Preparation method for improving liposome entrapment efficiency
A body encapsulation rate, lipid technology, applied in the field of medicine, can solve the problems of difficult to increase the encapsulation rate of lipid-water insoluble compounds, large toxic and side effects, etc., to improve solubility and bioavailability, eliminate side effects, and improve drug stability sexual effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
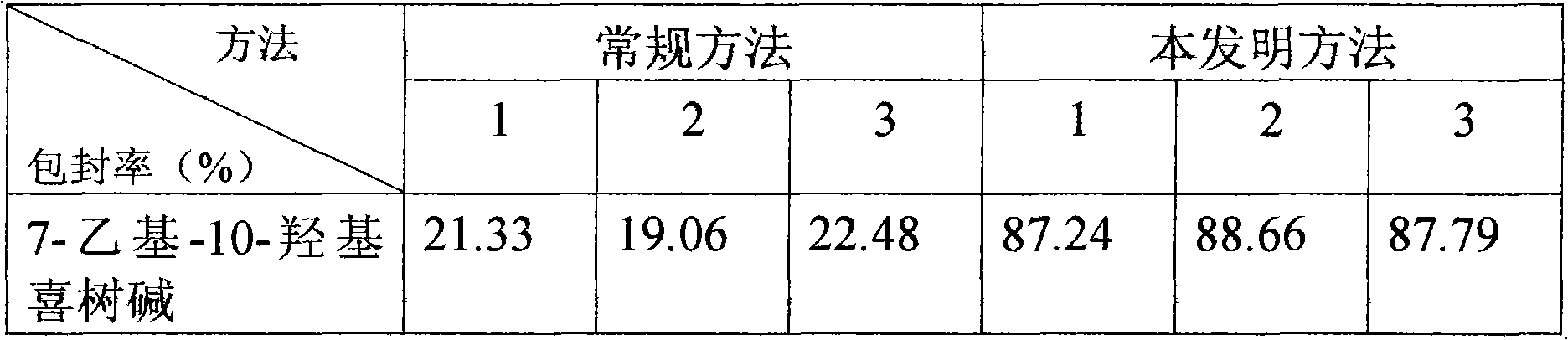
[0103] 7-Ethyl-10-Hydroxycamptothecin Liposomes
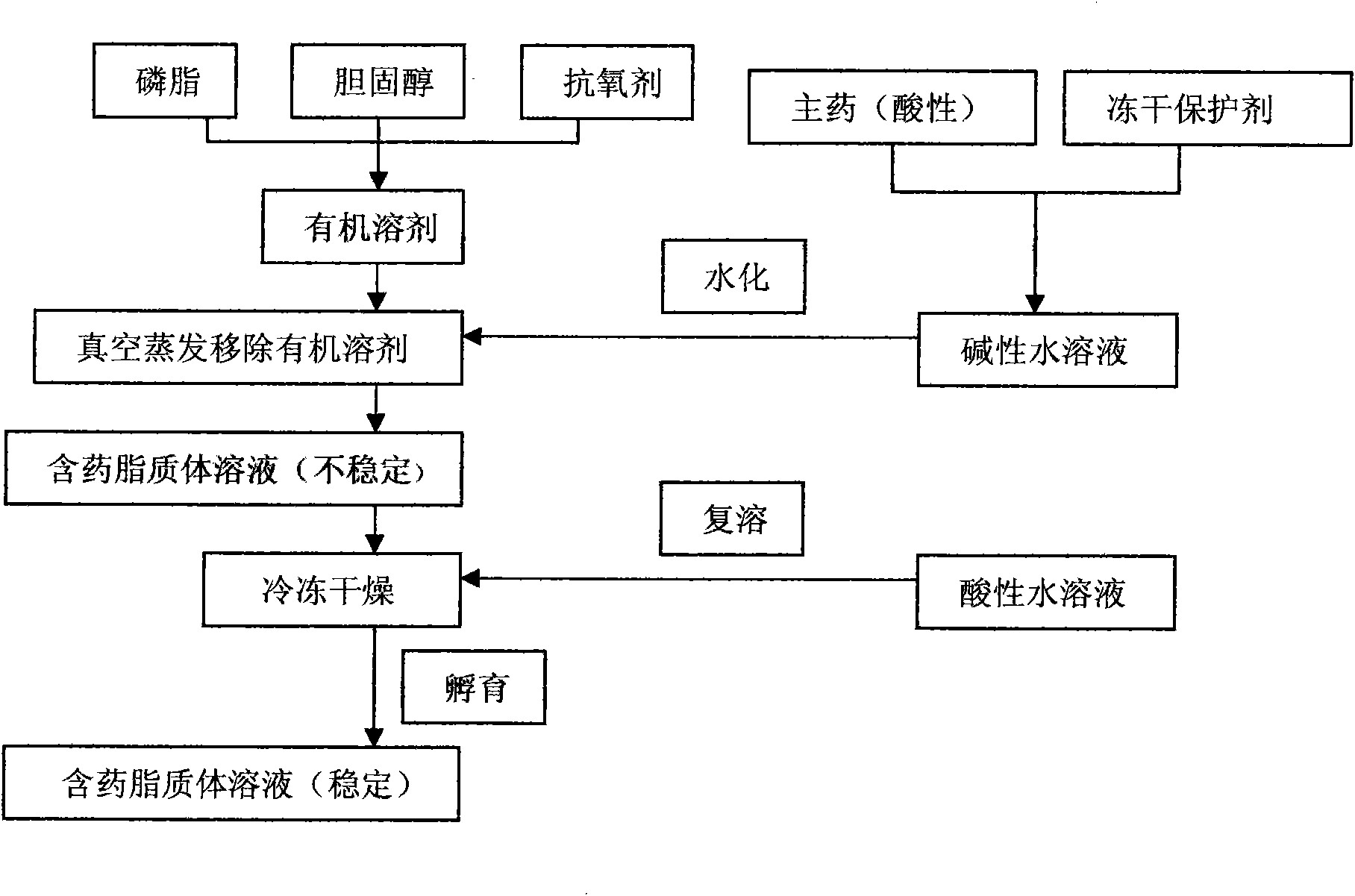
[0104] (1) Dissolve 30 grams of soybean lecithin (injection grade), 5 grams of cholesterol (injection grade), and 2 grams of α-tocopherol in 2000 ml of ethanol (analytical pure), vacuum rotary transfer organic solvent, and place in a vacuum constant temperature drying oven overnight The residual organic solvent was evaporated to prepare dry lipid film, and the above operations were all completed under sterile conditions.
[0105] (2) Dissolve 1.67 grams of 7-ethyl-10-hydroxycamptothecin and 100 grams of sucrose (injection grade) in 1000 ml of sodium carbonate-sodium bicarbonate buffered saline solution (pH=9.9) at a hydration temperature of 45°C Next, use the above-mentioned alkaline buffered saline solution to hydrate the lipid dry film, and after the hydration is complete, use a milk homogenizer to reduce the particle size to 100-200nm to obtain drug-containing liposomes. All the above operations were completed under sterile...
Embodiment 2
[0108] 10-Hydroxycamptothecin liposomes
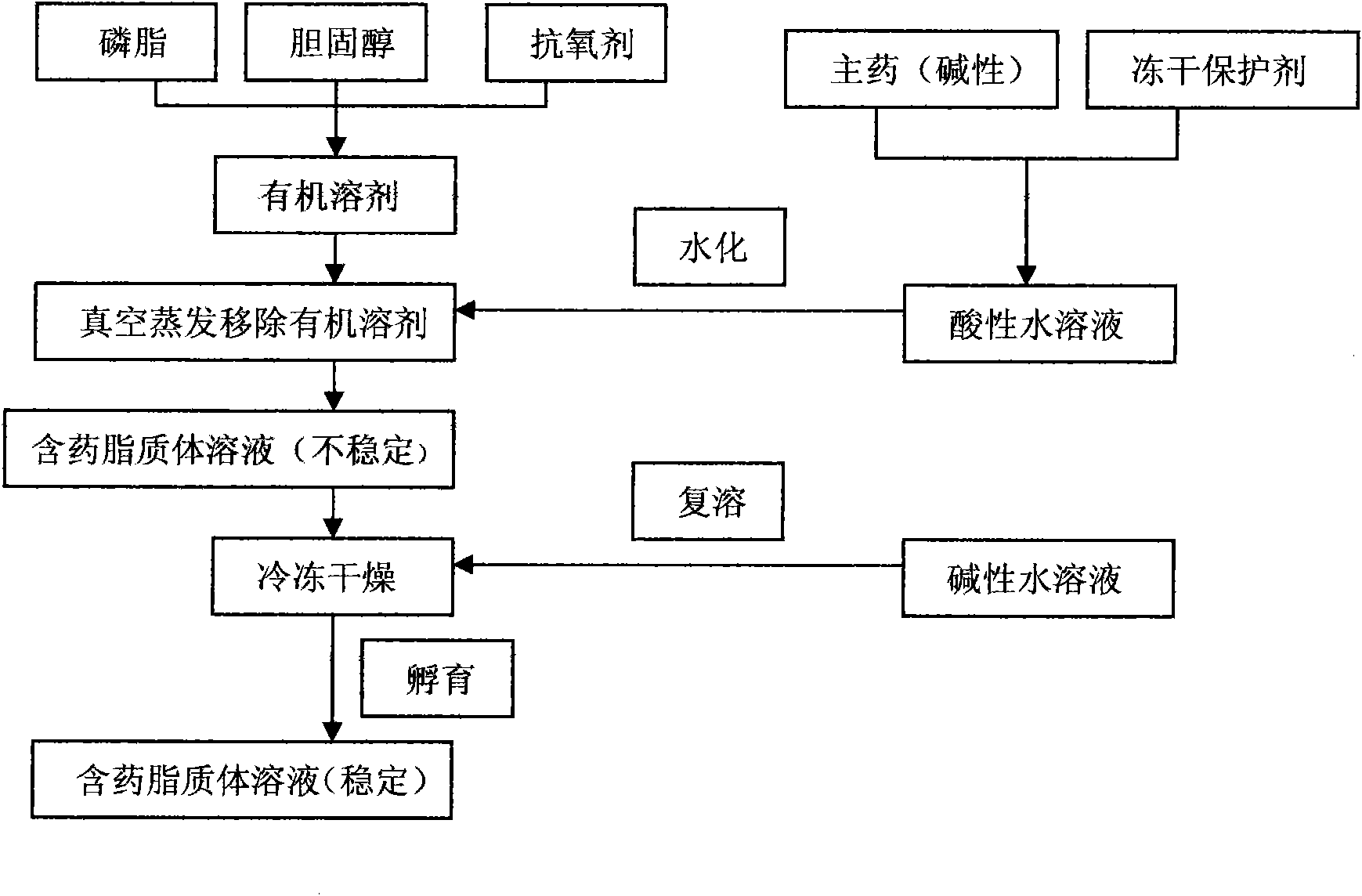
[0109] (1) Dissolve 40 grams of sphingomyelin (injection grade), 10 grams of cholesterol (injection grade), and 4 grams of α-tocopherol in 6000 ml of ethanol (analytical pure), vacuum rotary transfer organic solvent, and place in a vacuum constant temperature drying oven The residual organic solvent was evaporated overnight to prepare a dry lipid film. The above operations were all completed under sterile conditions.
[0110](2) Dissolve 2 grams of 10-hydroxycamptothecin and 100 grams of trehalose (injection grade) in 3000 ml of potassium dihydrogen phosphate-sodium hydroxide buffer solution (pH=10.0), and use the above-mentioned Alkaline buffered saline solution hydrates the dry lipid film, and after the hydration is complete, use a homogenizer to reduce the particle size to 100-200nm to obtain drug-containing liposomes. All the above operations were completed under sterile conditions.
[0111] (3) Vacuum freeze-drying the drug-cont...
Embodiment 3
[0114] (1) Dissolve 50 grams of phosphatidylserine (injection grade), 30 grams of cholesterol (injection grade), and 6 grams of α-tocopherol in 8000 ml of ethanol (analytically pure), vacuum rotary transfer organic solvent, and place in a vacuum constant temperature drying oven The residual organic solvent was evaporated overnight to prepare a dry lipid film. The above operations were all completed under sterile conditions.
[0115] (2) Dissolve 1.67 g of tegafur and 300 g of glycine (injection grade) in 4000 ml of sodium carbonate-sodium bicarbonate buffered saline solution (pH=10.0), and use the above-mentioned alkaline buffered salt at a hydration temperature of 45°C The solution hydrates the lipid dry film, and after the hydration is complete, reduce the particle size to 100-200nm with a homogenizer to obtain the drug-containing liposome. All the above operations were done under sterile conditions.
[0116] (3) Vacuum freeze-drying of the drug-c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com