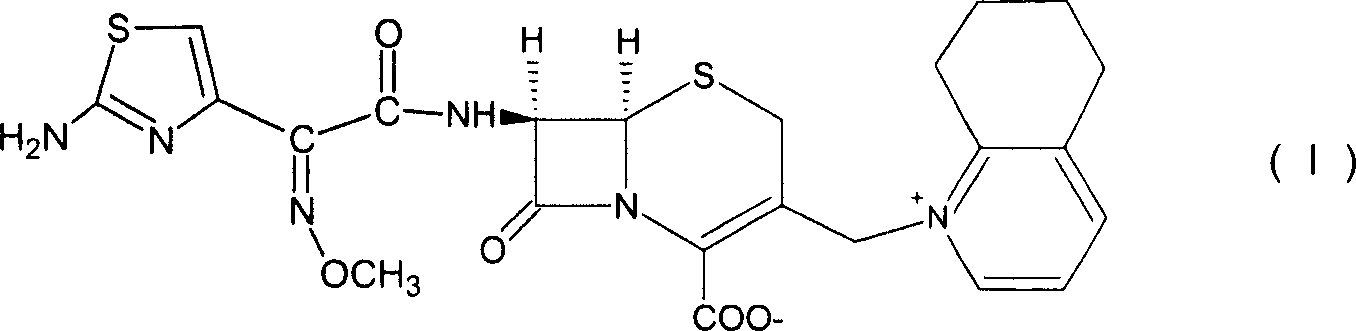
Cefquinome medicinal composition for injection capable of being stored stably and dissolved easily in water
A technology of cefquinome and composition, which is applied in the field of cefquinome pharmaceutical composition for injection, which can be stored stably and is easily soluble in water, can solve the problems of slow absorption speed, inconvenient use, high preparation process requirements, etc. The effect of rapid dissolution and stable quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Take 10g of cefquinome sulfate, add 100ml of freshly prepared cold water for injection, add 4g of L-arginine and 0.6g of mannitol under stirring, adjust the pH to 6.5 with 0.1N aqueous sodium hydroxide solution or 0.1N hydrochloric acid aqueous solution, add 0.1 g activated carbon and stirred for 20 minutes to decarbonize. Under aseptic conditions, after coarse filtration, use a 0.22-micron microporous membrane filter to filter and sterilize. The filtrate is divided into vials and freeze-dried to obtain a freeze-dried powder for injection, which is made into cefquinome-containing 0.1g / bottle specification.
[0033] The test sample of Example 1 was stored at 4°C ± 2°C for 6 months, and the solubility was detected by the same method before and after storage. The results showed that 0.2g of the sample before and after storage could be dissolved in 3ml of normal temperature injection within 10 seconds In water, without leaving any water-insoluble solid matter, to meet the ...
Embodiment 2
[0040] Under sterile environment, mix 100g cefquinome sulfate sterile powder and 24gNa 2 CO 3 The aseptic powder is mixed evenly, ground through a No. 6 sieve, and then aseptically divided into bottles to obtain cefquinome powder for injection, which is made into a specification containing 1 g of cefquinome per bottle.
[0041] The test sample of Example 2 was stored at 4°C ± 2°C for 6 months, and the solubility was detected by the same method before and after storage. The results showed that 0.2g of the sample before and after storage could be dissolved in 3ml of normal temperature injection within 20 seconds In water, without leaving any water-insoluble solid matter, to meet the needs of injection medicine.
[0042] The test samples of Example 2 were stored at 4°C±2°C for 6 months, and the relevant physical and chemical properties were measured at 0 month, 3 months, and 6 months respectively, as shown in Table 2.
[0043] Table 2. Stability data of cefquinome sulfate powde...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com