Cefquinome crystal and preparation method thereof
A technology for cefquinoxime and crystals, applied in the field of cefquinoxime crystals and preparation thereof, can solve the problems of cefquinoxime amorphous state, thermodynamic instability, large drug consumption, etc. The effect of cheap and easy-to-obtain raw materials and simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Step 1: At 3°C, add 0.088 g of activated carbon to the cefquinome solution containing 70 mL of water and 0.25 g / (mL of water), stirring for 30 minutes, and filtering. Step 2: Add the filtrate obtained in Step 1 to 490 mL of n-butanol within 60 minutes under stirring at 3°C and 300 rpm. After adding, continue to stir for 2h. Then, filter out the milky white soft "mud" cake. A small amount of ethanol is added multiple times to wash the filter cake, then filtered, the filter cake is ground into a powder, and dried at 45° C. under vacuum for 10 hours to obtain the cefquinoxime crystals.
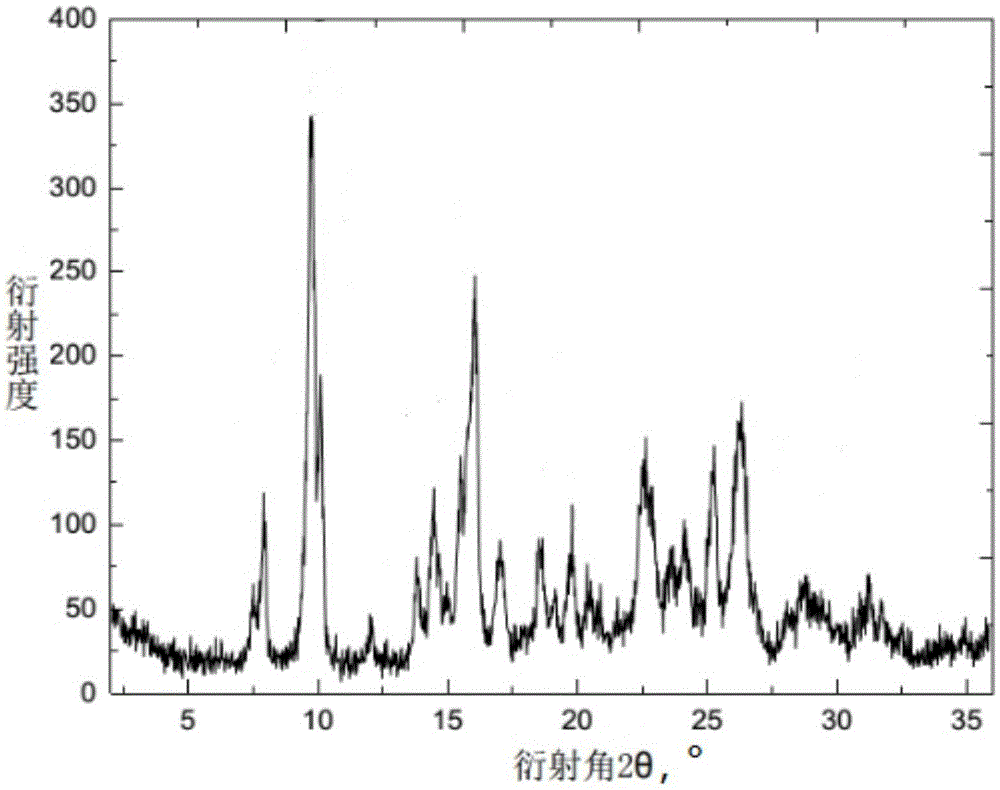
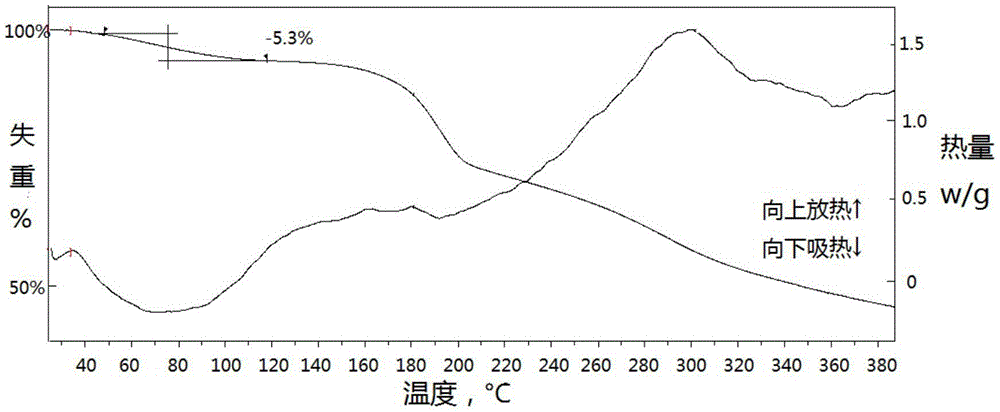
[0030] The X-ray powder diffraction pattern of the cefquinome crystal prepared in this example is at diffraction angle 2θ=7.49, 7.88, 9.62, 10.06, 11.94, 13.72, 14.42, 15.60, 16.00, 16.92, 18.43, 19.74, 20.52, 20.97, There are characteristic peaks at 22.64, 22.96, 23.66, 24.14, 25.24, 26.34, 28.72, 31.23 degrees. The TG-DSC curve has an endothermic weight loss of 5.3% at 40~110℃, and an ...
Embodiment 2
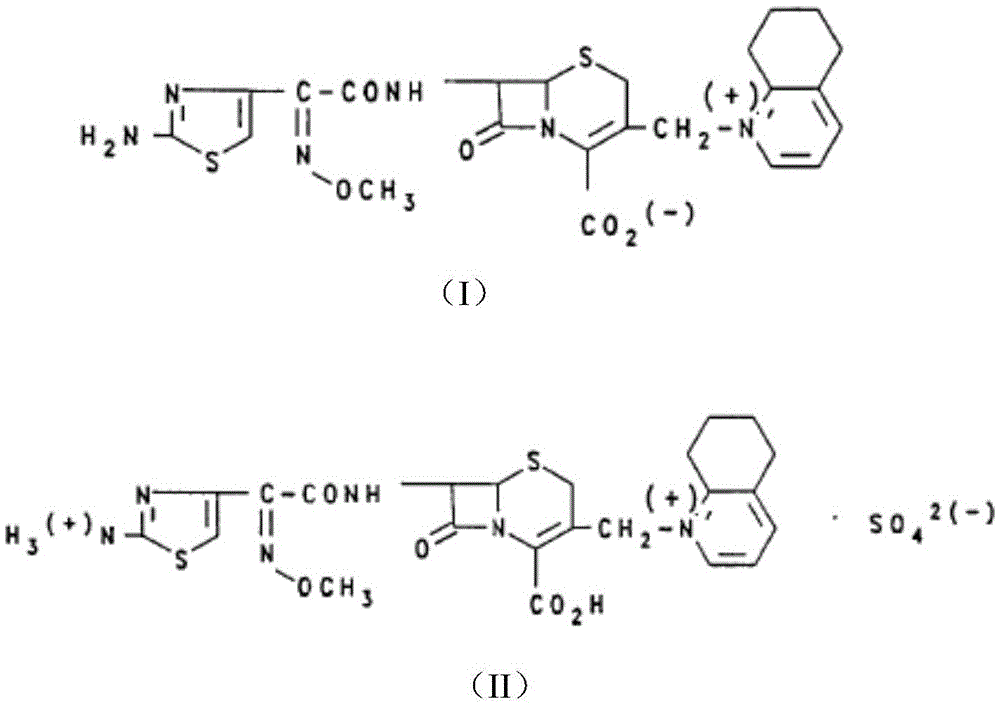
[0032] Step one: Dissolve 1.33 g of sodium carbonate in 30 mL of water. Weigh 5.24 g of cefquinome sulfate and add it to the sodium carbonate solution in small quantities under stirring at 30° C. and 300 rpm to perform acid-base neutralization reaction to generate cefquinome and sodium sulfate. After the reaction was completed, the system was clear and transparent, and the solution was golden yellow. Then, 45 mL of acetone was added to the solution, the flow acceleration rate was 3 mL / min, and sodium sulfate was precipitated. Filter and remove sodium sulfate to obtain a cefquinoxime solution with a concentration of 0.15 g / (mL water). At 30°C, add 0.055 g of activated carbon to the cefquinoxime solution, stir for 30 min, and filter.
[0033] Step 2: Add all the filtrate obtained in Step 1 to 300 mL of acetone within 10 minutes under stirring at 30°C and 300 rpm, and then stir for 12 hours. Then it was filtered and washed, and the filter cake was dried at 25° C. under vacuum for...
Embodiment 3
[0036] Step one: Dissolve 0.33 g of potassium carbonate in 20 mL of water. Weigh 1.18 g of the raw material Cefquinome Sulfate and slowly add it to the potassium carbonate solution under stirring at 17° C. and 300 rpm to carry out the acid-base neutralization reaction to generate Cefquinome and potassium sulfate. The reaction is complete after the system is clear and transparent, and the solution is golden yellow. Then, 60 mL of ethyl acetate was added to the solution at a flow rate of 3 mL / min to precipitate potassium sulfate. Filter to remove potassium sulfate to obtain a cefquinoxime solution with a concentration of 0.05 g / (mL water). At 17°C, add 0.02 g of activated carbon to the cefquinoxime solution, stir for 30 min, and filter.
[0037] Step two, prepare the crystal product of cefquinome: under the action of stirring at 17° C. and 300 rpm, all the filtrate obtained in step one is added to 170 mL of ethyl acetate within 35 minutes. Then, continue stirring for 7 hours. T...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com