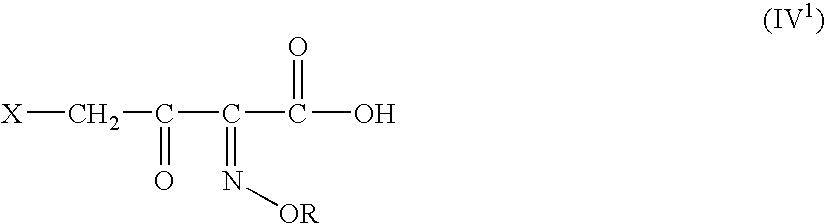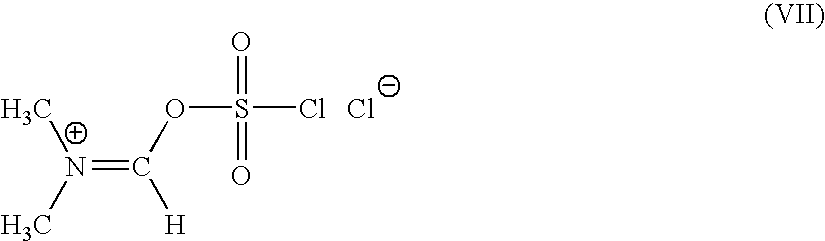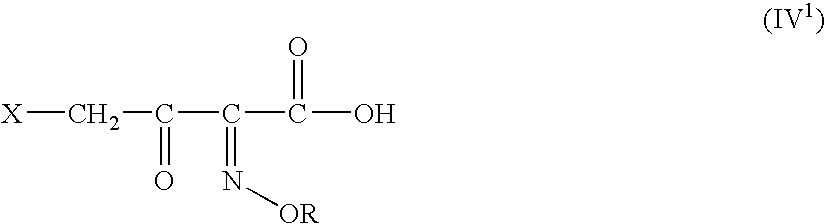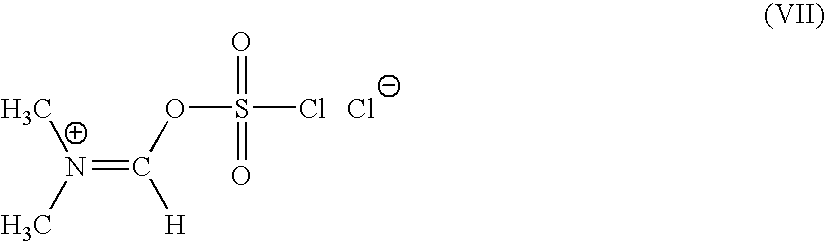Patents
Literature
38 results about "Cefquinome" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
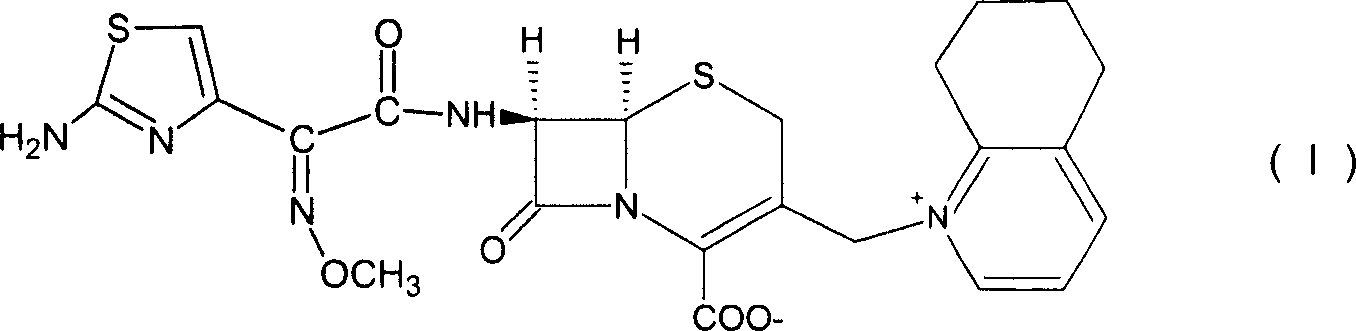
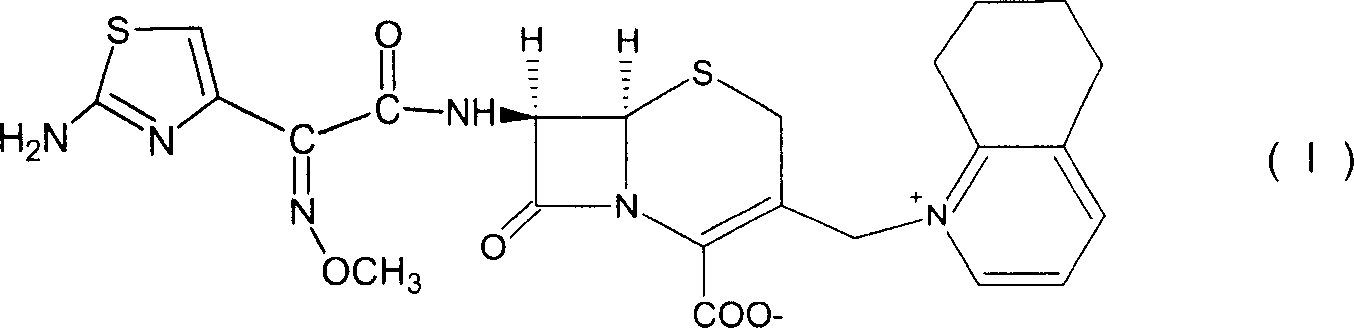
Cefquinome is a fourth-generation cephalosporin with pharmacological and antibacterial properties valuable in the treatment of coliform mastitis and other infections. It is only used in veterinary applications.
Novel intermediates for synthesis of cephalosporins and process for preparation of such intermediates
InactiveUS20060135761A1Easily hydrolysableSulfuric acid esters preparationBulk chemical productionCefmenoximeAntibiotic Y
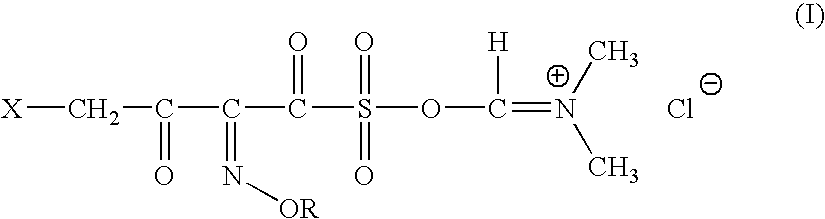
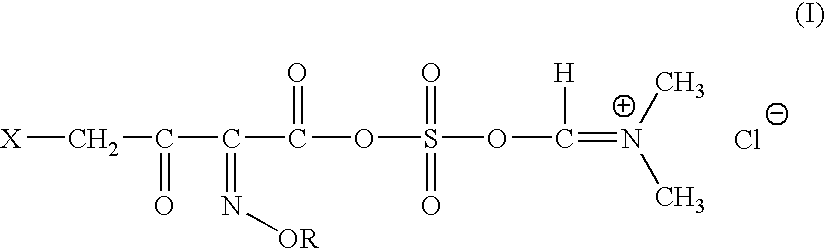
A novel 4-halo-2-oxyimino-3-oxo butyric acid-N,N-dimethyl formiminium chloride chlorosulfate of formula (I) useful in the preparation of cephalosporin antibiotics wherein X is chlorine or bromine; R is hydrogen, C1-4 alkyl group, an easily removable hydroxyl protective group, —CH2COOR5, or —C(CH3)2COOR5, wherein R5 is hydrogen or an easily hydrolysable ester group. The compound of formula (I) is prepared by reacting 4-halo-2-oxyimino-3-oxobutyric acid of formula (IV1), wherein X, R and R5 are as defined above, with N,N-dimethylformiminium chloride chlorosulphate of formula (VII) in an organic solvent at a temperature ranging from −30° C. to −15° C. The cephalosporins that may be prepared from the intermediate include cefdinir, cefditoren pivoxil, cefepime, cefetamet pivoxil, cefixime, cefmenoxime, cefodizime, cefoselis, cefotaxime, cefpirome, cefpodoxime proxetil, cefquinome, ceftazidime, cefteram pivoxil, ceftiofur, ceftizoxime, ceftriaxone and cefuzonam.
Owner:LUPIN LTD
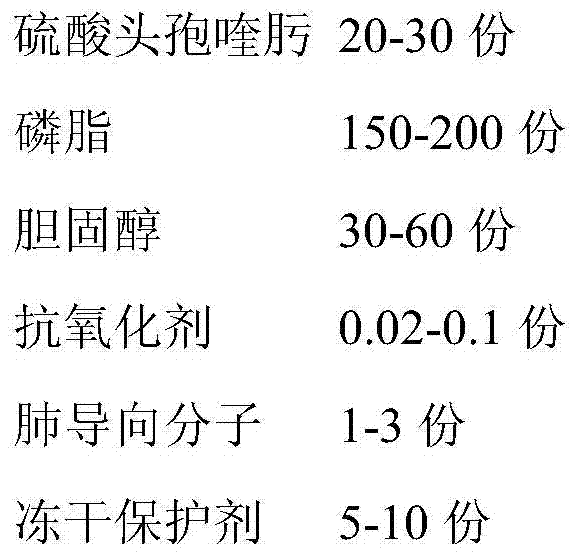
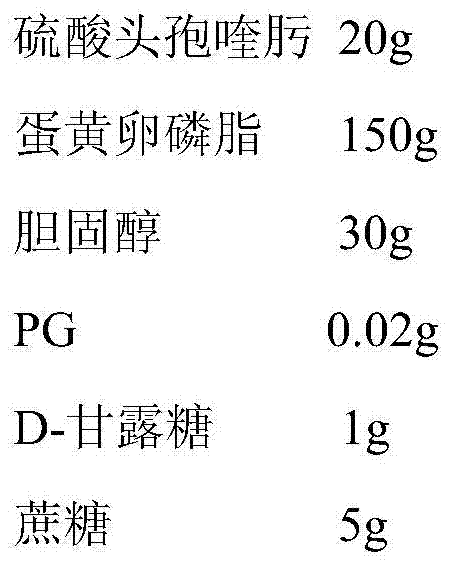
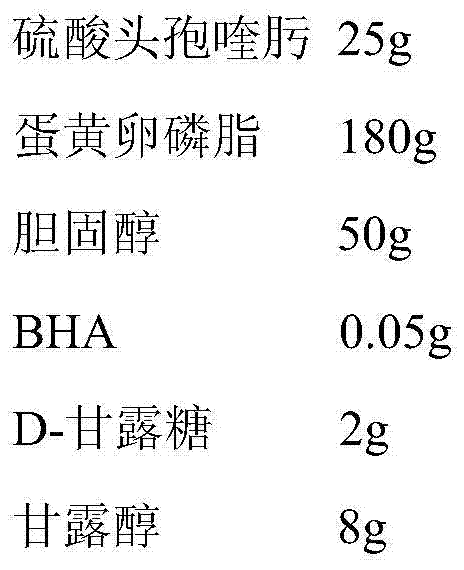
Livestock cefquinome lung-targeted microspheres and preparation method thereof
InactiveCN101536985AReduce releaseGood curative effectAntibacterial agentsOrganic active ingredientsParaffin waxSide effect
The invention belongs to the technical field of veterinary drugs. The invention discloses a veterinary drug of cefquinome microspheres serving as a lung-targeted preparation prepared by using cefquinome as a raw material and a preparation method thereof. The cefquinome is used as the raw material and gelatin is used as a carrier to form micropheres, and the ratio of the cefquinome to the gelatin is 1:2. The preparation method comprises the following steps: firstly, dissolving the cefquinome in a solution of the gelatin, uniformly mixing the cefquinome and the solution of the gelatin; secondly, dripping the mixed solution in sorbitanmonooleate and liquid paraffin and uniformly stirring the mixture till the mixture turns milky white; quickly cooling the mixture to below 5 DEG C in an ice bath, adding glutaric dialdehyde in form of thin flow, and continuing stirring for cross linking and curing; dehydrating the product obtained with isopropanol, and performing stirring and suction filtration; and washing the dehydrated product with a small amount of isopropanol and ether to remove the glutaric dialdehyde completely, washing away the liquid paraffin on the surface of the microspheres with petroleum ether and drying the microspheres at room temperature and under vacuum to obtain the cefquinome microspheres. The preparation effectively improves tissue selectivity of drug, slows drug release, improves treatment effect, reduces toxic and side effects, and achieves slow release, long-lasting effect and targeting.
Owner:TIANJIN RINGPU BIO TECH
Cefquinome liposome
ActiveCN104000783AReduce toxicityTargetedAntibacterial agentsOrganic active ingredientsPhospholipinSterol
Cefquinome liposome, which belongs to the field of pharmaceutics, has a particle size of less than 1000 nm, and is mainly prepared from the following raw materials by weight: 1 part of cefquinome, 1-40 parts of phospholipid, 0-15 parts of sterol or soy isoflavone glucoside or soyasapogenol, and 0-15 parts of additives. The cefquinome liposome can be prepared into a liquid preparation, and can also be prepared into a solid preparation by adding a proper amount of a support agent. The cefquinome liposome or cefquinome long circulating liposome prepared in the invention contains no irritant substances, and can relieve anaphylactic reaction; the obtained preparation has good stability, has an average liposome particle size of less than 1000 nm, and has encapsulation efficiency of more than 80%. The preparation method is mature in process, simple, practical, and suitable for industrial production.
Owner:HENAN SOAR VETERINARY PHARMA
Method for detecting cefquinome residual quantity in milk
The invention relates to a method for detecting cefquinome residual quantity in milk through ultra-high performance liquid chromatography-electric spraying tandem mass spectrometry. A technological method is supplied for monitoring the cefquinome residual quantity in milk.
Owner:INST OF AGRI PROD QUALITY SAFETY & STANDARD JIANGXI ACAD OF AGRI SCI
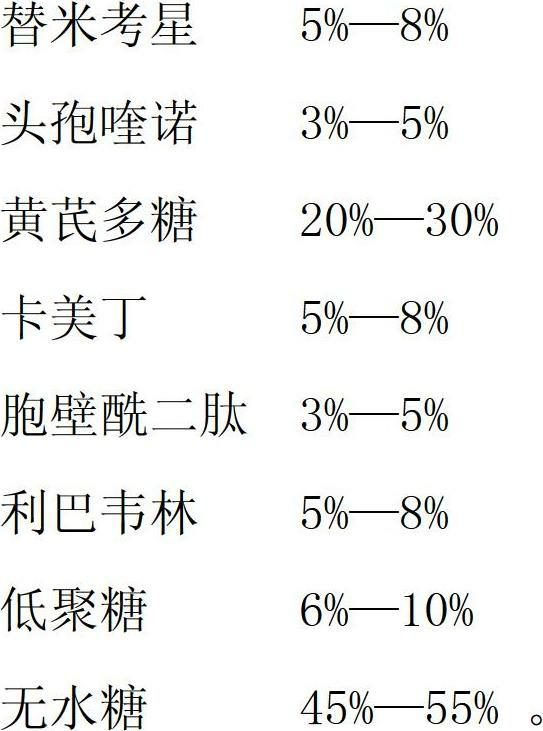
Medicinal composition for treating respiratory diseases of livestock
InactiveCN102688477AHeat-clearing and detoxifyingUnique curative effectDipeptide ingredientsTetracycline active ingredientsDiseaseRegimen
The invention provides a medicinal composition for treating respiratory diseases of livestock, which comprises the following active components in percentage by mass: 5 to 8 percent of tilmicosin, 3 to 5 percent of cefquinome, 20 to 30 percent of astragalus polysaccharides, 5 to 8 percent of kameiding, 3 to 5 percent of muramyl dipeptide, 5 to 8 percent of ribavirin, 6 to 10 percent of oligosaccharide, and 45 to 55 percent of anhydrous sugar. The drugs are mixed, and have the functions of clearing heat and removing toxicity, relieving cough and asthma, have unique curative effect for treating respiratory diseases of livestock, have no drug resistance, and have quick response, good curative effect and short course of treatment.
Owner:ZHENGZHOU AINUO BIOTECH
Cefquinome liposome and preparation method thereof
InactiveCN103585109AHigh encapsulation efficiencyUniform particle size distributionAntibacterial agentsOrganic active ingredientsFreeze-dryingAntioxidant
The invention discloses cefquinome liposome. The cefquinome liposome comprises the following raw materials by weight: 0.01-50% of cefquinome or a salt thereof, 10-99.99% of phospholipid, 0-65% of cholesterol, 0-60% of deoxysodium cholate, 0-89.99% of anionic lipid, and 0-40% of an antioxidant. The invention further discloses a preparation method of the liposome. The cefquinome liposome researched by the invention has the advantages of excellent packaging rate, uniform particle size distribution, achievement of intravenous injection or local application standards, excellent stability and further improvement of stability through preparation of freeze-dried products. Moreover, the blank liposome-ammonium sulfate gradient medicine carrying preparation technique adopted by the invention is very suitable for large-scale industrial production, and has a wide industrialization prospect.
Owner:HENAN SOAR VETERINARY PHARMA
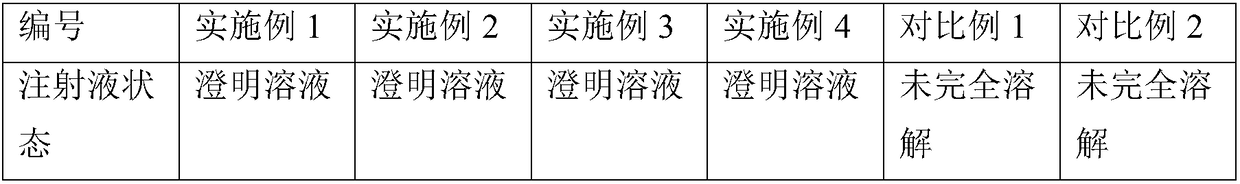
Cefquinome medicinal composition for injection capable of being stored stably and dissolved easily in water
InactiveCN101007007AImprove storage qualityImprove solubilityAntibacterial agentsOrganic active ingredientsWater solubleSolvent
The invention relates to the water soluble Cefquinome compounds which are used for injection. The compounds include Cefquinome (the active ingredient) and / or added salt with acid, one kind or many kinds of auxiliary solvent which can be used in medicine, and one kind or many kinds of additives commonly used for injections. The preparing method of the compounds is also mentioned in the invention. The product in the invention has a steady quality and can be dissolved in water easily.
Owner:QILU ANIMAL HEALTH PROD
Livestock cefquinome bletilla striata bioadhesive slow-release capsules
InactiveCN101537008AExtended stayGood treatment effectAntibacterial agentsOrganic active ingredientsBletilla striataBioadhesive
The invention relates to the technical field of veterinary drugs, and particularly relates to a veterinary drug of cefquinome bletilla striata bioadhesive slow-release capsule prepared by using cefquinome and bletilla striata as raw materials. An aqueous extraction and alcohol sedimentation method is adopted to extract the bletilla striata; the ratio of the cefquinome to the bletilla striata is 1:3; a 10 percent PVP ethanol solution is used as an adhesive; and the drug is made into 20 mesh grains and is filled in capsules. The contents of the cefquinome bletilla striata bioadhesive slow-release capsules can prolong the time for the detention of the drug at focuses, improve the local concentration of the drug in lesions, and have excellent bioadhesive property as well as the same drug release characteristic as a slow-release preparation.
Owner:TIANJIN RINGPU BIO TECH
Electrochemical receptor sensor for detecting beta-lactam antibiotics, and preparation method and application thereof
InactiveCN110243907AHigh sensitivityHigh precisionMaterial electrochemical variablesPenicillinAntibiotics beta lactam
Owner:HUAZHONG AGRI UNIV
Livestock cefquinome colon-targeted pills and preparation method thereof
InactiveCN101536992AFast absorptionShort peak timeAntibacterial agentsOrganic active ingredientsIntestinal structureTime lag
The invention belongs to the technical field of veterinary drugs and relates to livestock cefquinome colon-targeted pills. The livestock cefquinome colon-targeted pills comprise cefquinome serving as a main drug, and empty pellets are covered with a drug layer made of the cefquinome, 8 percent of a block layer made of cetanol, 2 percent of a time lag layer made of HPMC, and 15 percent of a pH sensitive film made of eudragit S100 respectively to form the livestock cefquinome colon-targeted pills. The livestock cefquinome colon-targeted pellets combining time lag and pH sensitivity can release drug at fixed positions of the colons of livestock, greatly improve the treatment effect on local infection and general infection, have low toxicity and little residue in edible animal tissues, and can be widely applied to clinic treatment of the livestock.
Owner:TIANJIN RINGPU BIO TECH
Cefquinome crystal and preparation method thereof
ActiveCN105646543AGood water solubilityImprove bioavailabilityOrganic chemistry methodsOrganic solventPowder diffraction
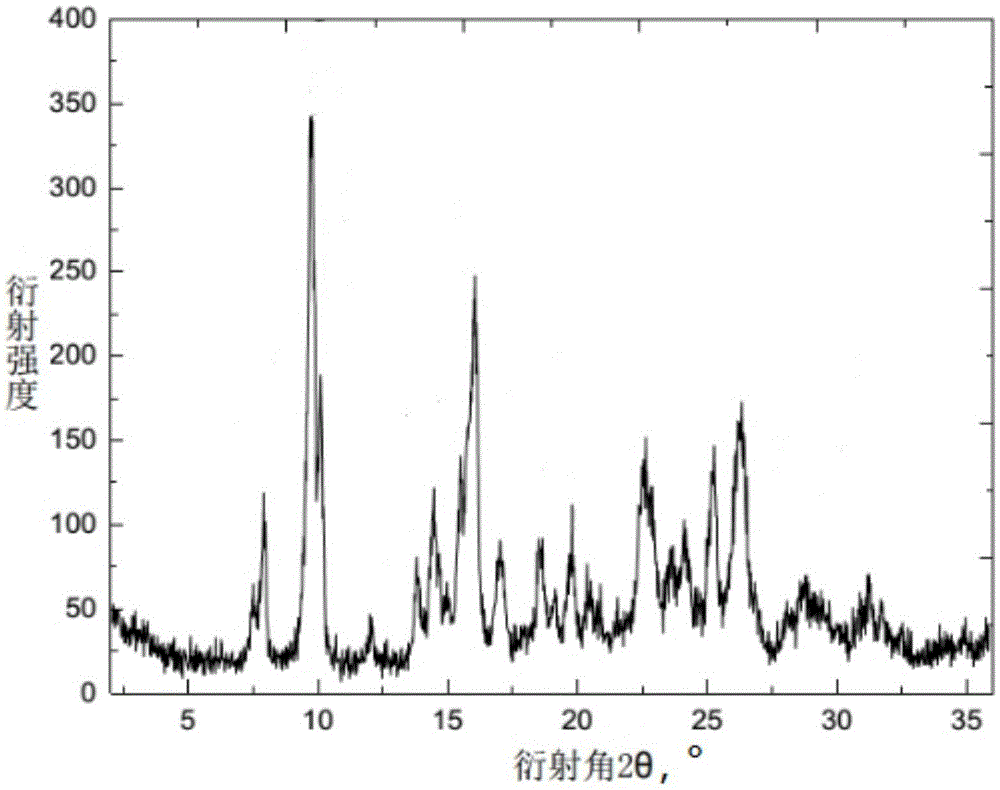
The invention discloses a cefquinome crystal and a preparation method thereof. An X-ray powder diffraction spectrum of the cefquinome crystal has characteristic peaks at places where a diffraction angle 2theta is equal to 7.5 plus / minus 0.1, 7.9 plus / minus 0.1, 9.7 plus / minus 0.1, 10.1 plus / minus 0.1, 12.0 plus / minus 0.1, 13.8 plus / minus 0.1, 14.5 plus / minus 0.1, 15.5 plus / minus 0.1, 16.0 plus / minus 0.1, 17.0 plus / minus 0.1, 18.5 plus / minus 0.1, 19.8 plus / minus 0.1, 20.5 plus / minus 0.1, 20.9 plus / minus 0.1, 22.6 plus / minus 0.1, 22.9 plus / minus 0.1, 23.7 plus / minus 0.1, 24.2 plus / minus 0.1, 25.3 plus / minus 0.1, 26.3 plus / minus 0.1, 28.7 plus / minus 0.1, and 31.2 plus / minus 0.1. The preparation method comprises the steps of adding activated carbon in a cefquinome solution, stirring and filtering; adding filtrate into an organic solvent, thus obtaining the cefquinome crystal. The method is simple, high in yield and low in cost; safety is realized when the cefquinome crystal is used as a drug.
Owner:TIANJIN UNIV +1
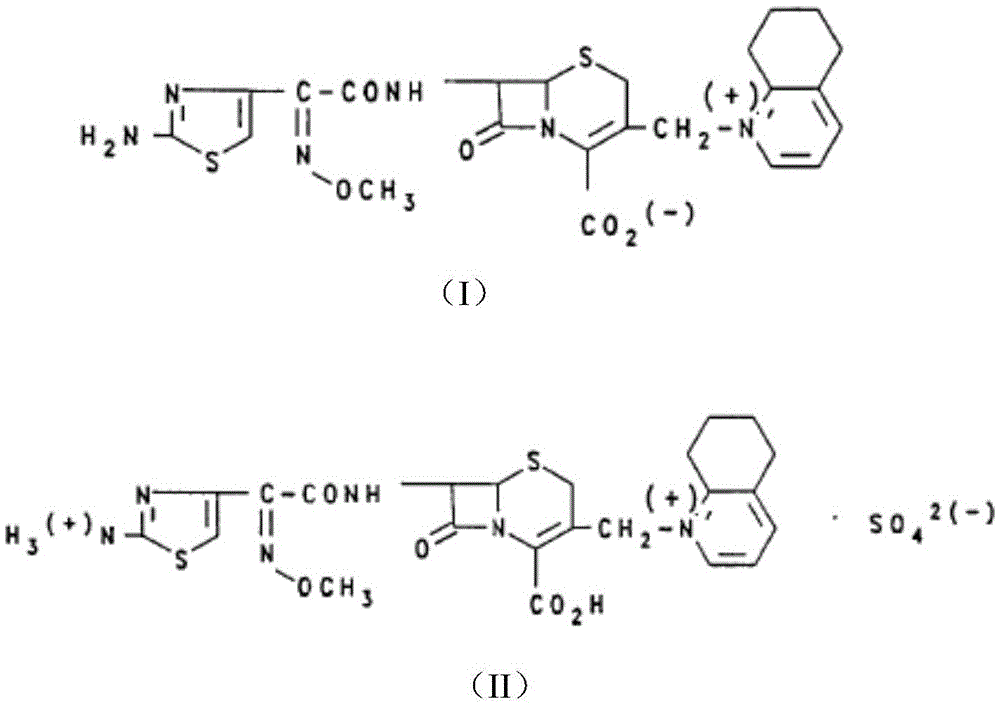
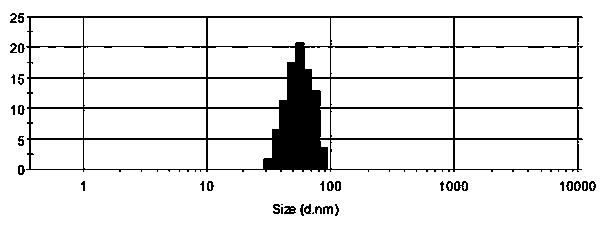
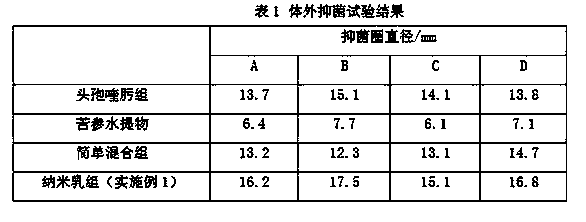
Compound cefquinome antibacterial agent
ActiveCN104352554AEvenly distributedSystem transparencyAntibacterial agentsOrganic active ingredientsAntimicrobial drugActive agent
The invention belongs to the technical field of medicines and provides a compound cefquinome antibacterial agent. The compound cefquinome antibacterial agent comprises the effective ingredients of cefquinome and radix sophorae flavescentis aqueous extract, the dosage form is an oil-in-water type nanoemulsion, the nanoemulsion comprises the following materials in parts by weight: 1-15 parts of the radix sophorae flavescentis aqueous extract, 15-40 parts of a surfactant, 0-20 parts of a cosurfactant, 1-20 parts of cefquinome, 1-20 parts of oil, and 20-70 parts of distilled water. The nanoemulsion is uniform in distribution, transparent in system, excellent in stability, relatively low in surface tension, excellent in mobility and convenient in eating, the nanoemulsion is rapidly devoured by reticuloendothelial cells after being taken, so that the medicine takes effects quickly, the constant blood concentration and the pharmacological effect are maintained, the bioavailability of the medicine is improved, the effect of the medicine is enhanced, and the amount of the medicine used and the use times are reduced.
Owner:HENAN SOAR VETERINARY PHARMA
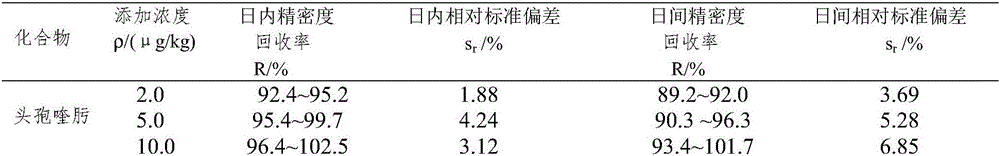
Specific anti-ceftiofur monoclonal antibody hybridoma cell strain 2E5 and application thereof
ActiveCN105505886AHigh detection sensitivityHigh affinityTissue cultureImmunoglobulinsAntibiotic YSpecific antibody
The invention discloses a specific anti-ceftiofur monoclonal antibody hybridoma cell strain 2E5 and the application thereof and belongs to the technical field of food safety immunodetection. The monoclonal cell strain 2E5 is preserved in the China General Microbiological Culture Collection Center, CGMCC for short, with the preservation number of CGMCC No.10873. The crude drug ceftiofur and BSA are coupled to prepare immunogen by means of the active ester method, and ceftiofur and OVA are coupled to prepare coating antigen. The specific anti-ceftiofur hybridoma cell strain is obtained after a mouse is immunized. The monoclonal antibody secreted by the cell strain only aims at ceftiofur specifically, the crossing-over rates between the monoclonal antibody and cefalexin and between the monoclonal antibody and cefquinome are 3.12% and 5.3% respectively, the crossing-over rates between the monoclonal antibody and other cephalosporin antibiotics are all smaller than 0.1%, and therefore specificity is high. The anti-ceftiofur monoclonal antibody hybridoma cell strain has high detection sensitivity and compatibility, the crossing-over rates with other cephalosporin antibiotics are low, and therefore specific detection of ceftiofur can be achieved. The invention also provides a new idea for preparing a specific antibody aiming at a specific cephalosporin so as to obtain a good specific monoclonal cell strain.
Owner:无锡迪腾敏生物科技有限公司
Composite cefquinome microsphere gel preparation and preparation method thereof
ActiveCN106474087AGood sustained release effectReduce clearanceAntibacterial agentsOrganic active ingredientsGel preparationBlood clearance
The invention discloses a composite cefquinome microsphere gel preparation and a preparation method thereof, and belongs to the technical field of medicines. The composite cefquinome microsphere gel preparation for veterinary use is prepared by adopting a spray drying method to prepare microspheres, adopting cefquinome as the medicine for the microspheres, adopting polylactic acid / polylactic acid-glycolic acid as a coating carrier material, and adopting hyaluronic acid and water as gel bases to prepare the composite cefquinome microsphere gel preparation. The microspheres are of 5-30umd in grain size, which is beneficial to lung targeting of the main medicine cefquinome, the cefquinome can easily gather at a target organ, blood clearance is reduced, long-time circulation is benefited, and pharmacological efficacy is improved. In addition, by the aid of the gel bases, contact of the medicine and interstitial fluid is reduced, the time of the medicine flowing into a bloodstream is prolonged, and a slow-release effect of the preparation is further increased.
Owner:RINGPU TIANJIN BIOLOGICAL PHARMA
Intermediates for synthesis of cephalosporins and process for preparation of such intermediates
Owner:LUPIN LTD
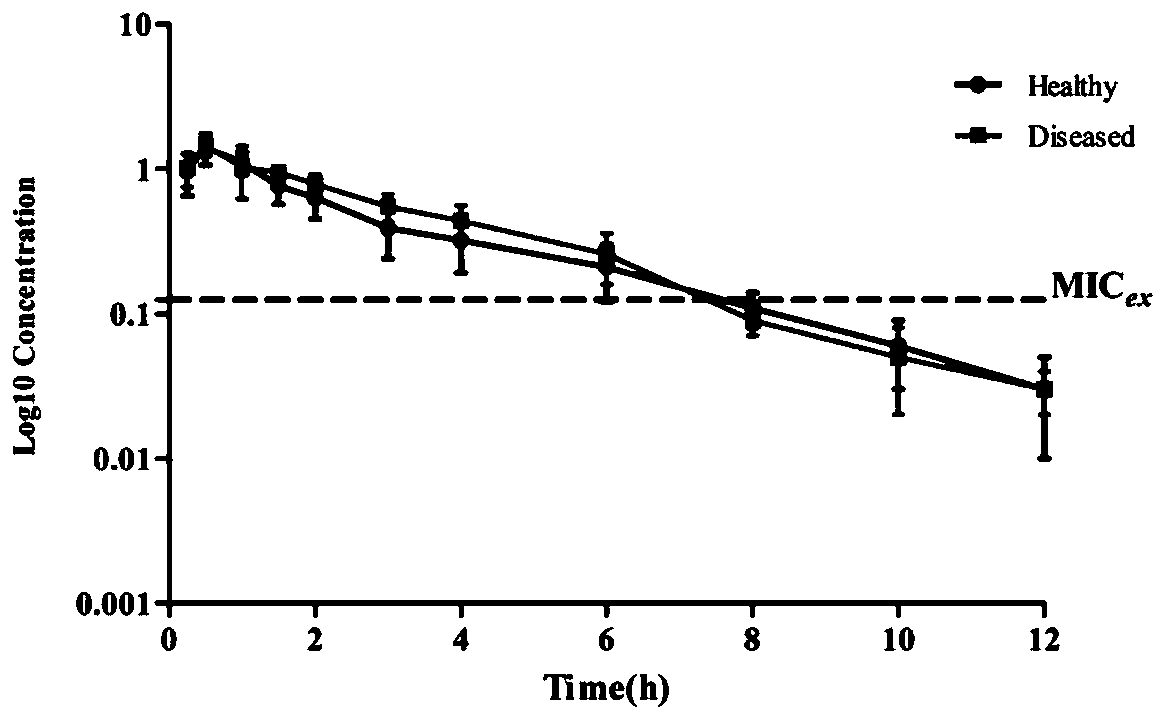
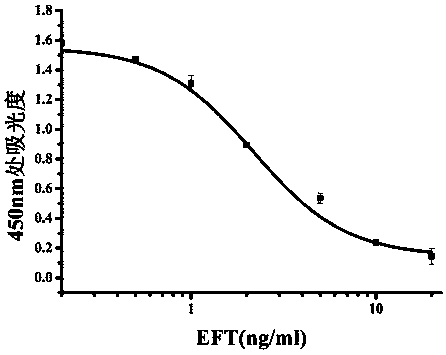
Method for constructing pharmacokinetic-pharmacodynamic (PK/PD) synchronization model of cefquinome and application thereof
The invention discloses a method for constructing a pharmacokinetic-pharmacodynamic (PK / PD) synchronization model of cefquinome and application thereof. The method specifically includes: detecting thedrug sensitivity of the antibacterial drug to the corresponding bacterial microorganism, and obtaining the MIC distribution range; using liquid chromatography detection method to determine the free drug concentration in the plasma samples obtained at different time points after administration of healthy and disease model animals to obtain a drug time curve; fitting the pharmacokinetic parametersof the drug by pharmacokinetic software to obtain PK parameters; studying, the antibacterial effect of antibacterial drugs on pathogenic bacteria under in vitro and semi-in vivo conditions, and obtaining PD parameters after fitting; establishing the semi-in vivo PK-PD model according to the Sigmoid Emax equation; and based on this model, using the dose calculation method and Mlxplore software to formulate different dosage regimen under the purpose of the medicine. The invention has guiding significance for the clinical application of cefquinome.
Owner:HUAZHONG AGRI UNIV
Cefquinome powder injection
ActiveCN108685852AInhibition of crystal changeCrystalline retentionAntibacterial agentsPowder deliveryPolyethylene glycolSURFACTANT BLEND
Cefquinome powder injection is characterized by comprising crystalline cefquinome, and polyethylene glycol and amino acid surfactant acting as crystal transition inhibitory auxiliaries; the mass ratioof the polyethylene glycol to the amino acid surfactant is 10:(0.5-2). A preparation method of the cefquinome powder injection includes the steps of (1) melting, to be specific, heating the polyethylene glycol to 50-65 DEG C for melting, adding the crystalline cefquinome and the amino acid surfactant, and mixing to obtain melt A; (2) cooling, to be specific, quickly cooling the melt A to solid state to obtain solid mixture B; (3) crushing the solid mixture B.
Owner:RINGPU TIANJIN BIOLOGICAL PHARMA
Compound cefquinome sulphate injection
ActiveCN104352500AImprove stabilityHigh antibacterial activityAntibacterial agentsOrganic active ingredientsSophocarpidineSulfite salt
The invention provides a compound cefquinome sulphate injection which has a synergetic antibacterial effect. The compound cefquinome sulphate injection disclosed by the invention comprises the following components in parts by weight: 1-4 parts of cefquinome sulphate, 1-2 parts of sophocarpidine, 0.5-1 part of sodium sulfite and 1-2 parts of hydroxypropyl-beta-cyclodextrin. The compound cefquinome sulphate injection disclosed by the invention is high in stability, simple in preparation process and accurate in curative effect on colibacillosis in pig, and has a good inhibiting effect on colibacillosis in pig; the dosage of a prescribed preparation can be reduced; the treatment cycle can be shortened; and thus, the compound cefquinome sulphate injection disclosed by the invention has wide market prospect.
Owner:CHONGQING TAITONG ANIMAL PHARMA
Cefquinome liposome
ActiveCN104000783BReduce toxicityTargetedAntibacterial agentsOrganic active ingredientsSterolSOY ISOFLAVONES
Cefquinome liposome, which belongs to the field of pharmaceutics, has a particle size of less than 1000 nm, and is mainly prepared from the following raw materials by weight: 1 part of cefquinome, 1-40 parts of phospholipid, 0-15 parts of sterol or soy isoflavone glucoside or soyasapogenol, and 0-15 parts of additives. The cefquinome liposome can be prepared into a liquid preparation, and can also be prepared into a solid preparation by adding a proper amount of a support agent. The cefquinome liposome or cefquinome long circulating liposome prepared in the invention contains no irritant substances, and can relieve anaphylactic reaction; the obtained preparation has good stability, has an average liposome particle size of less than 1000 nm, and has encapsulation efficiency of more than 80%. The preparation method is mature in process, simple, practical, and suitable for industrial production.
Owner:HENAN SOAR VETERINARY PHARMA
A cefquinome sulfate lung targeting liposome preparation and preparation method thereof
ActiveCN104825393BImprove targetingHigh encapsulation efficiencyOrganic active ingredientsPharmaceutical non-active ingredientsSide effectMedicine
The invention belongs to the field of medicine, and relates to a new dosage form of veterinary medicine, in particular to a cefquinome sulfate lung-targeting liposome preparation and a preparation method thereof. The cefquinome sulfate lung-targeting liposome preparation provided by the invention has the advantages of good targeting, stable quality, high encapsulation efficiency and the like. The cefquinome sulfate lung-targeted liposome provided by the present invention is easy to use, has active and passive targeting effects, and the drug concentrates in the target organ after injection to take effect, reduces the loss of the drug, improves the curative effect, and reduces the effect on the Toxic side effects in other organs.
Owner:AMICOGEN CHINA BIOPHARM CO LTD
A method to improve the detection recovery rate of cefquinome residues in food of animal origin and reduce the matrix effect
The invention discloses a method for improving the detection recovery rate of cefquinome residue in animal-derived food and reducing a substrate effect. According to the method, moisture is effectively removed through freeze drying; the recovery rate of the cefquinome residue in the animal-derived food is obviously improved; the substrate effect is reduced, so that a detection result is more accurate; the reasonable clinical application of cefquinome in veterinary medicine can be more effectively monitored; the safety of the animal-derived food for people is guaranteed.
Owner:JIANGXI AGRICULTURAL UNIVERSITY
Livestock cefquinome solution containing hexacyclic water
InactiveCN101537183ALow viscosityReduce surface tensionOrganic active ingredientsAntiinfectivesDiseaseDiffusion resistance
The invention belongs to the technical field of veterinary drugs and relates to a livestock antibacterial drug of broad spectrum antibiotic solution prepared by using cefquinome as a raw material. The cefquinome solution uses hexacyclic water as a carrier. The hexacyclic water prepared by refrigeration is filled in the cefquinome solution, so that the volume of water clusters is reduced and correspondingly the viscosity, surface tension and the like of water are reduced to reduce the diffusion resistance of the cefquinome and to promote drug release. The speed and amount of drug release of the cefquinome solution are improved. The hexacyclic water has high cellular affinity. The livestock cefquinome solution containing hexacyclic water is used for treating sensitive bacteria infection of livestock, obviously improves the treatment effect of the drug, and quickly promotes the restoration from diseases.
Owner:TIANJIN RINGPU BIO TECH
A kind of cefquinome powder injection
ActiveCN108685852BInhibition of crystal changeCrystalline retentionAntibacterial agentsPowder deliveryCrystallographyActive agent
Owner:RINGPU TIANJIN BIOLOGICAL PHARMA
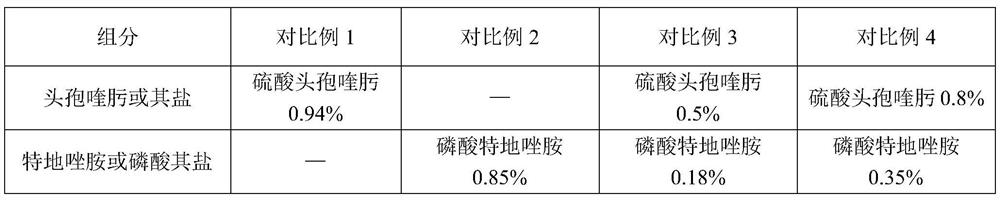
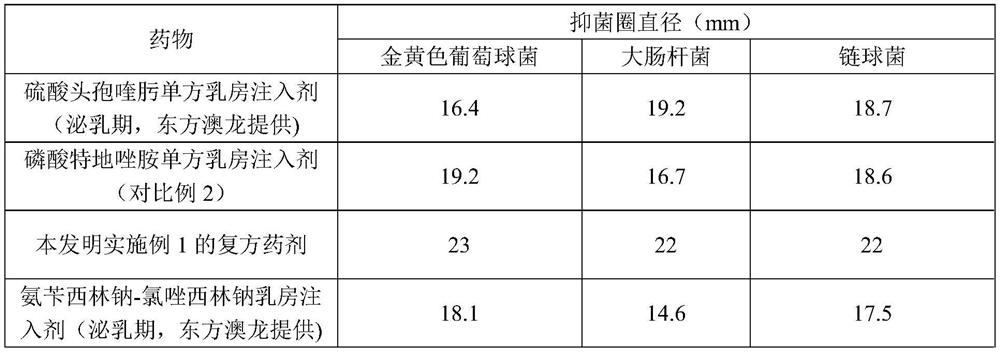
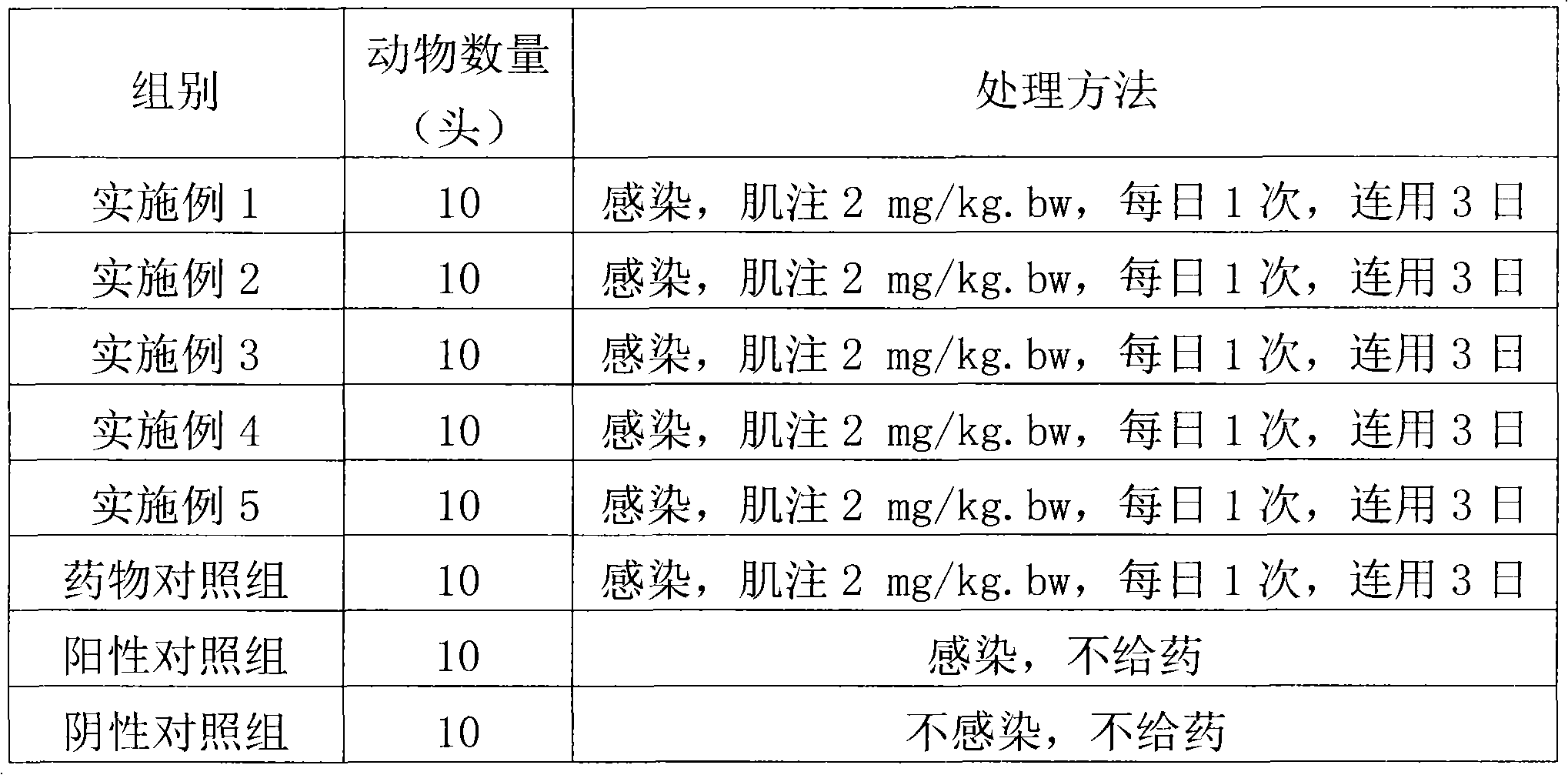
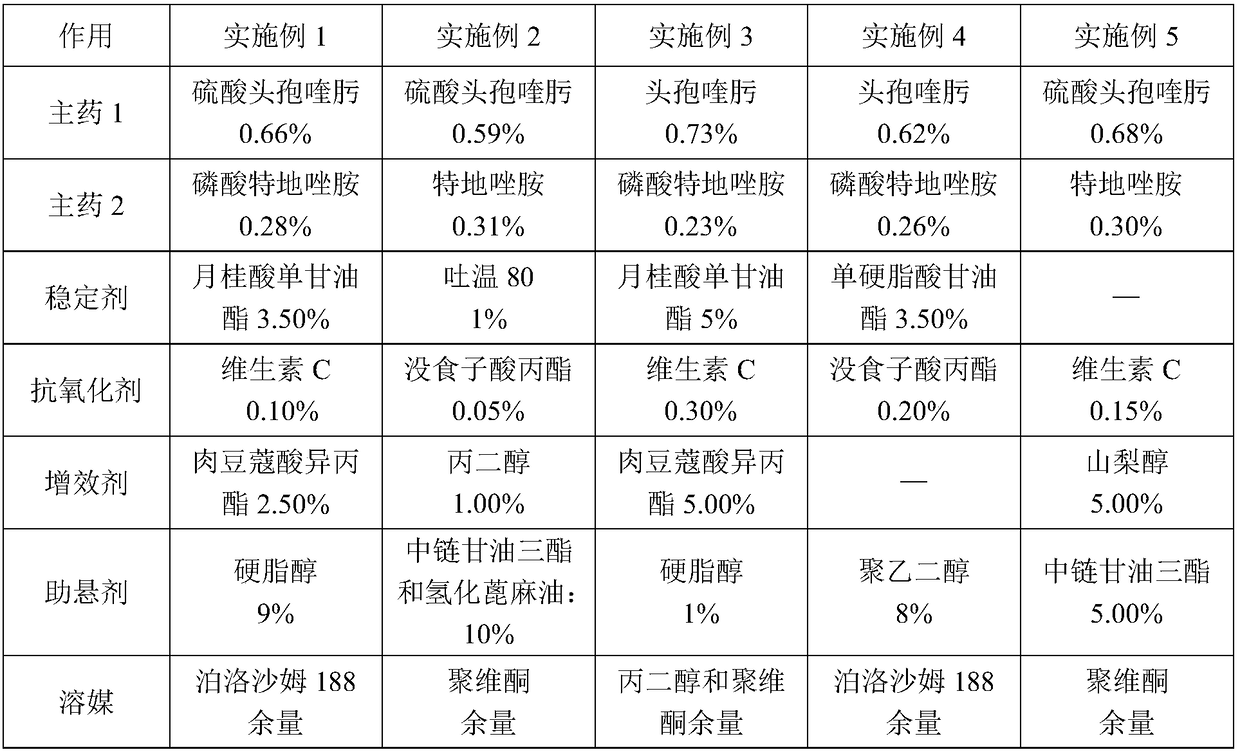
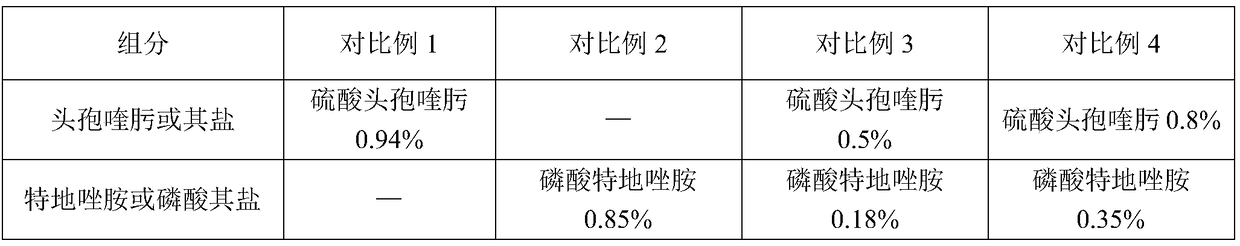
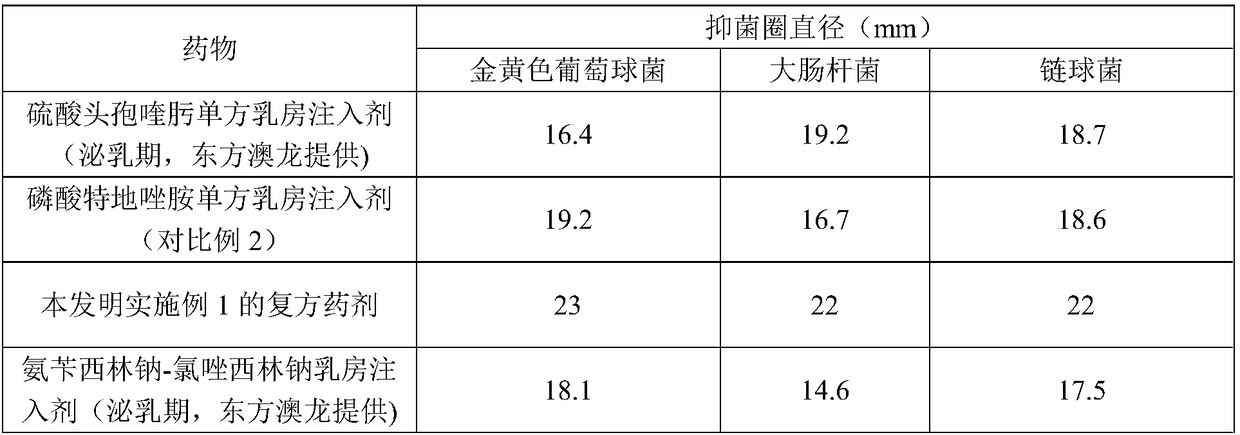
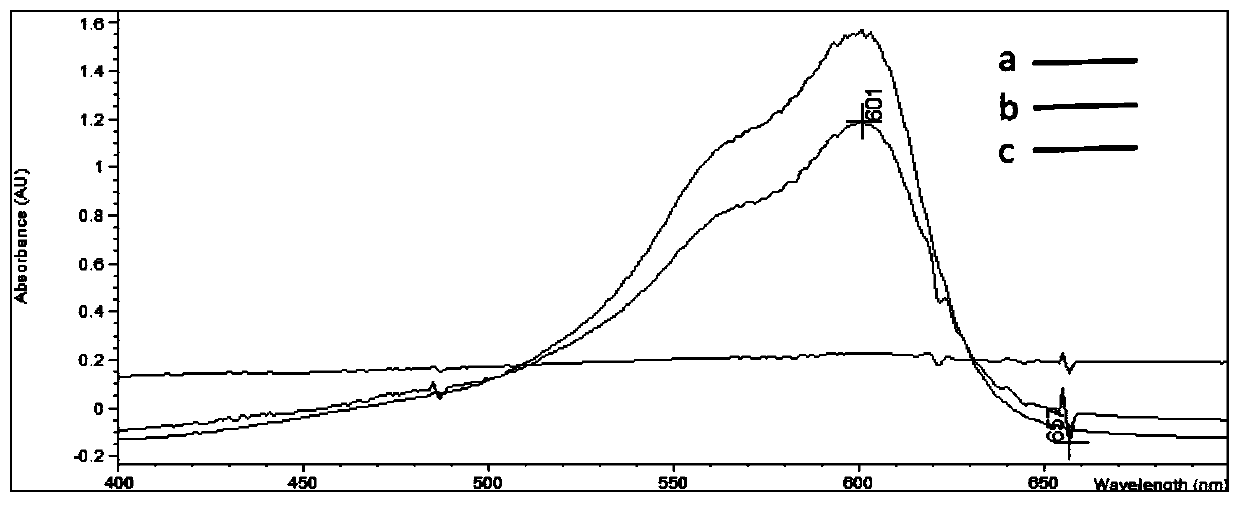
A compound medicament and its preparation method and use
ActiveCN109045044BImprove liquidityGood drug releaseAntibacterial agentsOrganic active ingredientsAntioxidantMilk cow's
The invention discloses a compound medicament. The compound medicament comprises a main drug, which is cefquinome or salt thereof and tedizolid or phosphate thereof. The compound medicament is a breast injectant in dosage form and is used for treating mastitis of cows in the lactation period. The invention further discloses a preparation method of the compound medicament, the method comprises thefollowing steps: mixing the main drug, a stabilizing agent and a solvent uniformly, and enabling the main drug and an ingredient to be dissolved to obtain a settled solution; enabling an antioxidant,a synergist and a suspending agent to be mixed with the settled solution, and stirring uniformly; afterwards, grinding for 20-50 min by using a colloid mill to obtain a finished product. The compoundmedicament realizes a synergistic anti-microbial effect through two kinds of mechanisms in which the cefquinome or the salt thereof and the tedizolid or the phosphate thereof are jointly combined, thesynthesis of a cell wall is jointly inhibited and the synthesis of protein is inhibited. The compound medicament further has the advantages of good fluidity, good drug release performance and convenient drug administration.
Owner:FOSHAN NANHAI EASTERN ALONG PHARMA CO LTD
A kind of cefquinome microsphere gel composite preparation and preparation method thereof
ActiveCN106474087BGood sustained release effectReduce clearanceAntibacterial agentsOrganic active ingredientsGel preparationBlood clearance
The invention discloses a composite cefquinome microsphere gel preparation and a preparation method thereof, and belongs to the technical field of medicines. The composite cefquinome microsphere gel preparation for veterinary use is prepared by adopting a spray drying method to prepare microspheres, adopting cefquinome as the medicine for the microspheres, adopting polylactic acid / polylactic acid-glycolic acid as a coating carrier material, and adopting hyaluronic acid and water as gel bases to prepare the composite cefquinome microsphere gel preparation. The microspheres are of 5-30umd in grain size, which is beneficial to lung targeting of the main medicine cefquinome, the cefquinome can easily gather at a target organ, blood clearance is reduced, long-time circulation is benefited, and pharmacological efficacy is improved. In addition, by the aid of the gel bases, contact of the medicine and interstitial fluid is reduced, the time of the medicine flowing into a bloodstream is prolonged, and a slow-release effect of the preparation is further increased.
Owner:RINGPU TIANJIN BIOLOGICAL PHARMA
A specific anti-ceftiofur monoclonal antibody hybridoma cell line 2e5 and its application
ActiveCN105505886BHigh detection sensitivityHigh affinityTissue cultureImmunoglobulinsAntibiotic YSpecific antibody
The invention discloses a specific anti-ceftiofur monoclonal antibody hybridoma cell strain 2E5 and the application thereof and belongs to the technical field of food safety immunodetection. The monoclonal cell strain 2E5 is preserved in the China General Microbiological Culture Collection Center, CGMCC for short, with the preservation number of CGMCC No.10873. The crude drug ceftiofur and BSA are coupled to prepare immunogen by means of the active ester method, and ceftiofur and OVA are coupled to prepare coating antigen. The specific anti-ceftiofur hybridoma cell strain is obtained after a mouse is immunized. The monoclonal antibody secreted by the cell strain only aims at ceftiofur specifically, the crossing-over rates between the monoclonal antibody and cefalexin and between the monoclonal antibody and cefquinome are 3.12% and 5.3% respectively, the crossing-over rates between the monoclonal antibody and other cephalosporin antibiotics are all smaller than 0.1%, and therefore specificity is high. The anti-ceftiofur monoclonal antibody hybridoma cell strain has high detection sensitivity and compatibility, the crossing-over rates with other cephalosporin antibiotics are low, and therefore specific detection of ceftiofur can be achieved. The invention also provides a new idea for preparing a specific antibody aiming at a specific cephalosporin so as to obtain a good specific monoclonal cell strain.
Owner:无锡迪腾敏生物科技有限公司
Cefquinome injection as well as preparation method and application thereof
InactiveCN113116817ANot easy to settleGood redispersibilityAntibacterial agentsOrganic active ingredientsGlycerol dioleateGlycerol
The invention provides a cefquinome suspension injection. The cefquinome suspension injection contains cefquinome, an anti-caking agent and a suspending aid, the anti-caking agent is nanoscale silicon dioxide, the suspending aid is dipolyglycerol dioleate, the content of the nanoscale silicon dioxide is 0.5 w / v%-3w / v%, and the content of the dipolyglycerol dioleate is 0.5 w / v%-3w / v%. The cefquinome suspension injection is not easy to settle, good in stability, good in redispersibility, easier to release, not easy to generate stress, and beneficial to clinical use.
Owner:LUOYANG HUIDE BIO ENG CO LTD
Sulfuric acid cefquinome frozen powder injection and preparation method thereof
ActiveCN102349872AImprove stabilityReduce stress responseAntibacterial agentsOrganic active ingredientsAnimal welfareFreeze-drying
The invention discloses a sulfuric acid cefquinome frozen powder injection, belonging to the field of veterinary drug preparation. The frozen powder injection comprises the ingredients of, by weight, 2.22-6.67 parts of sulfuric acid cefquinome, 5-20 parts of enveloping auxiliary material, 2-10 parts of freeze-dried excipient and the balance of injection water; wherein the weight ratio of the sulfuric acid cefquinome and the injection water is 1:5-20. The invention further discloses a preparation method of the sulfuric acid cefquinome frozen powder injection. Firstly, the sulfuric acid cefquinome frozen powder injection disclosed by the invention has good stability and high activity, and the appearance and the activity barely change after the sulfuric acid cefquinome frozen powder injection is laid aside for six months. Secondly, the sulfuric acid cefquinome frozen powder injection has high curative effect, accordingly, the injection times and the stress reactions of an animal can be reduced and the animal welfare can be improved. Moreover, the invention is more applicable for industrialization production.
Owner:JIANGSU NANJING AGRI UNIV ANIMAL PHARM CO LTD
Compound medicament as well as preparation method and application of compound medicament
ActiveCN109045044AHigh activitySmall doseAntibacterial agentsPharmaceutical delivery mechanismPhosphateAntioxidant
The invention discloses a compound medicament. The compound medicament comprises a main drug, which is cefquinome or salt thereof and tedizolid or phosphate thereof. The compound medicament is a breast injectant in dosage form and is used for treating mastitis of cows in the lactation period. The invention further discloses a preparation method of the compound medicament, the method comprises thefollowing steps: mixing the main drug, a stabilizing agent and a solvent uniformly, and enabling the main drug and an ingredient to be dissolved to obtain a settled solution; enabling an antioxidant,a synergist and a suspending agent to be mixed with the settled solution, and stirring uniformly; afterwards, grinding for 20-50 min by using a colloid mill to obtain a finished product. The compoundmedicament realizes a synergistic anti-microbial effect through two kinds of mechanisms in which the cefquinome or the salt thereof and the tedizolid or the phosphate thereof are jointly combined, thesynthesis of a cell wall is jointly inhibited and the synthesis of protein is inhibited. The compound medicament further has the advantages of good fluidity, good drug release performance and convenient drug administration.
Owner:FOSHAN NANHAI EASTERN ALONG PHARMA CO LTD
An electrochemical receptor sensor for detecting β-lactam antibiotics, preparation method and application thereof
InactiveCN110243907BHigh sensitivityHigh precisionMaterial electrochemical variablesReceptorEngineering
The invention belongs to the technical field of analytical chemistry and veterinary drug residue analysis, and particularly relates to an electrochemical receptor sensor for detecting beta-lactam antibiotics, and a preparation method and application thereof. The electrochemical receptor sensor comprises an electrochemical probe, a signal probe and a novel composite nano material, the electrochemical probe is thionine, the signal probe is HRP labeled ampicillin (HRP-AMP), and the composite nano material is graphene / thionine / glassy carbon electrode (GO / TH / GCE). The method disclosed by the invention can be used for detecting cephalexin, cefquinome, ceftiofur, penicillin G and ampicillin, thereby providing a rapid and sensitive method for the detection of such substances in foods. The detection method established by the invention has high accuracy and good sensitivity, and has the advantages of saving a lot of time and cost compared with the existing method, and thus has a better market application value.
Owner:HUAZHONG AGRI UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com