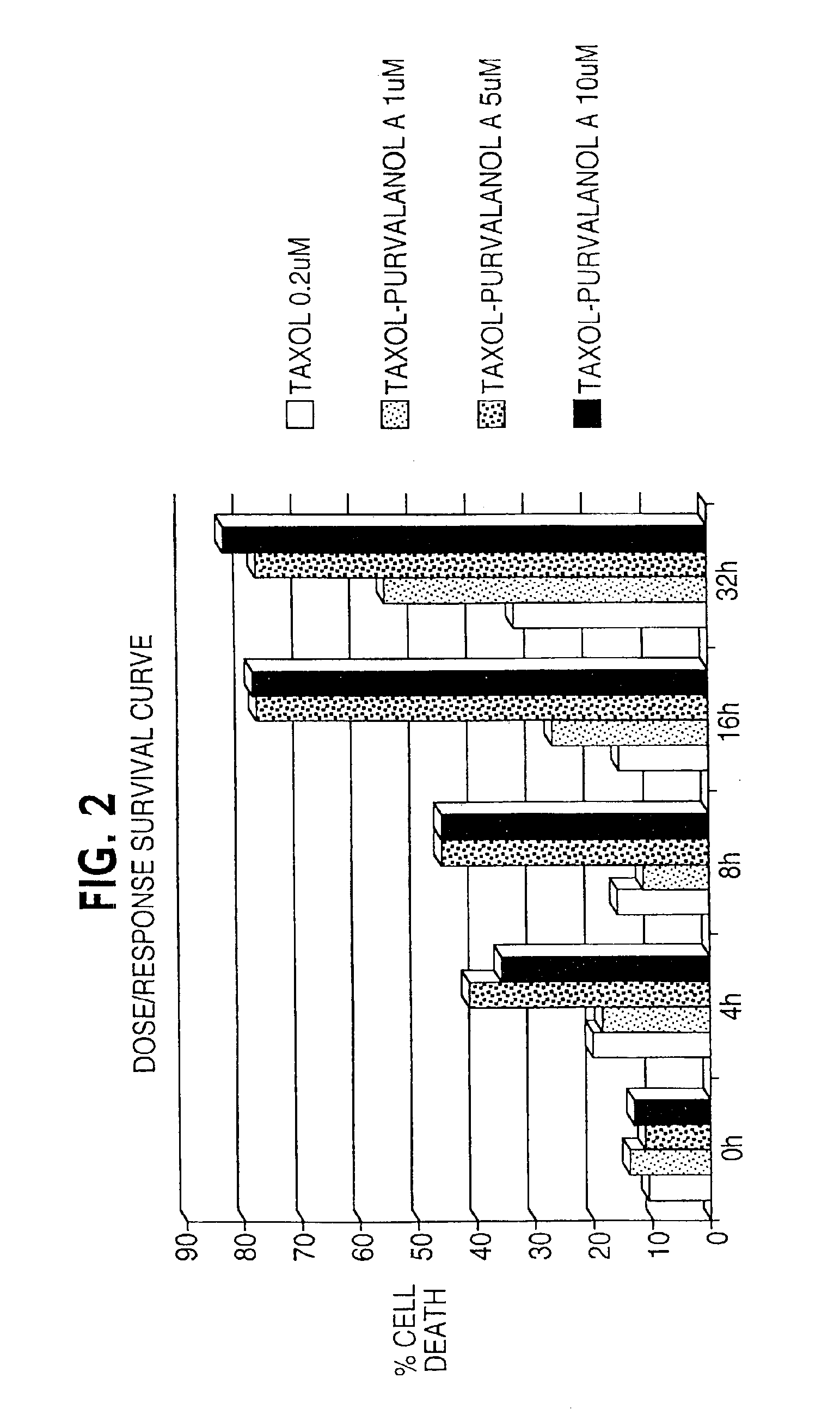
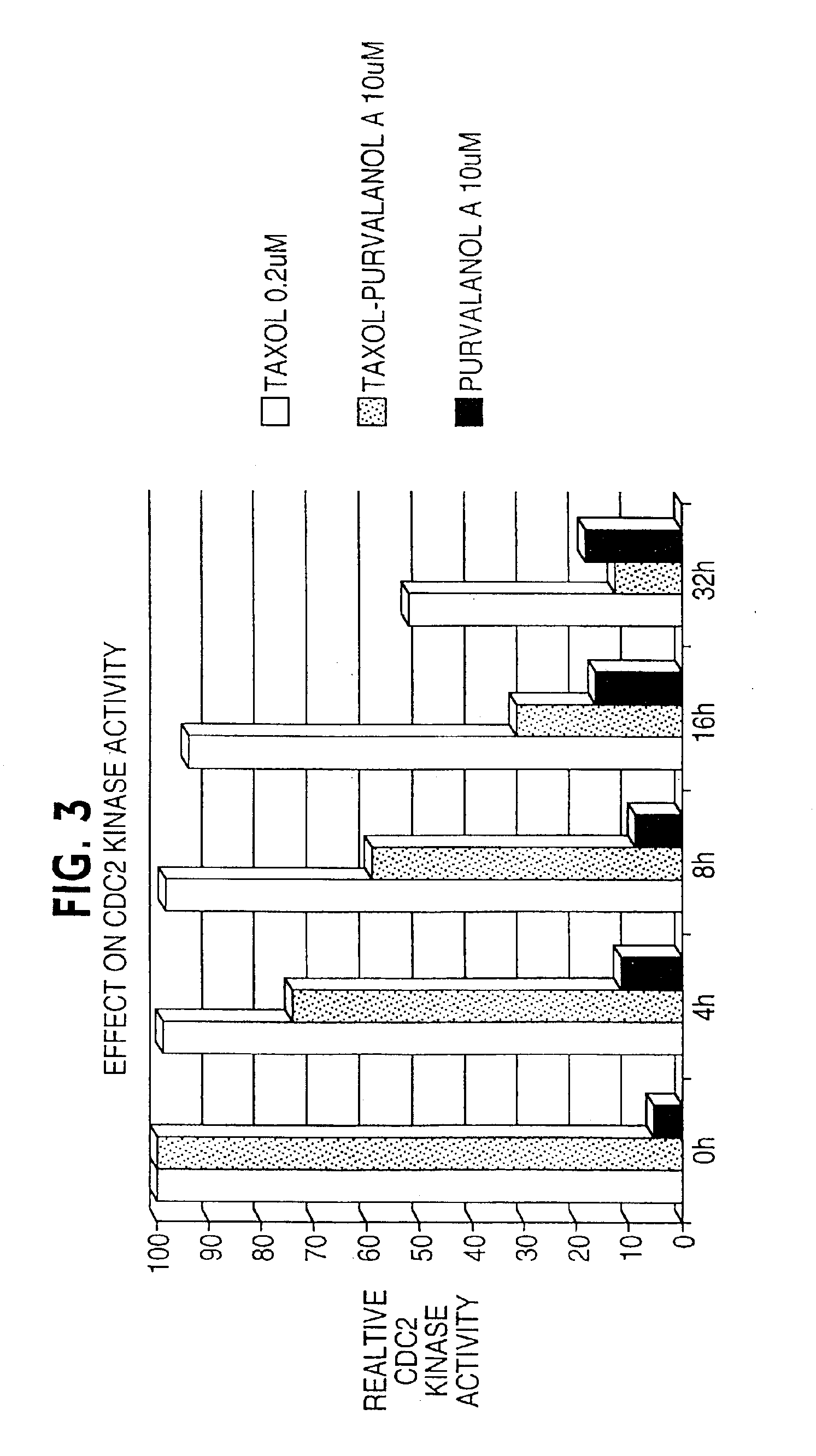
Enhancement of taxane-based chemotherapy by a CDK1 antagonist
InactiveUS6949558B2Faster and more effective kineticsReduce toxicityBiocideAnimal repellantsDrugWilms' tumor
The present invention provides a combination therapy for inhibiting the growth of tumor, for treating cancer, and for inducing cell death. The therapy comprises the sequential administration of taxane and a CDK1 antagonist. The present invention also provides pharmaceutical compositions comprising taxane and a CDK1 antagonist and kits comprising taxane and CDK1 antagonist.
Owner:YALE UNIV
Polyene taxol liposome and its prepn process
InactiveCN1931157AAltered pharmacokinetic propertiesContinuous Healing EffectOrganic active ingredientsAntineoplastic agentsSide effectCancer cell
The present invention provides one kind of polyene taxol liposome capable of being used for injection and oral taking and its solid preparation, and features that polyene taxol is coated with phospholipid matter and additive so as to prepare polyene taxol liposome preparation with small size, high coating rate, high stability and less toxic side effect. The polyene taxol liposome of the present invention has raised targeting effect on cancer cell, enhanced tumor cell killing effect, high curative effect and less toxic side effect. The present invention also provides several preparation processes of polyene taxol liposome, and the preparation processes are simple, low in cost and suitable for industrial production.
Owner:SHANGHAI INST OF MATERIA MEDICA CHINESE ACAD OF SCI
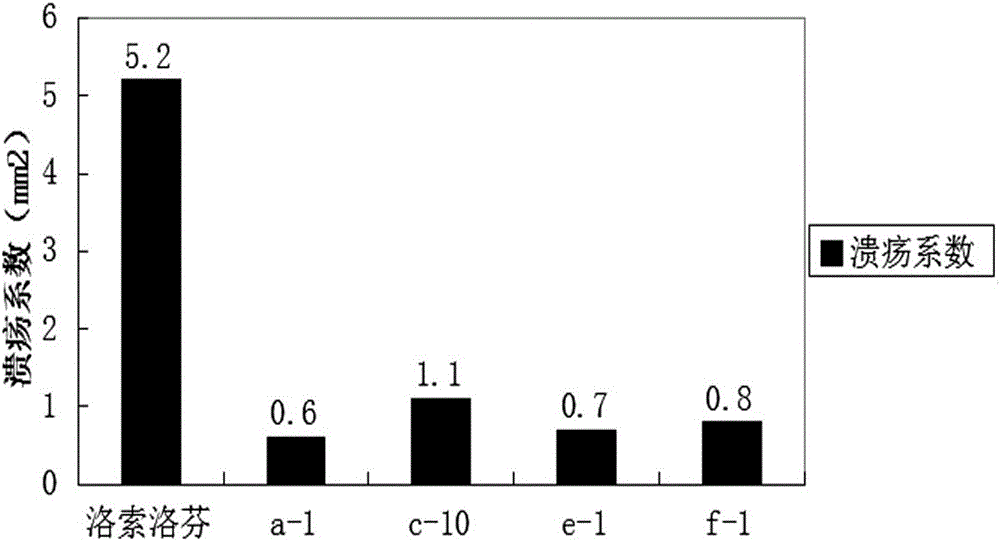
Aryl propionic acid derivative composition and pharmaceutical purpose
ActiveCN106661061ALess irritatingImprove medication complianceOrganic active ingredientsAntipyreticMetabolitePhosphate
The invention discloses an aryl propionic acid derivative composition and a pharmaceutical purpose. Alcohol metabolites of Loxoprofen having highest activity in metabolism is used as a nuclear parent to produce a series of derivatives of phosphate, sulphonate, carbonic ester, and amino-acid ester. The derivatives are used to enhance pesticide effect of anti-inflammatory and analgesic effects, and reduce toxic reaction, and have good pharmacokinetic property. And at the same time, the stimulation of the medicine on the gastrointestinal tract, and medication compliance of patients is improved, and therefore the aryl propionic acid derivative composition is the non-steroidal anti-inflammatory drugs having potentials.
Owner:NANJING HERON PHARMA SCI & TECH CO LTD +1
Separately packed structural fatty milk, aminoacid and glucose injection composition and the prepn process
ActiveCN101019823AMeet metabolic needsAvoid Metabolic AcidosisMetabolism disorderPharmaceutical delivery mechanismAmino Acid InjectionMedical prescription
The present invention is one kind of three-cavity packed injection composition, and has structural fatty milk injection, compound amino acid injection and glucose injection packed separately in three diaphragm separated cavities. Before intravenous infusion, the structural fatty milk injection, the compound amino acid injection and the glucose injection are squeezed out and mixed in common operation condition. The injection composition can be used to patient for meeting the requirement in protein and saccharide.
Owner:费森尤斯卡比华瑞制药有限公司
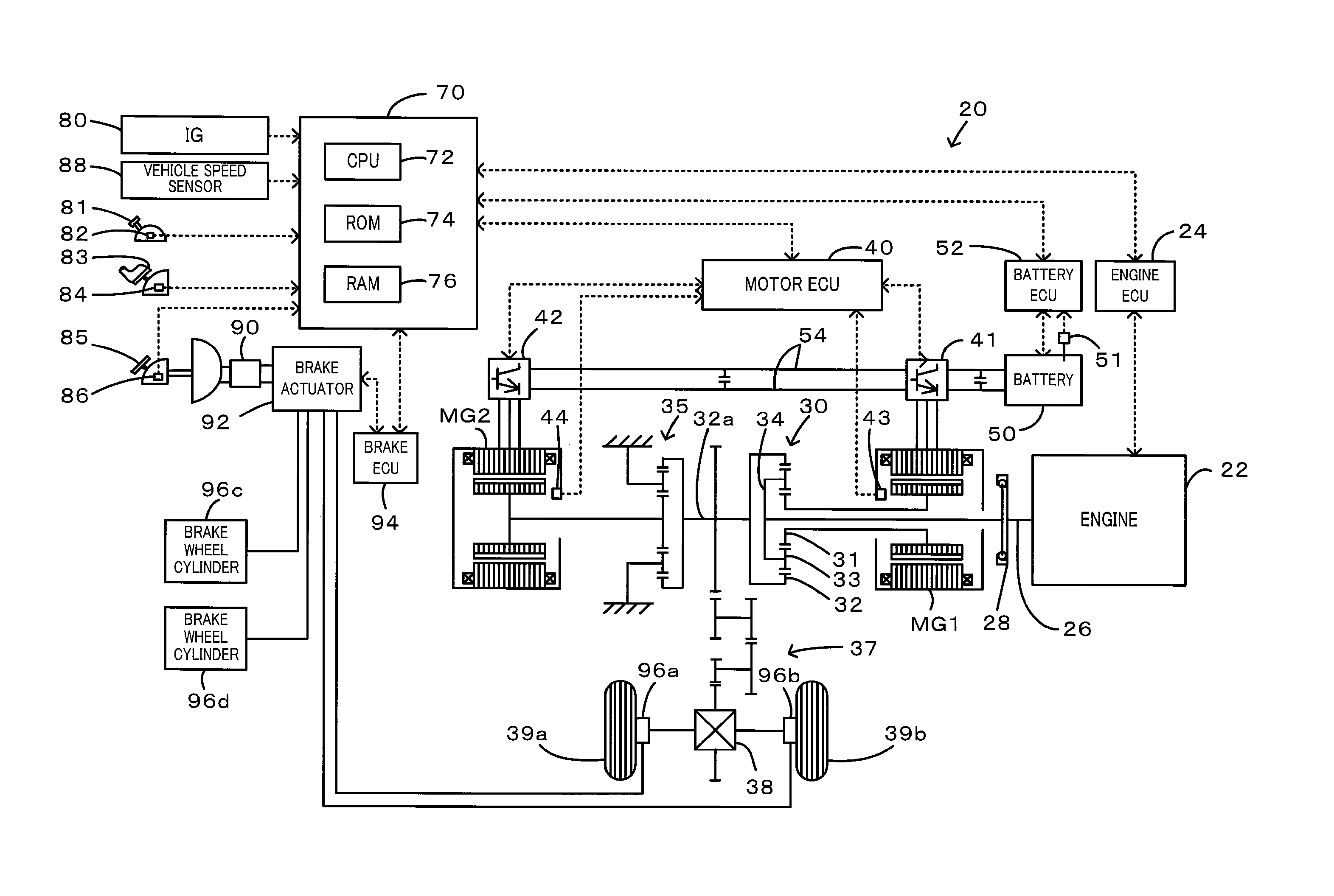
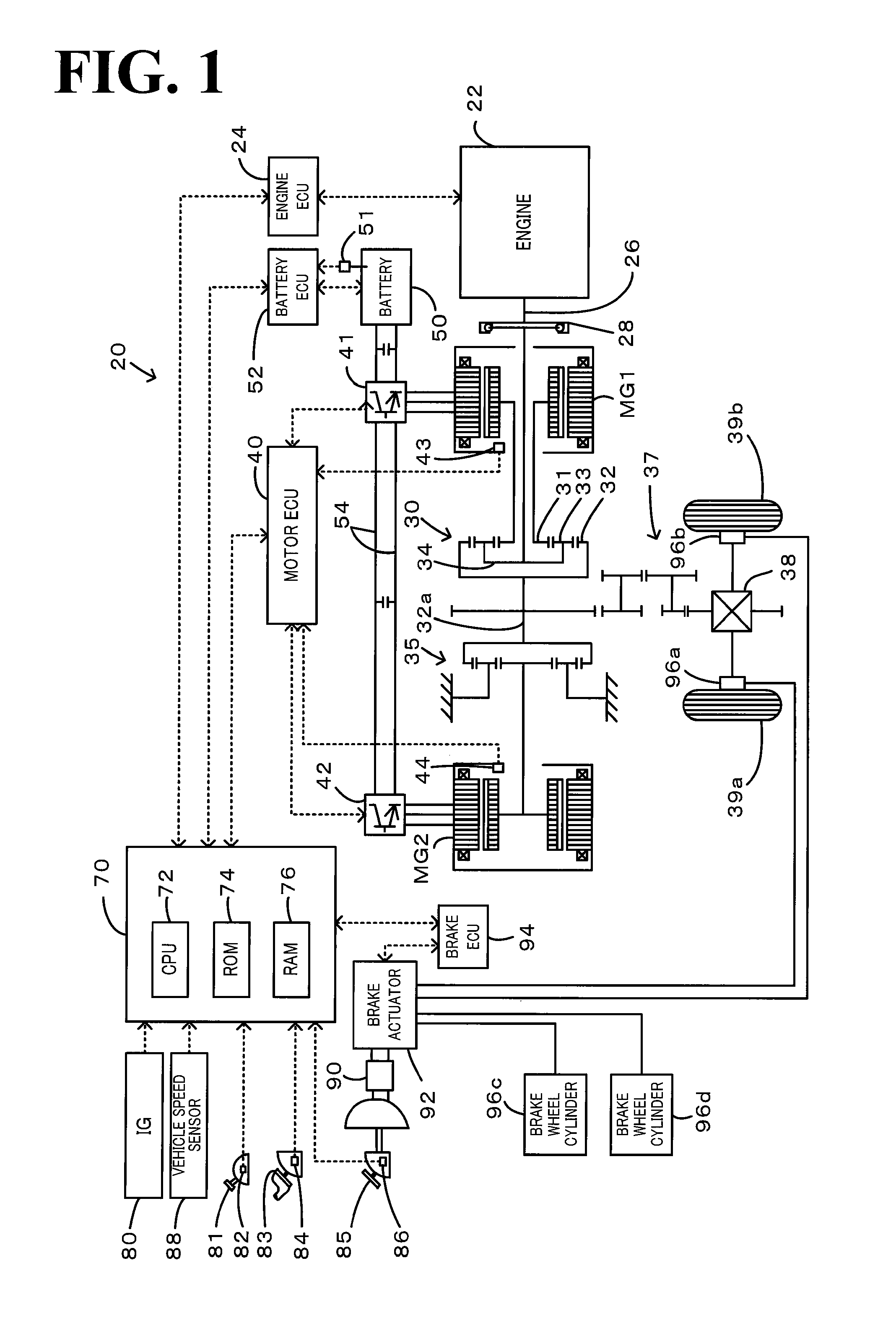
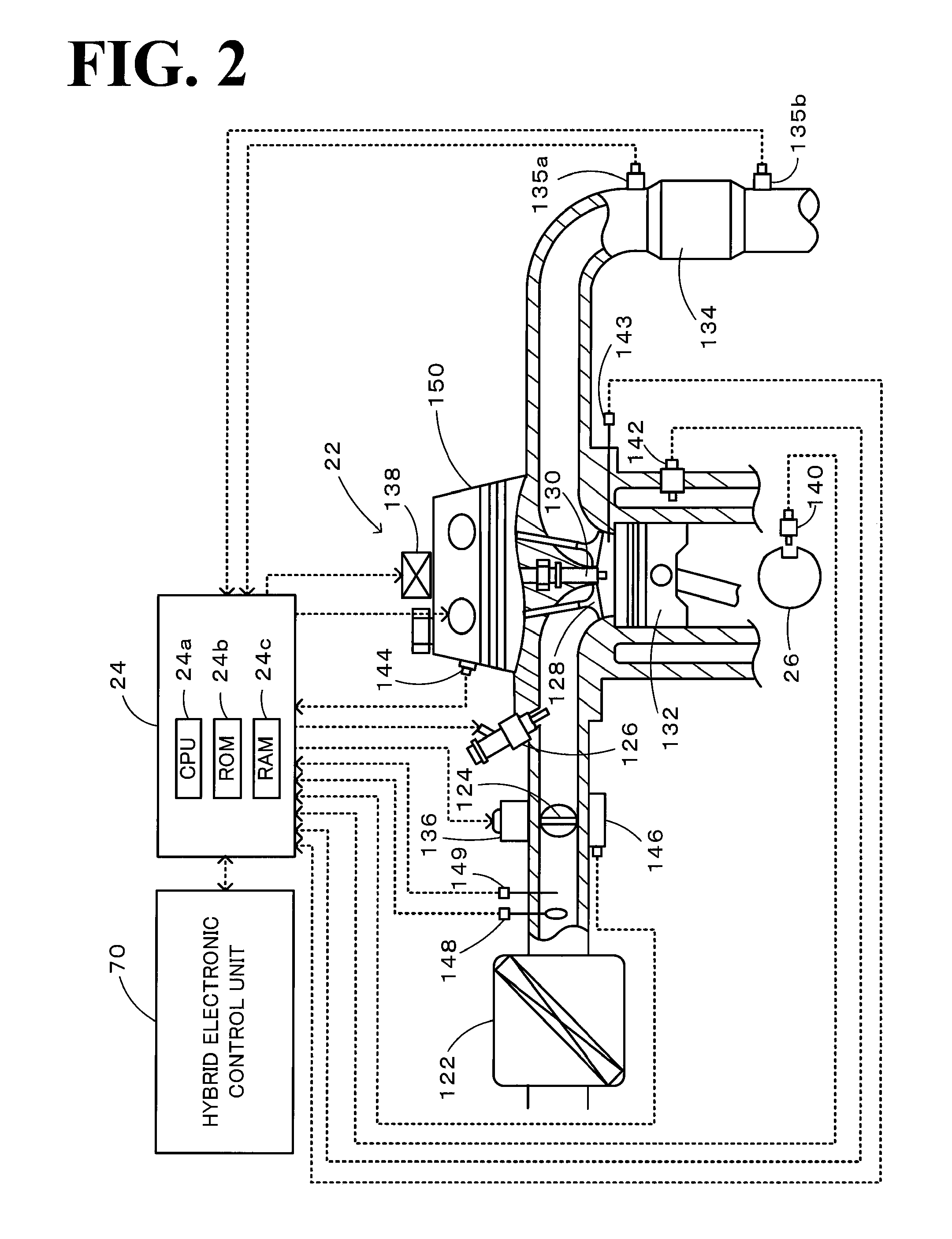
Power output apparatus, internal combustion engine system, and control methods thereof
InactiveUS20100036589A1Reduce toxicityHigh oxygen storage capacityInternal combustion piston enginesPlural diverse prime-mover propulsion mountingFuel supplyExternal combustion engine
On the occasion of a cutoff of fuel supply to an engine in an accelerator-off state, the invention expands a throttle opening over a specific throttle opening set in the state of idling of the engine at the reference rotation speed. On resumption of fuel injection to the engine, the invention reduces the throttle opening to the specific throttle opening set in the state of idling of the engine at the reference rotation speed. Under no control of lowering the rotation speed of the engine by a motor or under the condition of low vehicle speed with control of lowering the rotation speed of the engine by the motor, the invention resumes fuel injection to the engine resumed with setting of a smaller correction amount to a fuel increase correction amount.
Owner:TOYOTA JIDOSHA KK
Resin Composition Containing Release-Controlled Agricultural Chemical, Production Method Thereof, and Agricultural Chemical Formulation
InactiveUS20090093364A1Reduce frequencyReduce toxicityBiocidePharmaceutical non-active ingredientsPesticideChemistry
A resin composition containing an agricultural chemical in which the release of an agricultural chemical active ingredient is controlled; a production method thereof; and an agricultural chemical formulation; in other words, an agricultural chemical-containing resin composition which is a composition containing an agricultural chemical active ingredient, a resin, and a fatty acid metal salt, and characterized in that the composition is either in a compatible state or forming a matrix; its production method; and an agricultural chemical formulation including at least one of the agricultural chemical-containing resin compositions; in addition to an agricultural chemical-containing resin composition which is a composition containing an agricultural chemical active ingredient, a (meth)acrylate based resin, and a release controlling agent, and characterized in that the composition is either in a compatible state or forming a matrix; its production method; and an agricultural chemical formulation including at least one of the agricultural chemical-containing resin compositions, are provided.
Owner:NIPPON SODA CO LTD
Cefquinome liposome
ActiveCN104000783AReduce toxicityTargetedAntibacterial agentsOrganic active ingredientsPhospholipinSterol
Cefquinome liposome, which belongs to the field of pharmaceutics, has a particle size of less than 1000 nm, and is mainly prepared from the following raw materials by weight: 1 part of cefquinome, 1-40 parts of phospholipid, 0-15 parts of sterol or soy isoflavone glucoside or soyasapogenol, and 0-15 parts of additives. The cefquinome liposome can be prepared into a liquid preparation, and can also be prepared into a solid preparation by adding a proper amount of a support agent. The cefquinome liposome or cefquinome long circulating liposome prepared in the invention contains no irritant substances, and can relieve anaphylactic reaction; the obtained preparation has good stability, has an average liposome particle size of less than 1000 nm, and has encapsulation efficiency of more than 80%. The preparation method is mature in process, simple, practical, and suitable for industrial production.
Owner:HENAN SOAR VETERINARY PHARMA
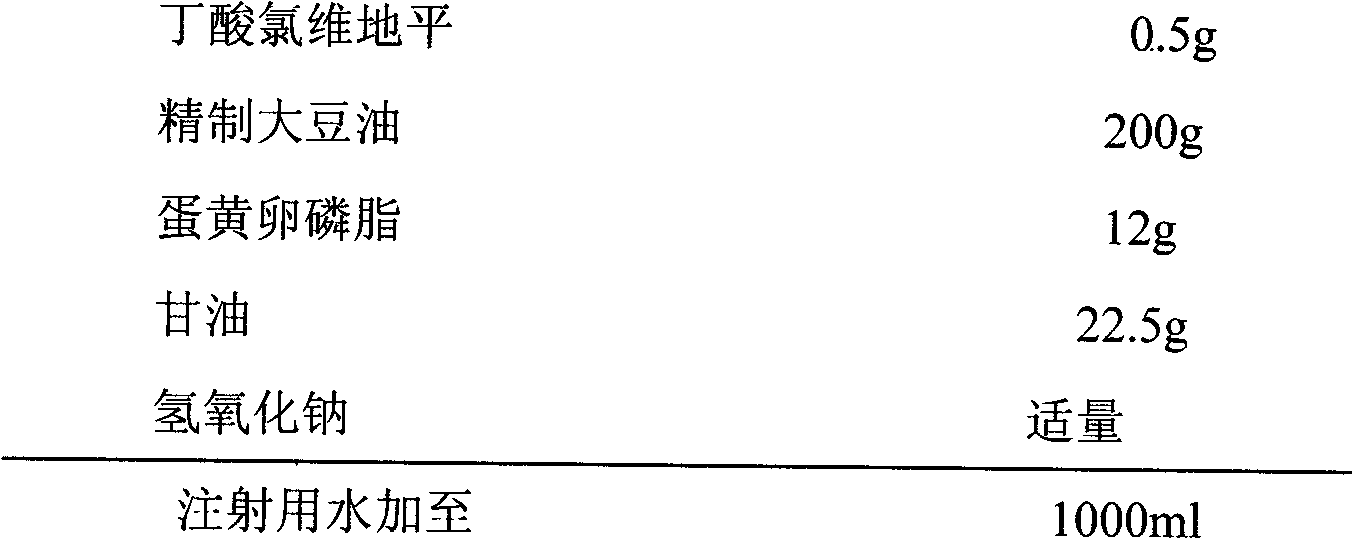
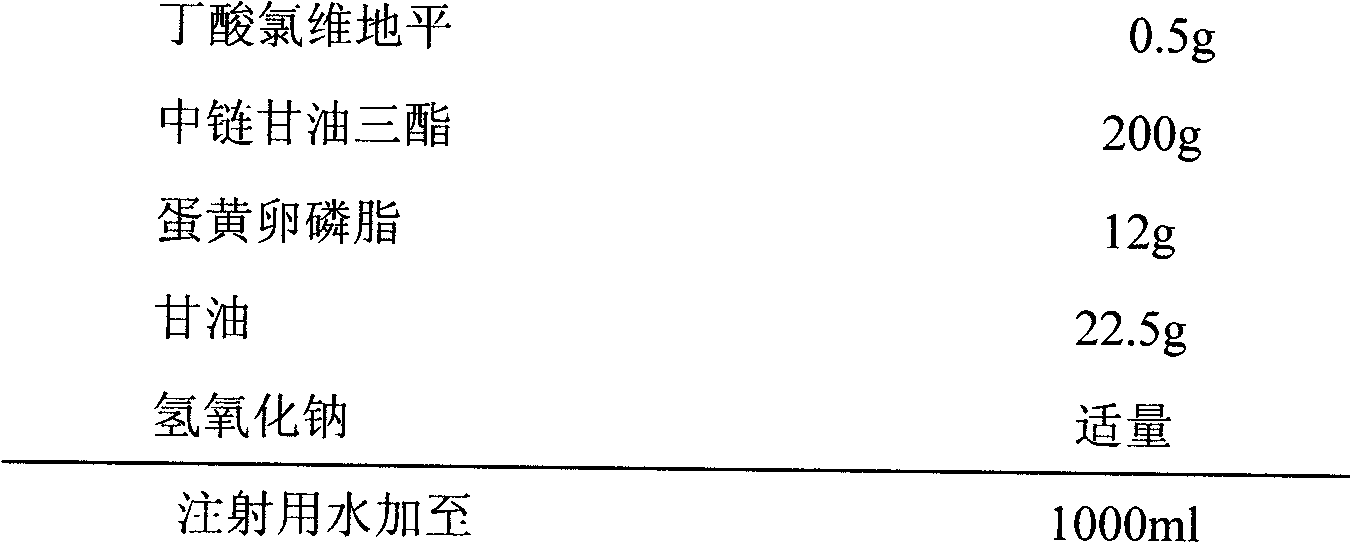
Clevidipine butyrate structured lipid emulsion and preparation method thereof
InactiveCN102319212ATo promote metabolismImprove technical effectOrganic active ingredientsEmulsion deliveryEmulsionMedicine
The invention relates to a clevidipine butyrate structured lipid emulsion which comprises clevidipine butyrate, structured triglyceride, an emulsifier, an isotonic agent, a pH regulator, and injection water, wherein the weight volume percentage of clevidipine butyrate is 0.05-0.1% (w / v); the weight volume percentage of structured triglyceride is 5-30% (w / v); the weight volume percentage of the emulsifier is 1.0-2.0% (w / v); and the weight volume percentage of the isotonic agent is 1.0-5.0% (w / v).
Owner:辽宁中海康生物制药股份有限公司
Bone peptide injection preparation technology
InactiveCN101103999AGrowth inhibitionReduce toxicityPeptide/protein ingredientsSkeletal disorderUse medicationToxicant
Disclosed is a preparation process of ossotide injection (gutai), belonging to the medical technical field. To solve the defects of the existing ossotide injection (gunning), the novel ossotide injection (gutai) preparation process is produced by the change of the existing ossotide injection (gunning) process. In the invention, four bones of a pig is picked up, degreased, condensed, and then precipitated and condensed, and then after acid precipitation and alkaline precipitation, the heated liquid is cooled to be in the room temperature and then is placed in a refrigerator; the liquid is taken out from the refrigerator and acidic protein is removed from the liquid is filtrated and hyperfiltrated to become the ossotide stock solution which is mixed with water, filtrated, potted and sterilized, thus the ossotide injection (gutai) is prepared well. The invention removes sensitizer and toxicant, largely reduces toxic reaction for the ossotide injection (gutai), and makes medicine for intravenous injection possibly used.
Owner:石海
Tripeptide prodrug compounds
InactiveUS20070275903A1High specificity of actionReduce toxicityOrganic active ingredientsAntipyreticEnzymeThimet oligopeptidase
The prodrug of the invention is a modified form of a therapeutic agent and comprises a therapeutic agent, an oligopeptide of three amino acids, a stabilizing group and, optionally, a linker group. The prodrug is cleavable by a trouase enzyme such as Thimet oligopeptidase. Also disclosed are methods of making and using the prodrug compounds.
Owner:MEDAREX LLC
Antifungal medicine composition
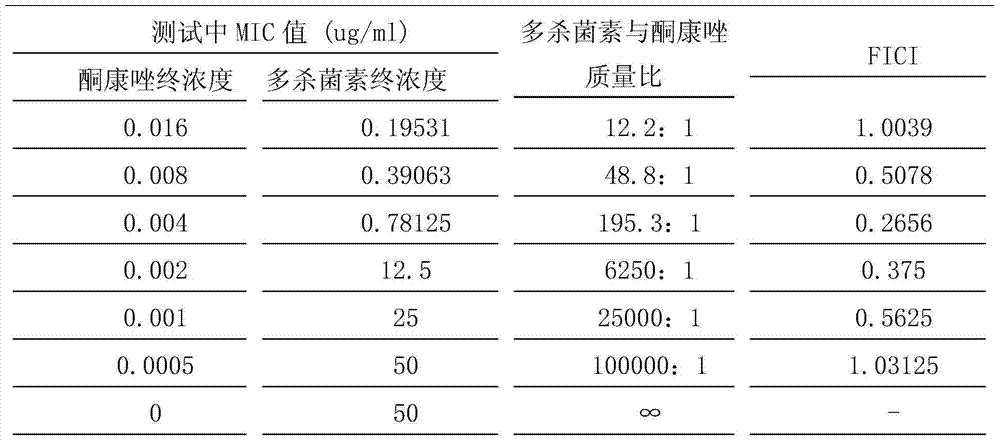
ActiveCN103536613AReduce doseReduce toxicityOrganic active ingredientsAntimycoticsToxic reactionDrug resistance
The invention discloses an antifungal medicine composition composed of A and B. The A is any one of the substances of: macrolide antibiotic, analogue thereof, derivative thereof, prodrug thereof, metabolite thereof, and pharmaceutically active salt thereof. B is any one of the substances of: triazole compound, analogue thereof, derivative thereof, prodrug thereof, metabolite thereof, and pharmaceutically active salt thereof. As a result of in-vitro antifungal experiments, when ketoconazole and spinosad are applied in combination, high synergetic activity can be developed. The composition disclosed by the invention is effective against intractable invasive fungal infections. With the composition, ketoconazole dose can be reduced, such that patient economic burden can be reduced, ketoconazole toxic reaction can be reduced, and fungal drug resistance development can be effectively reduced.
Owner:INST OF MICROBIOLOGY - CHINESE ACAD OF SCI
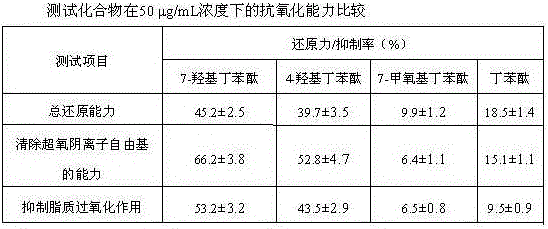
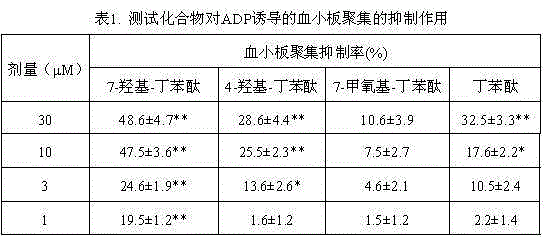
Medical use of 7-hydroxy-butylphthalide
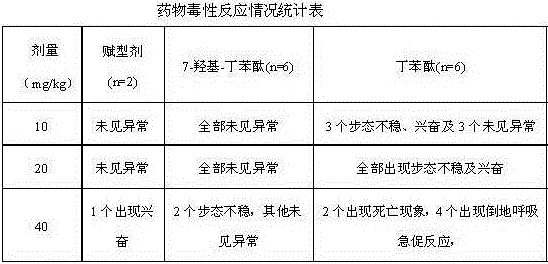
ActiveCN106214674AImprove protectionStrong medicinal effectOrganic active ingredientsAntinoxious agentsVascular diseaseButylphthalide
The invention belongs to the technical field of pharmaceutical chemistry and particularly relates to medical use of 7-hydroxy-butylphthalide in preparation of drugs for preventing and / or treating cardiac and cerebral vascular diseases.
Owner:NCPC NEW DRUG RES & DEV
Ketogenic-diet composition containing active polysaccharide, and preparation and application methods thereof
InactiveCN106376934ACorrect the imbalancePromote proliferationOrganic active ingredientsAntineoplastic agentsAdditive ingredientKetogenic diet
The invention discloses a ketogenic-diet composition containing active polysaccharide, and preparation and application methods thereof; the composition is a food prepared through adding dietary fibers and the polysaccharide component having immuno-enhancing activity based on a general ketogenic-diet formula and through combination preparation and supplied for patients to conveniently eat; specifically, the ketogenic-diet composition comprises the used raw material by mass: 1 part of fats, 0.2-1 part of proteins, 0.2-0.4 part of the dietary fibers, and 0.01-0.2 part of the active polysaccharide. The preparation method comprises 5 steps of raw material cleaning, drying, mixing, sterilizing and packaging. The application method comprises that the ketogenic-diet composition is used as a dietary therapy substance to be directly eaten by cancer patients. In the ketogenic-diet composition, the dietary fibers required to be added are cleared up, and the proportion of the dietary fibers and the active polysaccharide is also cleared up; the tumor cells are selectively hungered through ketogenic-diet, physiological ketonemia is generated to regulate a key path of tumor generation and development through ketogenic-diet, and an antitumor action body is generated through ketogenic-diet. The ketogenic-diet composition is suitable for dietary therapy ofor all kinds of cancer patients.
Owner:GUIZHOU MEDICAL UNIV
Sildenafil citrate sublingual tablet and its preparation method
InactiveCN101057850ARich blood vesselsImprove permeabilityPill deliverySexual disorderCITRATE ESTERMedicine
The invention discloses an acidum citricum hypoglossal tablet and the preparing method, relating to a tablet and the preparation method. The invention means to solve problems: current acidum citricum are all oral tablets and capsule, the content is large and increases liver injury, and it is easy to generate adverse effect. The comprised component and their weight proportion are as follows: acidum citricum 20 -30 units, manna sugar 45-65 units, lactin 65-105 units, sweetener 0. 5-1. 5 units, ethanol solution with concentration being 5%PVPK30 30 -40 units and dolomol 4-8 units. The preparation method comprises following steps: sifting sot material with nylon screen of 18-20 order to produce particular, drying at 55-65 Deg. C; sifting granular with screen of 14-16 order and placing for 5-7 hours for sheeting. The tablet is characterized by small content, increased biological utilization rate, low adverse effect rate, and the method is characterized by short and simple process.
Owner:HEILONGJIANG UNIV
7-ethyl-10-hydroxycamptothecine liposome freeze-dried powder injection and preparation method thereof
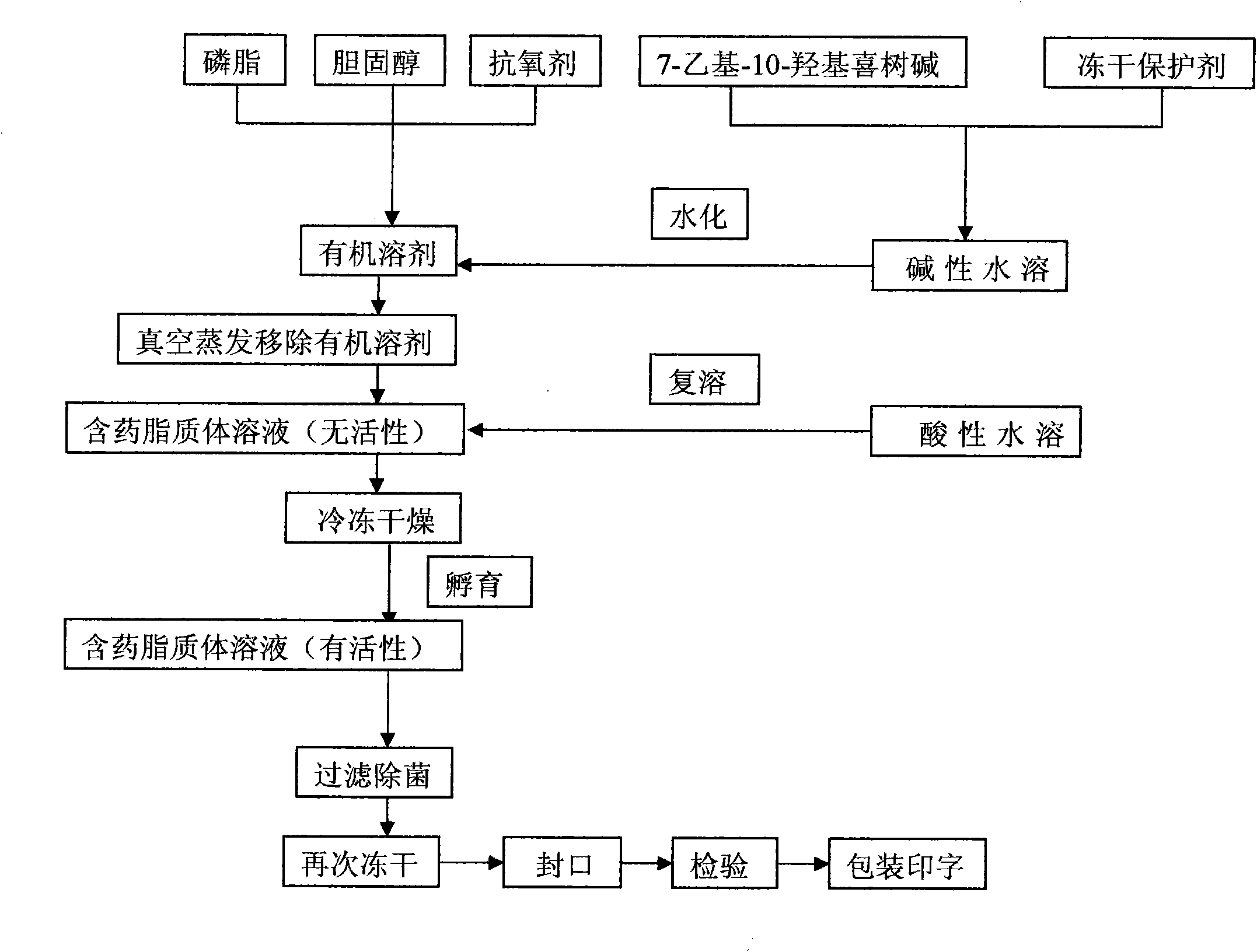
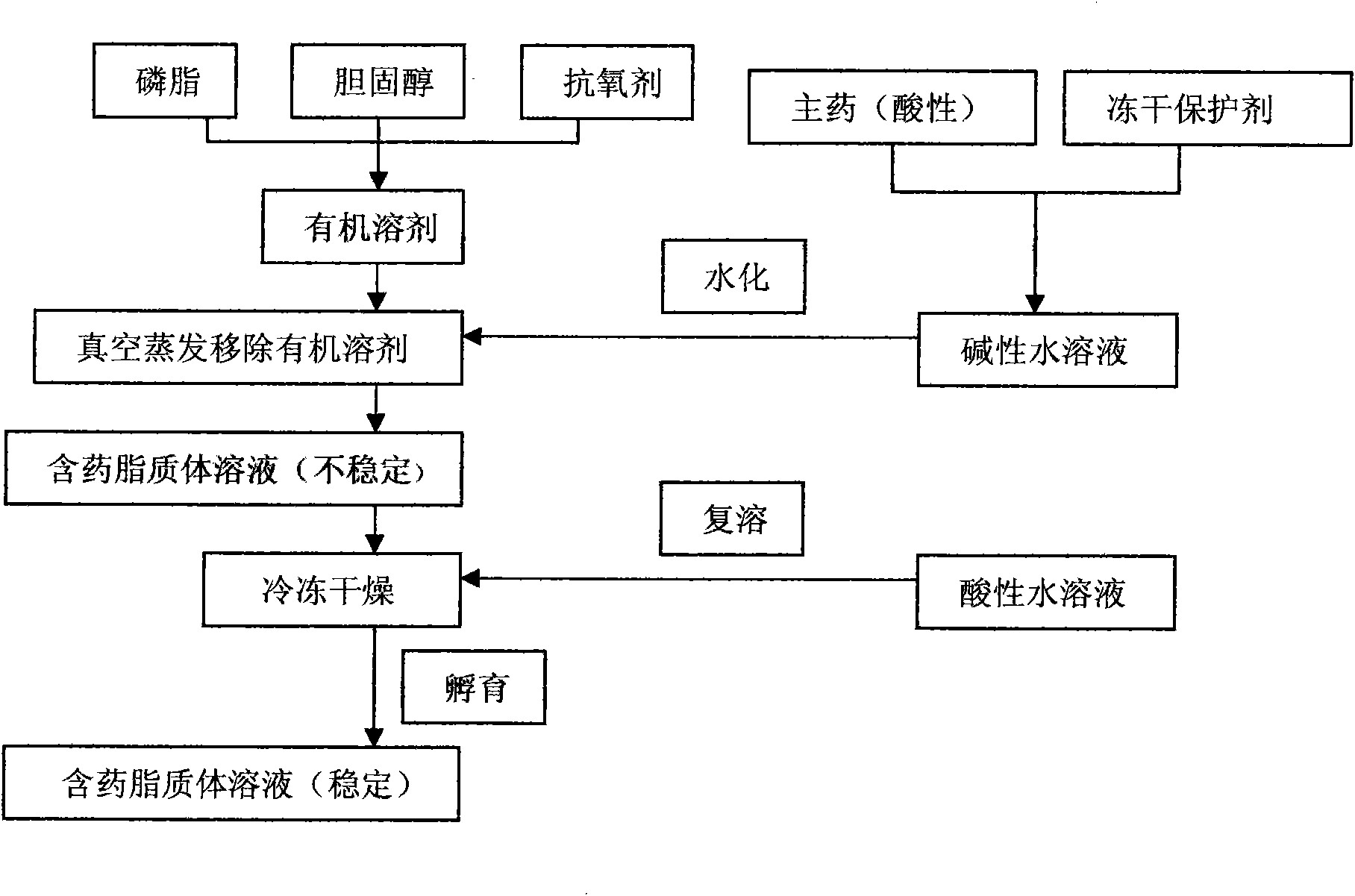
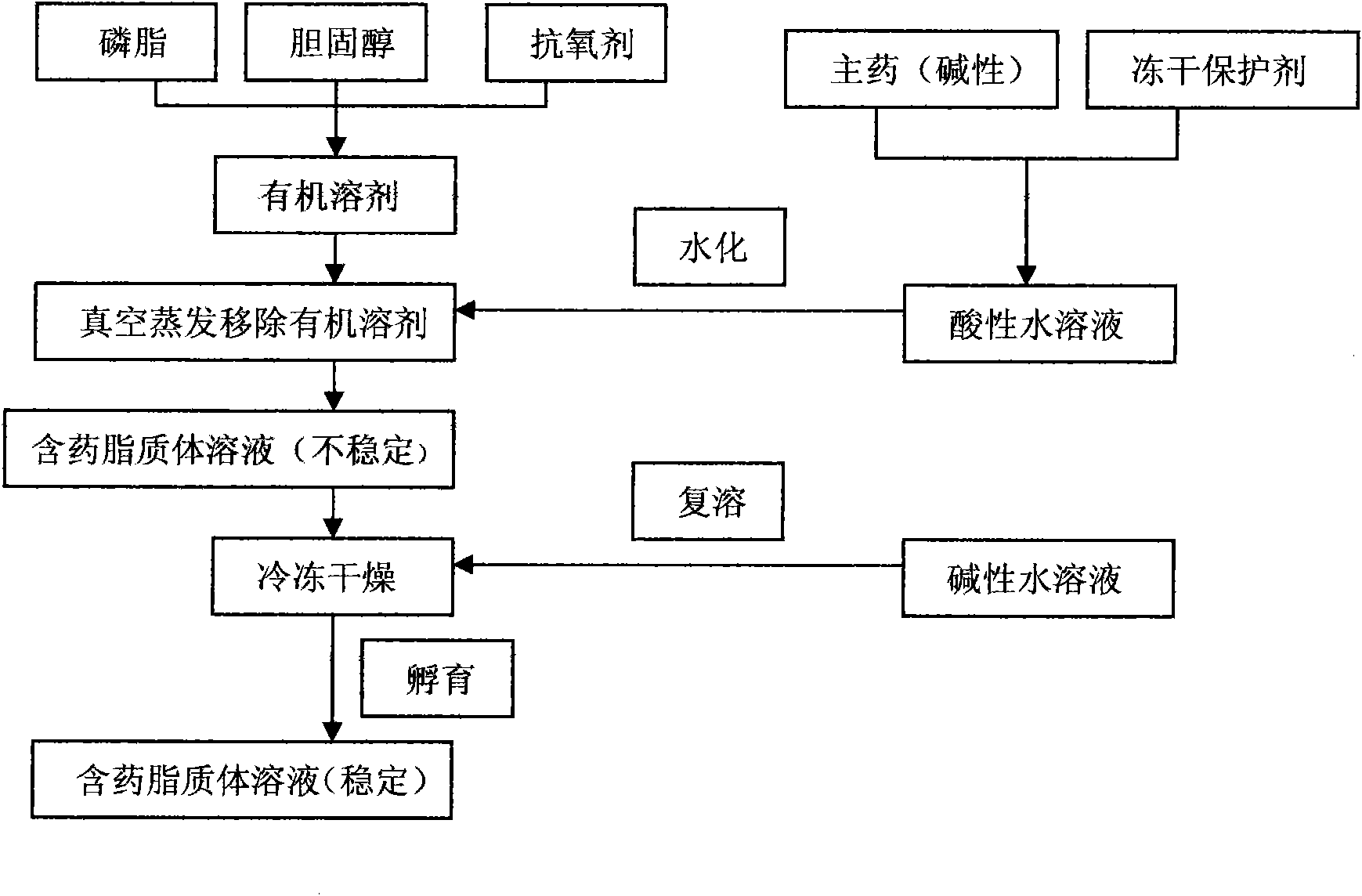
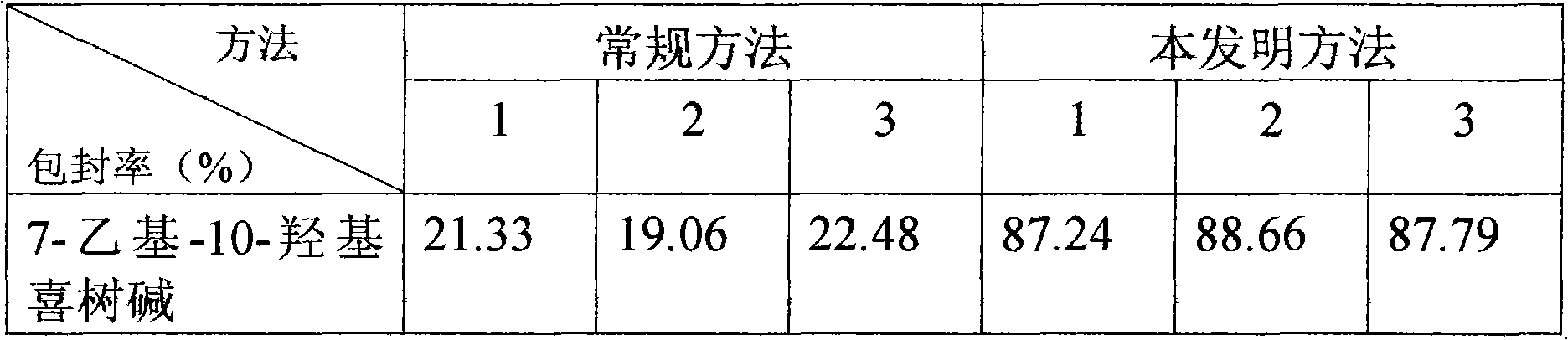
InactiveCN101874788AImprove stabilityImprove in vivo stabilityPowder deliveryOrganic active ingredientsSolubilityFreeze-drying
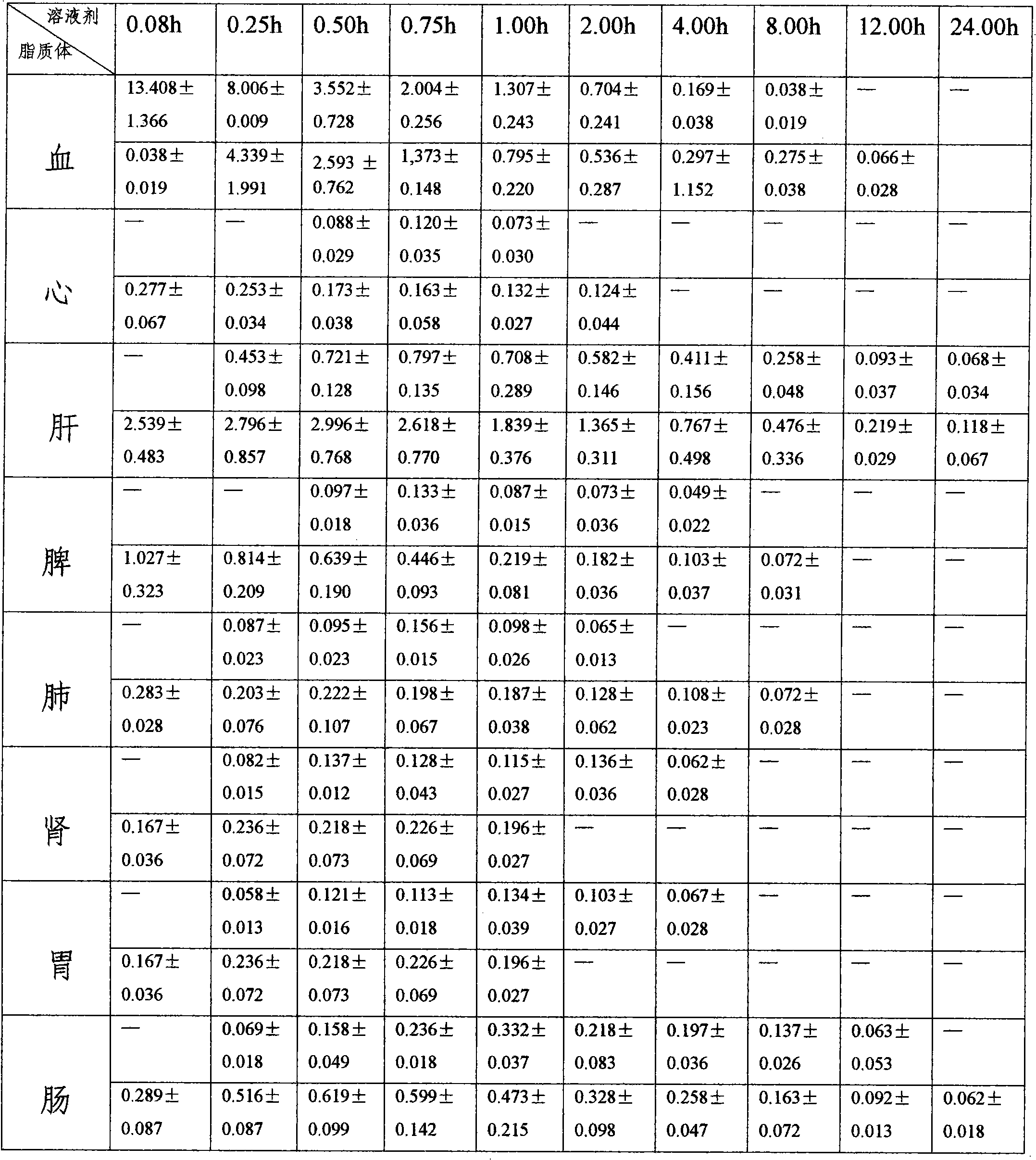
The invention belongs to the medical technical field, and discloses 7-ethyl-10-hydroxycamptothecine liposome freeze-dried powder injection and a preparation method thereof. The 7-ethyl-10-hydroxycamptothecine liposome freeze-dried powder injection comprises the following components: 1-10g of 7-ethyl-10-hydroxycamptothecine, 30-60g of phospholipids, 10-40g of cholesterol, 2-8g of VE, 100-300g of a freeze drying protectant, 2000-8000ml of an organic solvent, 1000-4000ml of alkaline buffer salt solution and 1000-4000ml of acid buffer salt solution. The preparation method comprises the following steps: dissolving liposoluble components in the organic solvent and water-soluble components in the alkaline buffer salt; transferring the organic solvent, and then adding the alkaline buffer salt for hydration; and carrying freeze drying in vacuum, re-dissolving with the acid buffer salt, incubating, filtering, sterilizing, and carrying out freeze drying again to obtain the 7-ethyl-10-hydroxycamptothecine liposome freeze-dried powder injection for injection. The invention solves the problems of low solubility and fast in-vivo metabolism of the 7-ethyl-10-hydroxycamptothecine, thus lowering toxic reaction, eliminating side reaction, having higher target distribution characteristics, prolonging metabolism time and improving solubility and bioavailability.
Owner:SHENYANG PHARMA UNIVERSITY
Preparation method of high-purity refined salinomycin sodium salt
The invention relates to a preparation method of high-purity refined salinomycin sodium salt. The high-purity refined salinomycin sodium salt is prepared from salinomycin fermentation liquor prepared by the prior art, and the preparation method comprises the following steps: regulating pH value by virtue of sodium hydroxide so as to generate sodium salt of salinomycin, removing water in the fermentation liquor by virtue of plate-frame pressure filtration, then extracting salinomycin sodium from mycelia by virtue of methanol, and carrying out a series of processing steps including concentrating, crystallizing, refining and drying, so that the refined salinomycin sodium is prepared. The preparation method disclosed by the invention can be used for extracting the active ingredients of the salinomycin from the fermentation liquor obtained in the production process, effectively removing remaining medium residues, water and other possible impurities in the production process, improving the yield and purity of the product, relieving possible toxic reaction generated due to the existence of other impurities in a process of using the product and enhancing the safety of medication.
Owner:山东齐发药业有限公司
Antifungal formulation and manufacturing method thereof
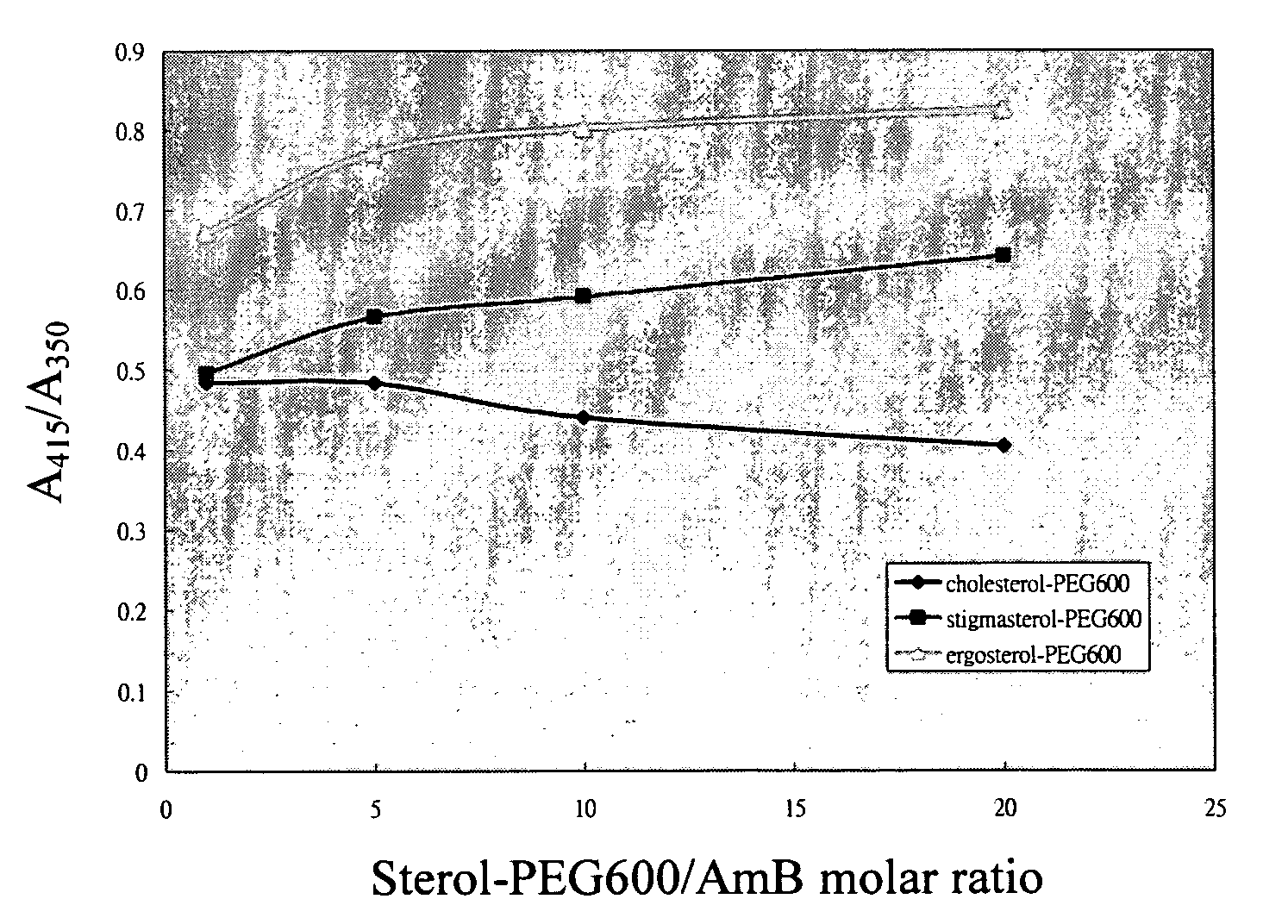
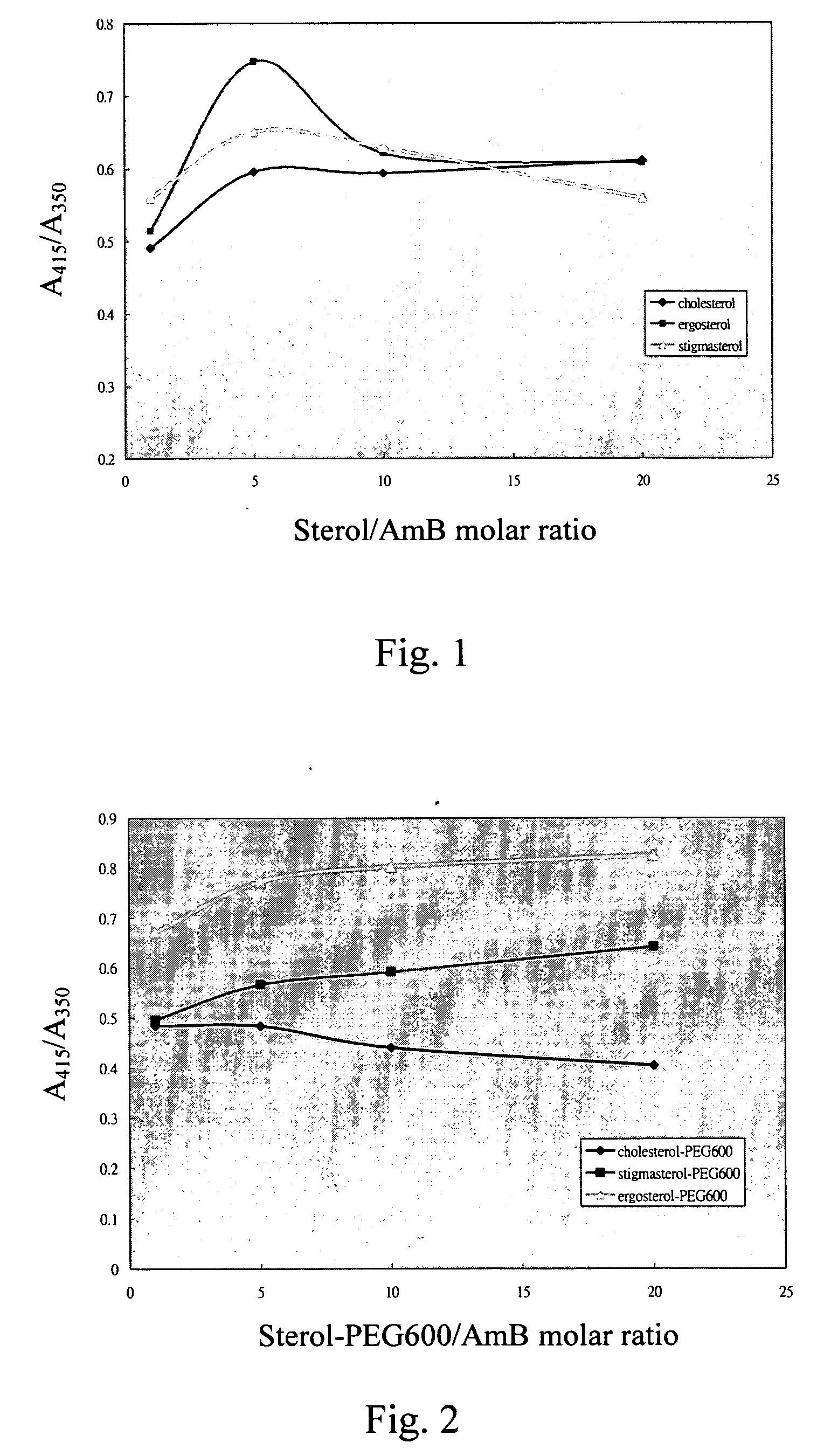
InactiveUS20050129767A1Reduce toxicityMaintain effectivenessBiocidePowder deliverySterolSelf-assembly
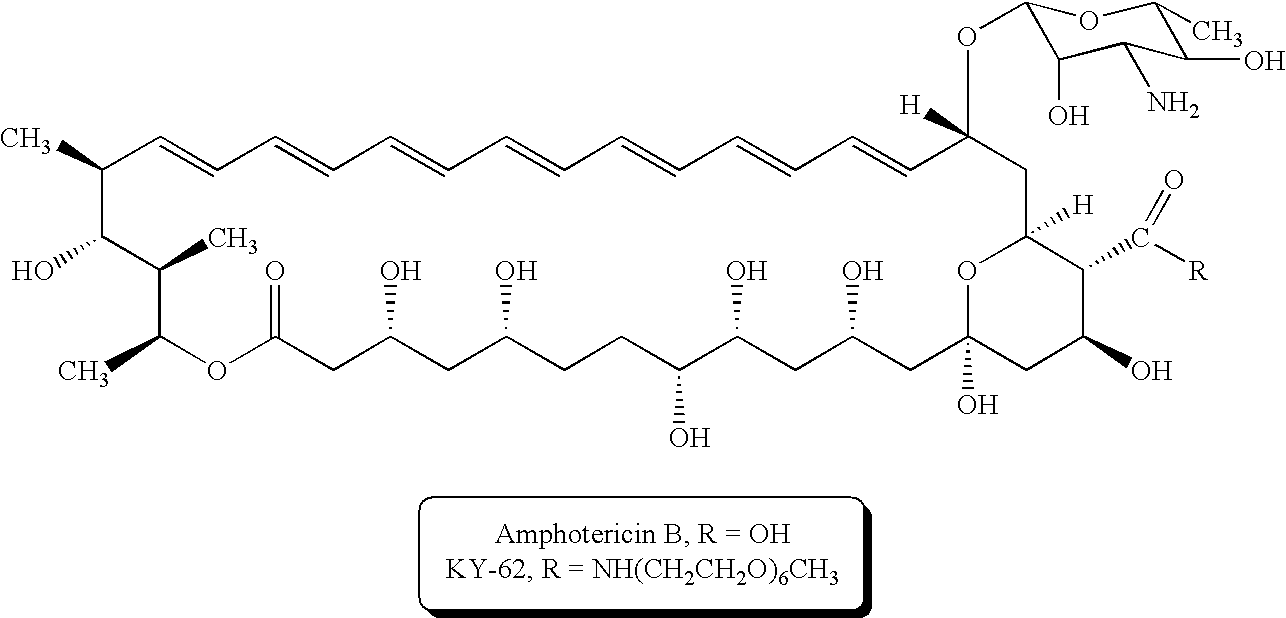
A new antifungal formulation is provided. The present invention uses a sterol modified with polyethylene glycol (PEG) as a drug carrier. The drug carrier encapsulates Amphotericin B (AmB) by self-assembly to form polymeric micelles. The polymeric micelles can reduce toxicity of Amphotericin B and control release of Amphotericin B. The polymeric micelles of Amphotericin B are used as a new antifungal formulation.
Owner:IND TECH RES INST
Traditional Chinese medicine fermented health-care food beneficial to sleep and preparation method thereof
PendingCN111903964APalliative propertiesSafe and Effective FormulaNervous disorderNatural extract food ingredientsSleep functionsLicorice roots
The invention discloses a traditional Chinese medicine fermented health-care food beneficial to sleep. The traditional Chinese medicine fermented health-care food beneficial to sleep is prepared fromthe following components in parts by weight of 2.72-4.08 parts of spina date seeds, 2.72-4.08 parts of lily bulbs, 2.72-4.08 parts of poria cocos, 4.08-6.12 parts of wheat, 1.36-2.04 parts of Chinesejujubes, 1.28-1.92 parts of licorice roots and 2.72-4.08 parts of rhizoma polygonati through extraction, fermentation and concentration shaping. According to the formula, medicinal and edible traditional Chinese medicine raw materials are used, the dosage of the traditional Chinese medicine fermented health-care food is smaller than 1 / 3 of the conventional dosage lower limit, the product is prepared through combination of optimized process extraction and probiotic fermentation technologies, and according to regulations on the sleep improving function evaluation procedure of health-care foods,the sleep improving function test result is positive, which illustrates that the product has the health-care function of improving sleep within the recommended edible dosage; and toxicological tests also show that the product is safe and non-toxic and has a wide application prospect.
Owner:HUBEI GOLDEN EAGLE BIOTECH
Medicament composition containing ginsenoside and cantharidin and application of medicament composition
ActiveCN102526146AReduce toxicityLow incidence of adverse reactionsAnthropod material medical ingredientsAntineoplastic agentsDrugToxicity
The invention relates to the field of Chinese medicine, and discloses a medicament composition containing ginsenoside and cantharidin and application of the medicament composition. The medicament composition containing the ginsenoside and the cantharidin contains the cantharidin or a Chinese medicinal material extract containing the cantharidin and the ginsenoside or a Chinese medicinal material extract containing the ginsenoside, wherein the ginsenoside or the Chinese medicinal material extract containing the ginsenoside is enough to reduce the toxic or side effect produced by the cantharidin. According to the medicament composition containing the ginsenoside and the cantharidin, the toxicity of the cantharidin on the kidney is obviously weakened, the adverse effect rate is obviously reduced, and the using safety of the cantharidin is greatly improved; and the medicament composition has the effect of improving tumor resistance, is safe in clinical use and low in toxic or side effects, and can be widely applied to preparation of medicaments for treating various cancers.
Owner:SHANGHAI GREEN VALLEY PHARMA
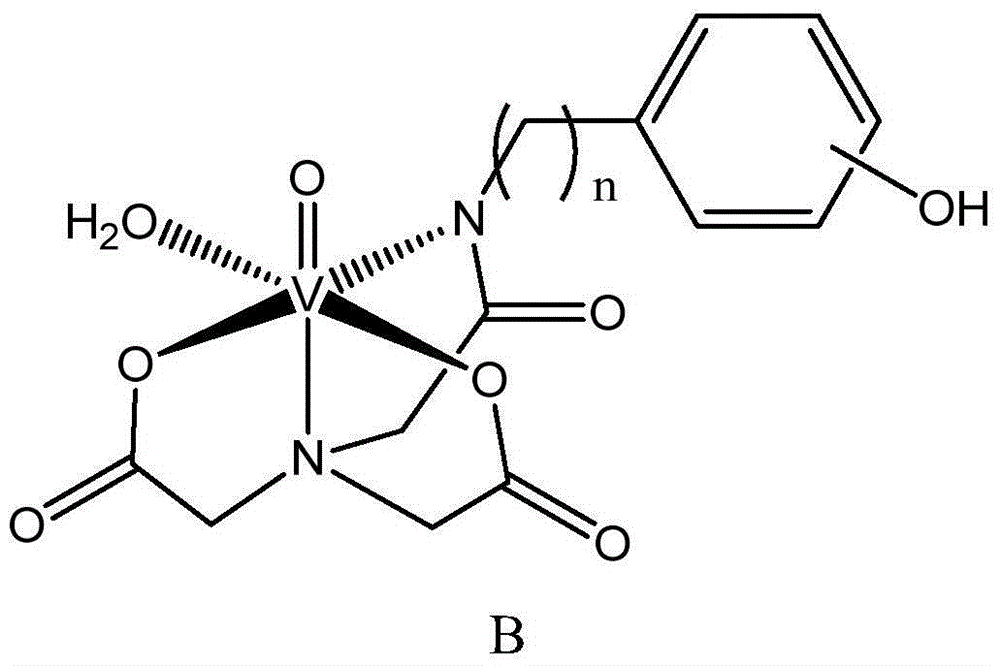
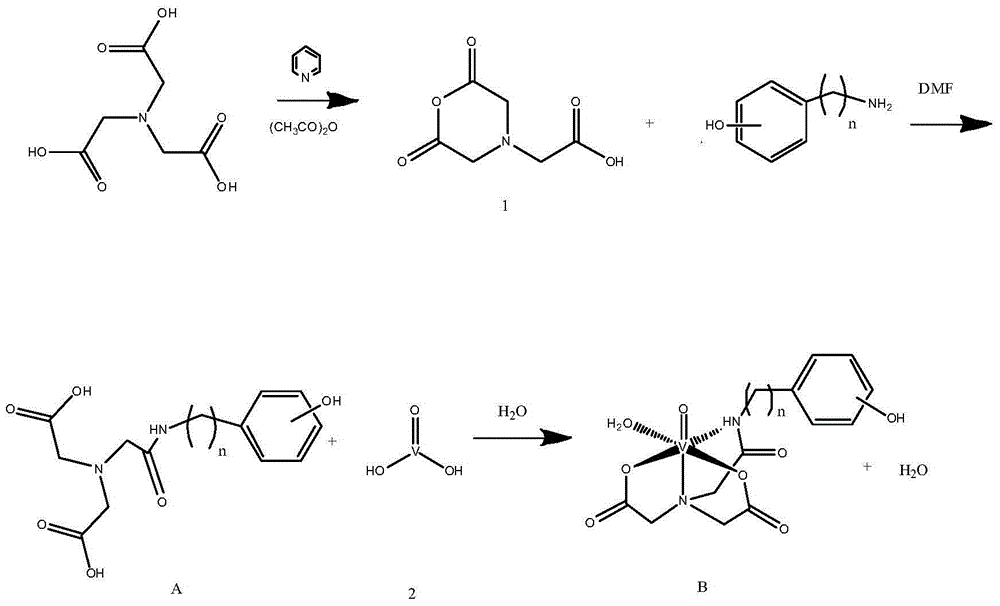
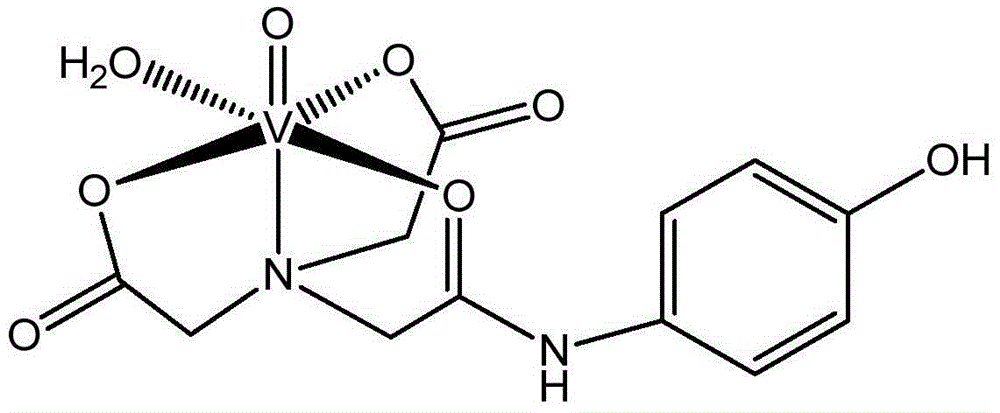
Vanadium coordination compound using aminotriacetic acid derivative as ligand, and preparation method and application thereof
InactiveCN104892663AGood metabolic properties in vivoEasy to derivatizeMetabolism disorderGroup 5/15 element organic compoundsSolubilityVanadyl sulfate
The invention relates to a vanadium coordination compound using aminotriacetic acid derivative as a ligand, and a preparation method and application thereof. The preparation method comprises the following steps: 1. reacting aminotriacetic acid in anhydrous pyridine to obtain a compound 1; 2. reacting the compound 1 with aminophenol or hydroxyaniline to obtain a compound A; 3. dissolving vanadyl sulfate in water, and regulating the pH value with NaOH to obtain a compound 2; and 4. dissolving the compound A in water, and reacting with the compound 2 to obtain a compound B. The compound is used in preparing antidiabetic drugs. The animal experiment proves that compared with the vanadyl compound using kojic acid as the ligand, the vanadyl coordination compound using aminotriacetic acid as the ligand has the advantages of lower molecular weight, higher water solubility, higher bioavailability and simpler preparation method.
Owner:PEKING UNIV
Preparation method for improving liposome entrapment efficiency
ActiveCN101874780AImprove stabilitySolve the problem of light and heat sensitivity and poor stabilityLiposomal deliverySolubilityOrganic solvent
The invention aims to provide a preparation method for improving liposome entrapment efficiency. The preparation method comprises the following steps: dissolving liposoluble components in an organic solvent, and dissolving water-soluble components in alkalescent or acidulous buffer salt; transferring the organic solvent, and adding buffer salt solution for hydration; and after vacuum freeze drying, redissolving the components in the alkalescent or acidulous buffer salt, and incubating to obtain a medicament-containing liposome. Acid compounds are easily dissolved in alkaline solution, and alkaline compounds are easily dissolved in acid solution; and in the method, the liposome is prepared by utilizing the characteristic of solubility change due to the acid-base difference, so that the entrapment efficiency of the compounds in the liposome is improved greatly, and a novel idea for preparing the liposome for indissoluble compounds is provided.
Owner:SHENYANG PHARMA UNIVERSITY +1
Method for preparing bowel-lubricating, face-beautifying soybean milk sheet with micron pine pollen, cactus powder, glossy ganoderma powder and medlar
The invention relates to a process for preparing intestine-moisturizing and face-beautifying soybean milk liquid, which is prepared from various conventional soybean milk liquids and air flow super fine disintegrated micrometer pine pollen powder, micrometer bitter orange fruit powder, micrometer cactus powder, micrometer glossy ganoderma powder, micrometer wolfberry powder and oligosaccharide as raw materials through combination. The invention achieves the functions of intestine-moisturizing, face-beautifying, fatigue-resisting, and human body immunity-improving.
Owner:余内逊
Monoclonal antibody-antigen binding segment-T-2 toxin conjugate
InactiveCN103330937AReduce toxicityRaise the dose rangeOrganic active ingredientsAntibody ingredientsCross-linkAntigen binding
The invention discloses a monoclonal antibody-antigen binding segment-T-2 toxin conjugate which is formed by connecting a monoclonal antibody-antigen binding segment with T-2 toxin by using one of a peptide chain, 1,4-butanediol diglycidyl ether, N-hydroxy succinimido-3-(2-pyrazolyl dithio)-propionate as a cross-linking agent. The invention further provides a preparation method of the monoclonal antibody-antigen binding segment-T-2 toxin conjugate and application of the monoclonal antibody-antigen binding segment-T-2 toxin conjugate in preparation of a targeted anti-tumor drug. According to the experiments, the conjugate disclosed by the invention has an obvious targeting performance for tumor tissues, and can be used for lowering the toxicity of the T-2 toxin and improving the inhibiting effect of the conjugate on the tumors.
Owner:济南环肽医药科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com