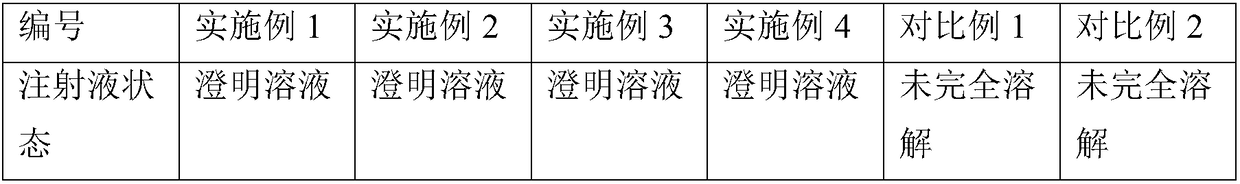
Cefquinome powder injection
A technology of cefquinome and powder injection, which is applied in the field of powder injection and can solve the problems of decreased water solubility and unstable crystal form, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Prescription: crystalline cefquinome: 10g; polyethylene glycol 4000: 5g; N-acyl sarcosine: 0.5g.
[0021] Production process: (1) Melting: Heat polyethylene glycol 4000 to 55 degrees to melt, add crystalline cefquinome and amino acid surfactant, mix and stir for 15 minutes. Get liquid A.
[0022] (2) Cooling: Rapidly cool liquid A to below 15 degrees to obtain solid mixture B.
[0023] (3) Pulverization: Ultrafine pulverization of the solid mixture B.
[0024] (4) Mixing: The pulverized material was mixed for 30 minutes.
[0025] (5) Subpackage: Subpackage in a sterile room, the specification of subpackage is 1g / cartridge (calculated as cefquinome).
Embodiment 2
[0027] Prescription: Crystalline cefquinome: 10g; polyethylene glycol 4000: 10g; N-acyl sarcosine: 0.8g
[0028] Production process: (1) Melting: Heat polyethylene glycol 4000 to 55 degrees to melt, add crystalline cefquinome and amino acid surfactant, mix and stir for 15 minutes. Get liquid A.
[0029] (2) Cooling: Rapidly cool liquid A to below 15 degrees to obtain solid mixture B.
[0030] (3) Pulverization: The solid mixture B is mechanically pulverized.
[0031] (4) Mixing: The pulverized material was mixed for 15 minutes.
[0032] (5) Subpackage: Subpackage in a sterile room, the specification of subpackage is 1g / cartridge (calculated as cefquinome).
Embodiment 3
[0034] Composition: crystalline cefquinome: 10g; polyethylene glycol 4000: 10g; N-acyl sarcosine: 1.2g
[0035] Production process: (1) Melting: Heat polyethylene glycol 4000 to 55 degrees to melt, add crystalline cefquinome and amino acid surfactant, mix and stir for 15 minutes. Get liquid A.
[0036] (2) Cooling: Rapidly cool liquid A to below 15 degrees to obtain solid mixture B.
[0037] (3) Pulverization: The solid mixture B is mechanically pulverized.
[0038] (4) Mixing: The pulverized material was mixed for 15 minutes.
[0039] (5) Subpackage: Subpackage in a sterile room, the specification of subpackage is 1g / cartridge (calculated as cefquinome).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com