Aryl propionic acid derivative composition and pharmaceutical purpose
A technology of aryl propionic acids and compounds, applied in the field of medicinal chemistry, can solve the problems of lower ulcer rate and adverse reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
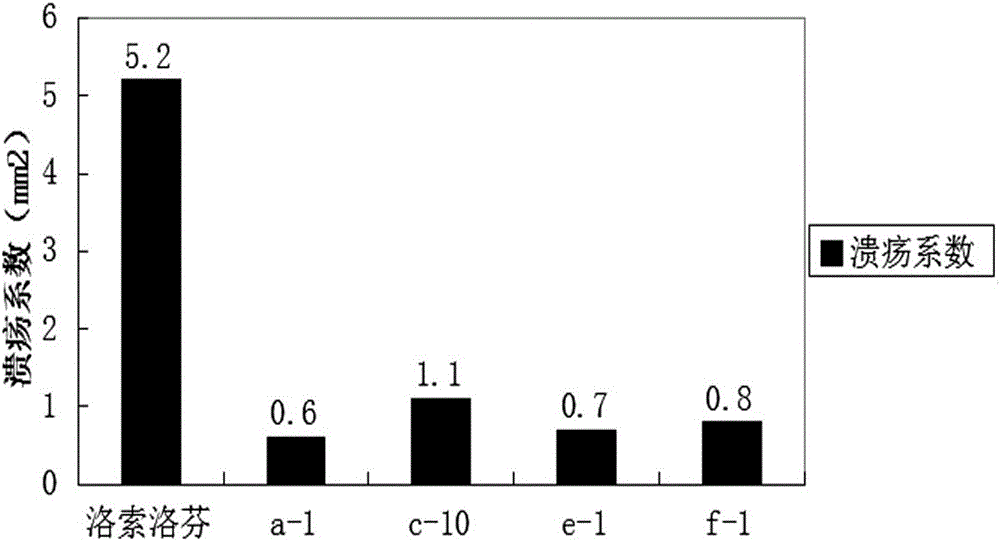
Examples
Embodiment 1
[0080] The synthesis of embodiment 1 compound a-1
[0081]
[0082] Preparation method using chlorophosphoric acid: Dissolve 25g of the compound of formula II in 70mL of anhydrous acetonitrile, add 13g of diisopropylethylamine, cool down to -5~0°C, slowly add 20mL of anhydrous dichloromethane solution of 12g of chlorophosphoric acid dropwise , after dropping, keep stirring for 1 h, slowly rise to room temperature and stir for 2 h, TLC monitors that the reaction is complete, remove the reaction solvent under reduced pressure, add 150 mL of water and 150 mL of dichloromethane and stir, leave to separate layers, concentrate the dichloromethane layer, and wash the residue with methanol / ethyl acetate recrystallization to obtain 14 g of white solid.
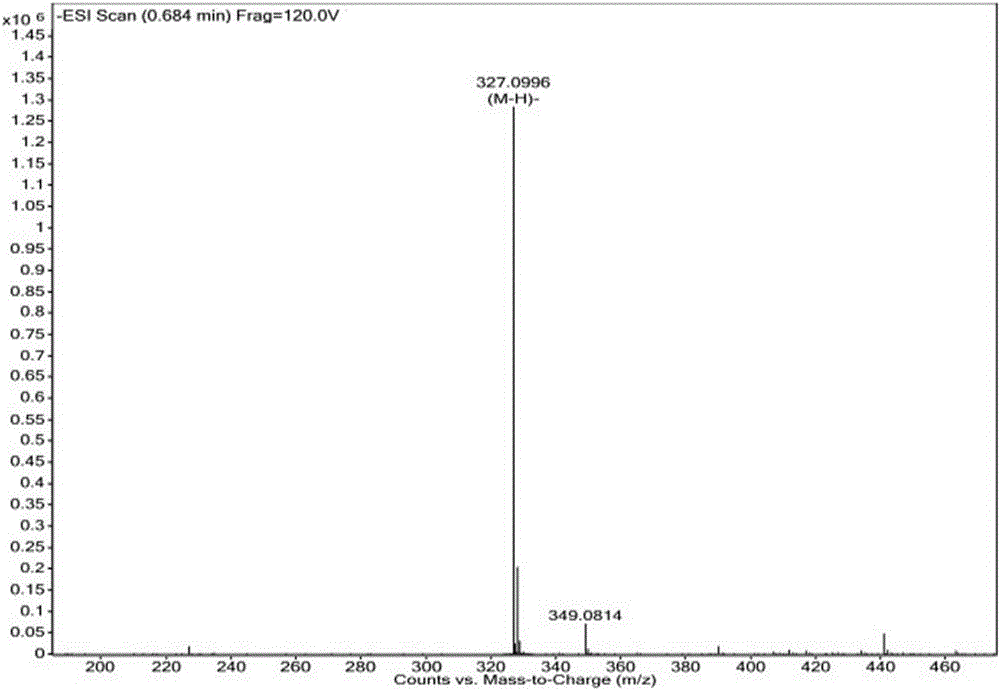
[0083] Among them, the structure of chlorophosphoric acid is Its preparation refers to the synthesis method of "Methyl(S)-4-(chloromethoxyphosphinyl)-3(tert-butyldiphenylsiloxy)butyrate" on page 6 of the specification of patent d...
Embodiment 2
[0086] The synthesis of embodiment 2 compound a-1
[0087] The preparation method using phosphorus oxychloride: dissolve 7.2g of the compound of formula II in 100mL of anhydrous tetrahydrofuran, add 14mL of pyridine, cool down and keep the internal temperature at -10 to -15°C, add dropwise 10mL of phosphorus oxychloride to dissolve in The solution in 30mL of tetrahydrofuran was dropped, stirred at 0-5°C for 1h, removed to room temperature, added 2mL of phosphorus oxychloride, stirred at room temperature until the reaction was complete, and the developer monitored by TLC was ethyl acetate: glacial acetic acid = 10: 1. After the reaction is completed, filter with suction, drop the filtrate into about 600mL of pure water kept at 0-5°C, after the drop is complete, stir at room temperature 25-30°C for 1h, add 300mL of ethyl acetate and stir for 0.5h, let stand to separate layers, The organic phase was washed with pure water and saturated brine, dried over anhydrous sodium sulfate, ...
Embodiment 3
[0090] The synthesis of embodiment 3 compound a-3
[0091]
[0092] The preparation was carried out with reference to the method of Example 1, except that an anhydrous dichloromethane solution of diethyl chlorophosphate was added dropwise.
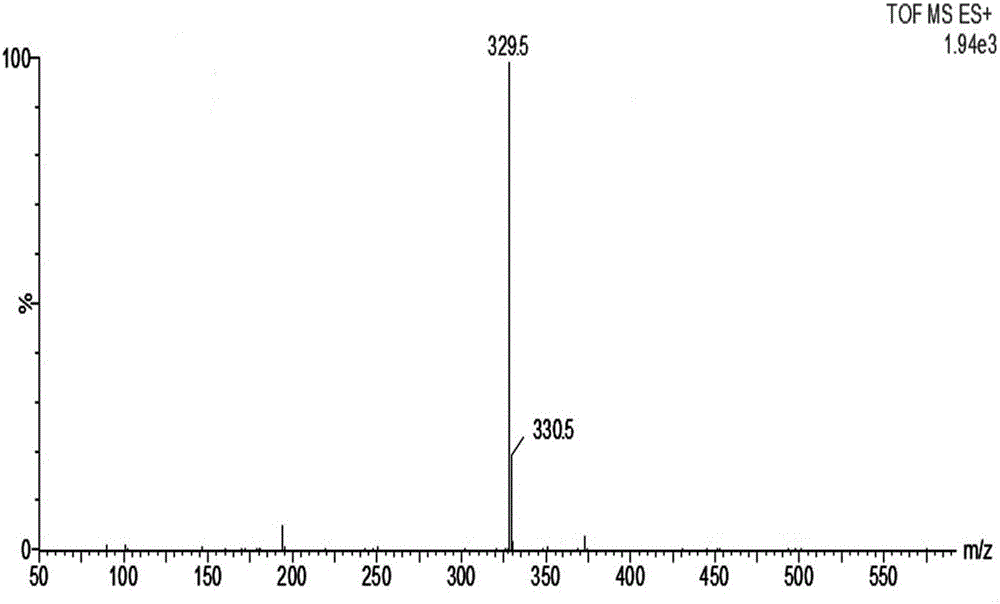
[0093] Mass Spectrum ESI-MS m / z=385.6, [M+H] + .
[0094] H NMR 1 H-NMR: δppm 7.25(d, 2H), 7.16(d, 2H), 4.04(m, 4H), 3.85(m, 1H), 3.64(m, 1H), 2.74(m, 1H), 2.46(m , 1H), 2.03 (m, 2H), 1.93-1.66 (m, 5H), 1.55 (m, 6H), 1.44 (d, 3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com