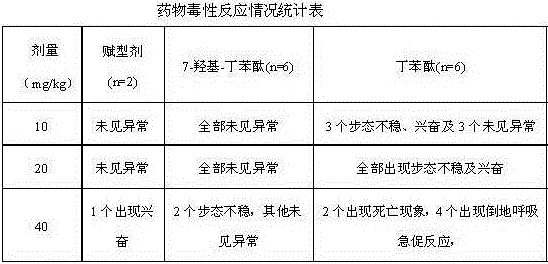
Medical use of 7-hydroxy-butylphthalide
A technology of butylphthalide and hydroxyl, which is applied in the field of medicinal chemistry, can solve the problems that the compound has no anti-oxidation, anti-thrombotic and anti-cerebral ischemia effects, and achieve superior anti-platelet aggregation effects, excellent drug effects, Effect of high platelet aggregation inhibition rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Embodiment 1. Preparation of 7-hydroxyl-butylphthalide, 4-hydroxyl-butylphthalide and 7-methoxyl-butylphthalide
[0032] The preparation of 7-hydroxy-butylphthalide, 4-hydroxy-butylphthalide and 7-methoxy-butylphthalide refers to the patent CN201510113631.9, and Penicillium vulpeides strain NCC3421 (preserved in the China General Microorganisms Collection Management Center, the deposit number is CGMCC No.9094) was inoculated in a fermenter with 30L of fermentation medium, the tank temperature was 26°C, stirring at 220rpm, and cultured for 120 h. The obtained culture was centrifuged at 4000 rpm for 20 minutes to collect mycelia, leached with ethanol for processing, and filtered to obtain a filtrate. Dilute the above filtrate with water to an ethanol concentration of 40%, absorb it with a column filled with 2L D312 resin, and then elute it with 40%, 55%, and 70% ethanol respectively, receive it in sections, combine the parts containing the target object, and depressurize ...
Embodiment 2
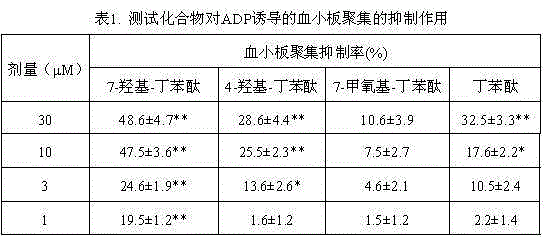
[0038] Example 2. In Vitro Anti-Platelet Aggregation Activity
[0039] (1) Experimental method: Evaluation of anti-platelet aggregation activity, using ADP as inducer, using Born turbidimetric method. Wistar rats (Animal Center of Hebei Medical University, experimental animal qualification certificate number: 1304152) were taken from orbital blood, anticoagulated with 3.8% sodium citrate (whole blood to anticoagulant volume ratio 9:1), centrifuged at room temperature ( 500-800 rpm, 10 min), prepare platelet-rich plasma (PRP), separate the PRP, and then centrifuge (3000 rpm, 15 min), prepare platelet-poor plasma (PPP), adjust to 0 with PPP. Take PRP (200 μL) and add 5 μL of DMSO solution of equimolar concentration of the target compound, add equal volume of DMSO to the solvent control group, incubate for 2 min, use 10 μM ADP as inducer, and measure platelet aggregation by Born nephelometric method. The anti-platelet aggregation activity of the target compound was determined in...
Embodiment 3
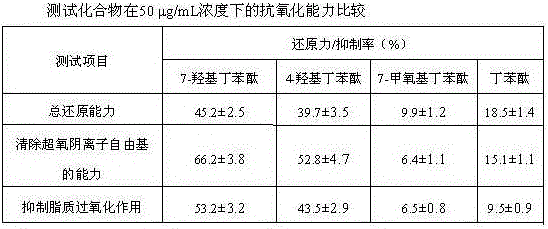
[0044] Example 3. In vitro antioxidant capacity
[0045] (1) Experimental materials and methods
[0046] Determination of total reducing ability: Add 2.5 mL of 0.2 mol / L pH 6.6 phosphate buffer solution and 2.5 mL of potassium ferricyanide with a mass fraction of 1% to 1 mL of samples with different concentrations, and place in a water bath at 50 °C for 20 min. After rapid cooling in an ice bath, add 2.5 mL of 10% trichloroacetic acid solution, centrifuge at 3000 r / min for 10 min, take 1.0 mL of the supernatant, add 1.0 mL of distilled water and 0.1% FeCl 3 0.2 mL, mix well, let stand at room temperature for 10 min, measure the absorbance value at a wavelength of 700 nm, measure the reducing power according to the absorbance value, and calculate the reducing power %.
[0047] The ability to scavenge superoxide anion free radicals: take different volumes of samples and add 50 mM Tris-HCl buffer solution with a pH value of 8.2 to prepare solutions with different concentrations...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com