Patents
Literature
36 results about "Diethyl chlorophosphate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Diethyl chlorophosphate is an organophosphorus compound with the formula (C₂H₅O)₂P(O)Cl. As an reagent in organic synthesis, it is use the convert alcohols to the corresponding diethylphosphate esters. It is a colorless liquid with a fruity odor. It is a corrosive, and as a cholinesterase inhibitor, highly toxic through dermal absorption. The molecule is tetrahedral.
Cardanol-based phosphatefire-retardant plasticizer and preparation method thereof
InactiveCN104610571AImprove flame retardant performanceGood thermal stabilityGroup 5/15 element organic compoundsDiethyl phosphatePhosphoric acid
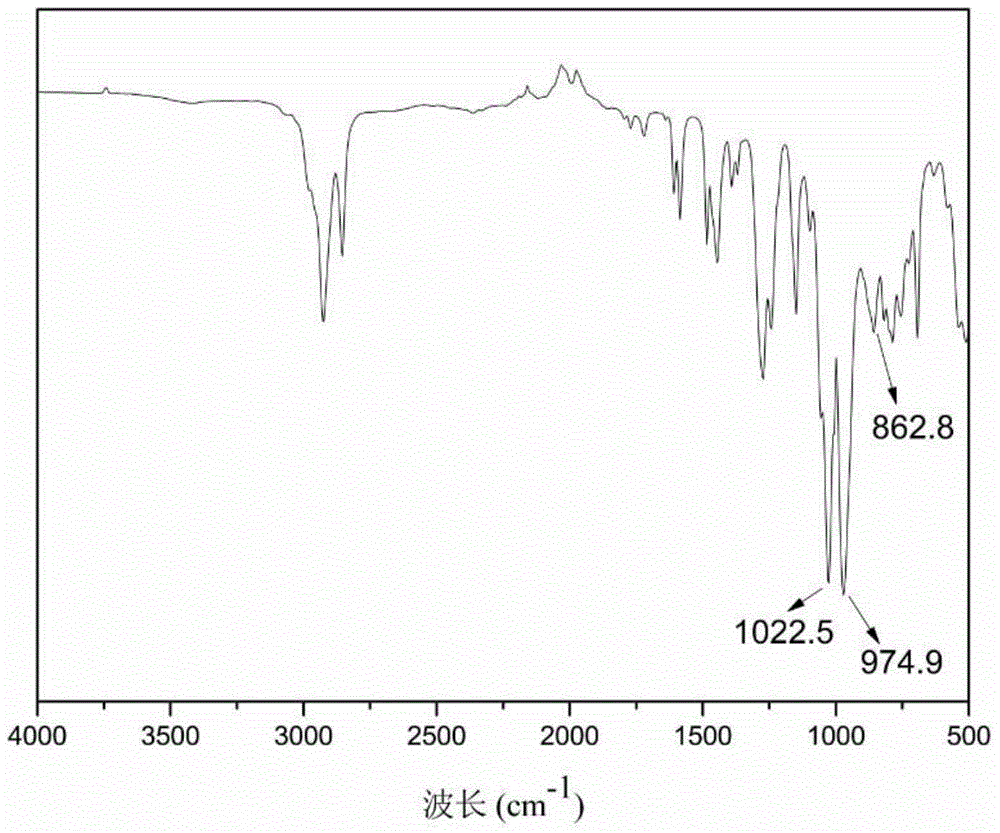
The invention discloses a cardanol-based phosphatefire-retardant plasticizer and a preparation method thereof. The preparation method comprises the following steps: with cardanol and diethyl chlorophosphate as main raw materials, carrying out substitution reaction in the presence of an acid binding agent to synthesize cardanol-based diethyl phosphate; further with cardanol-based phosphoric acid as a raw material, in the presence of a peracetic phosphotungstate phase transfer catalyst, adding a peracetic source dropwise for epoxidation to prepare crude epoxy cardanol-based diethyl phosphate, and filtering, washing and dehydration to obtain a finished epoxy cardanol-based glycidyl ether plasticizer. According to the cardanol-based phosphatefire-retardant plasticizer and the preparation method disclosed by the invention, as the cardanol is taken as the raw material, the cost is low, and the source is wide; the preparation process is simple, the energy consumption is low; the product is low in toxicity, is environmentally friendly, is capable of endowing a polymer substrate with excellent flame retardancy and flexibility and has good application prospect.
Owner:INST OF CHEM IND OF FOREST PROD CHINESE ACAD OF FORESTRY
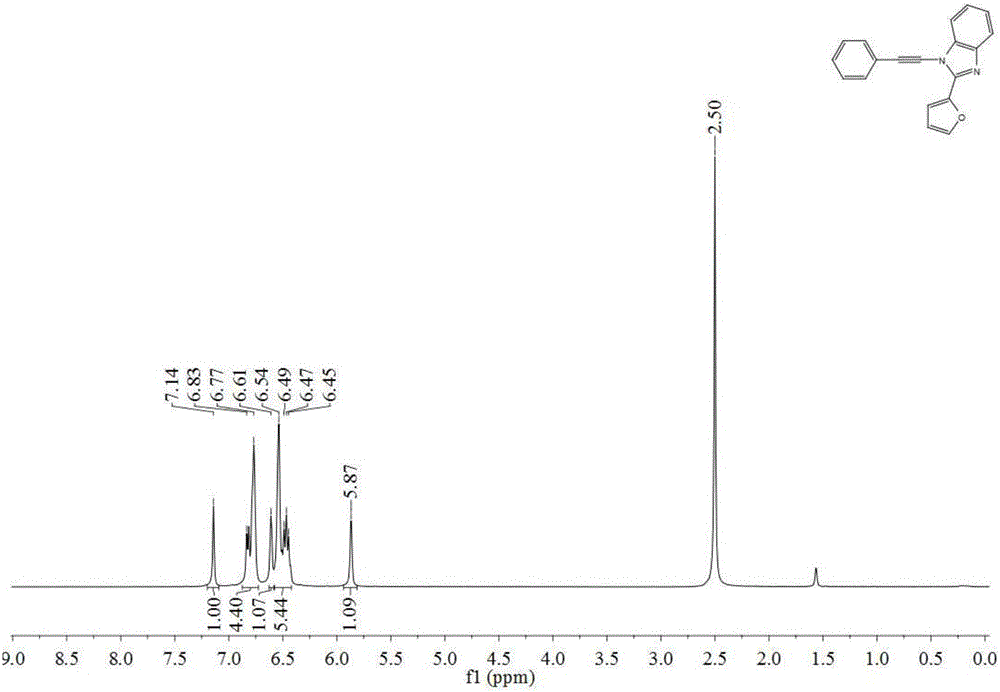
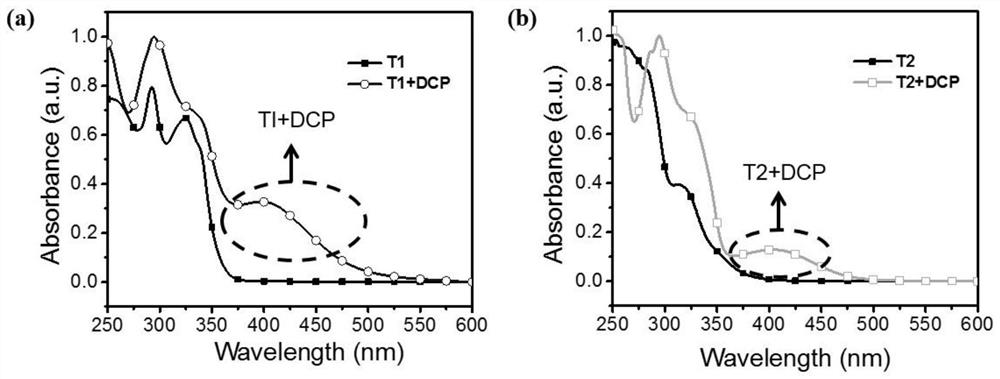
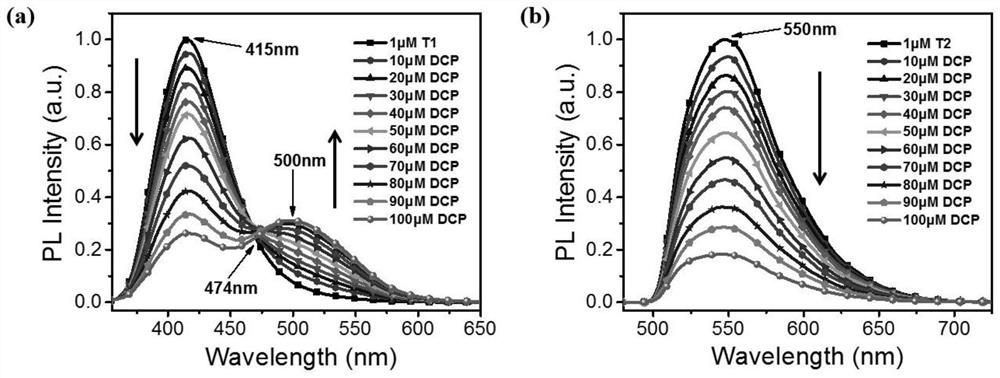
Fluorescent probe for detecting sarin and analogue thereof as well as synthesis method and application of fluorescent probe
ActiveCN106748976AThe synthesis method is simpleOptimize detection conditionsOrganic chemistryFluorescence/phosphorescenceGas phaseSynthesis methods
The invention relates to a fluorescent probe for detecting sarin and an analogue thereof. The fluorescent probe comprises a compound which is loaded on a substrate and has a structure shown as a formula I, wherein the formula I is shown in the description. The invention further provides a synthesis method and application of the fluorescent probe for detecting the sarin and the analogue thereof. According to the application provided by the invention, when a compound with the structure shown as the formula I in the fluorescent probe is in contact with the sarin or the DCP (Diethyl Chlorophosphate) in air, the fluorescence intensity and the emission wavelength are both changed, and gas-phase detection of the sarin and the DCP can be realized. The compound with the structure shown as the formula I can have one or more terpyridines; when the sarin or the DCP reacts with one certain pyridine in one certain terpyridine in a molecule, charge transferring from the fluorescent probe to the sarin or the DCP occurs; meanwhile, energy transferring from a polymer core to a peripheral radical occurs, and fluorescent quenching of the whole molecule is caused, so that the fluorescent probe has excellent sensing performance.
Owner:SHANGHAI INST OF MICROSYSTEM & INFORMATION TECH CHINESE ACAD OF SCI
Preparation and application of boron-dipyrromethene-based diethyl chlorophosphate fluorescent probe
InactiveCN106588968AInnovative designGood choiceGroup 3/13 element organic compoundsFluorescence/phosphorescenceDiethyl phosphatePhosphoric acid
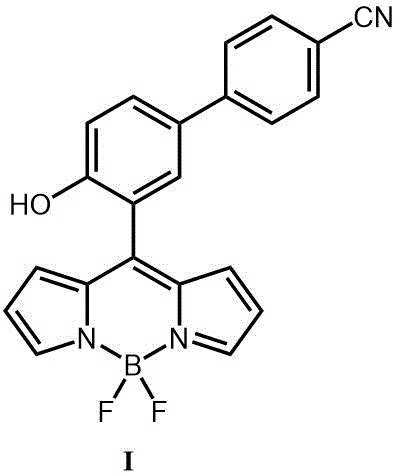
The invention relates to a detection diethyl chlorophosphate (DCP) fluorescent probe compound based on boron dipyrromethene (BODIPY) and preparation and application of the detection diethyl chlorophosphate (DCP) fluorescent probe compound. The detection diethyl chlorophosphate (DCP) fluorescent probe compound has the structure shown in the formula I. A preparation method includes the steps that an acetonitrile solution containing 4-(4-hydroxyphenyl)benzonitrile, anhydrous magnesium chloride, paraformaldehyde and triethylamine is subjected to heating and refluxing under protection of nitrogen to obtain white solid III; the obtained solid III and pyrrole are mixed and dissolved in dichloromethane; grey white solid II is obtained under catalysis of trifluoroacetic acid; and the obtained grey white solid II is subjected to DDQ oxydehydrogenation and boron trifluoride diethyl etherate coupling to obtain orange solid I. The probe compound has very good selectivity and sensitivity for DCP, the detection limit is low, and the probe compound can be applied to DCP content measurement.
Owner:UNIV OF JINAN
Intumescent flame retardant functionalized hydrotalcite flame retardant and preparation method thereof
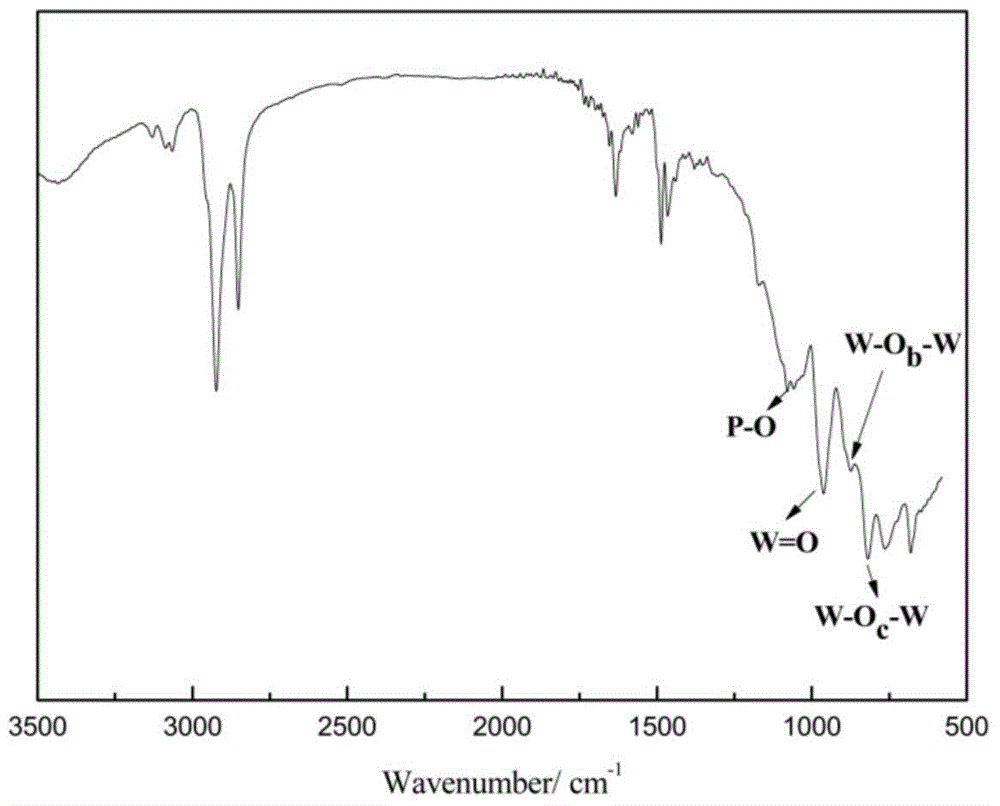
The invention provides an intumescent flame retardant functionalized hydrotalcite flame retardant. The flame retardant is good in flame retardant effect, and adopts a halogen-free system, so that secondary harm during combustion of materials is reduced. The intumescent flame retardant functionalized hydrotalcite flame retardant is prepared through the steps as follows: a silane coupling agent containing amine groups and hydrotalcite powder particles react with each other to prepare silane coupling agent modified hydrotalcite, and then, the silane coupling agent modified hydrotalcite and diethyl chlorophosphate react with each other. The flame retardant is wide in application range, good in flame resistant effect, and stable in performance; the preparation process of the flame retardant is simple and convenient in operation, the cost is low, the solvent recycling is convenient, and obtained products are easy to separate, so that the industrial production is facilitated.
Owner:TAIZHOU UNIV
Chitosan oligosaccharide derivative containing thiourea and diethoxyphosphamide structure and preparation method thereof
ActiveCN109021033AImprove biological activityLow reaction temperatureSugar derivativesFungicidesHydrazine compoundThiourea
Owner:INST OF OCEANOLOGY - CHINESE ACAD OF SCI
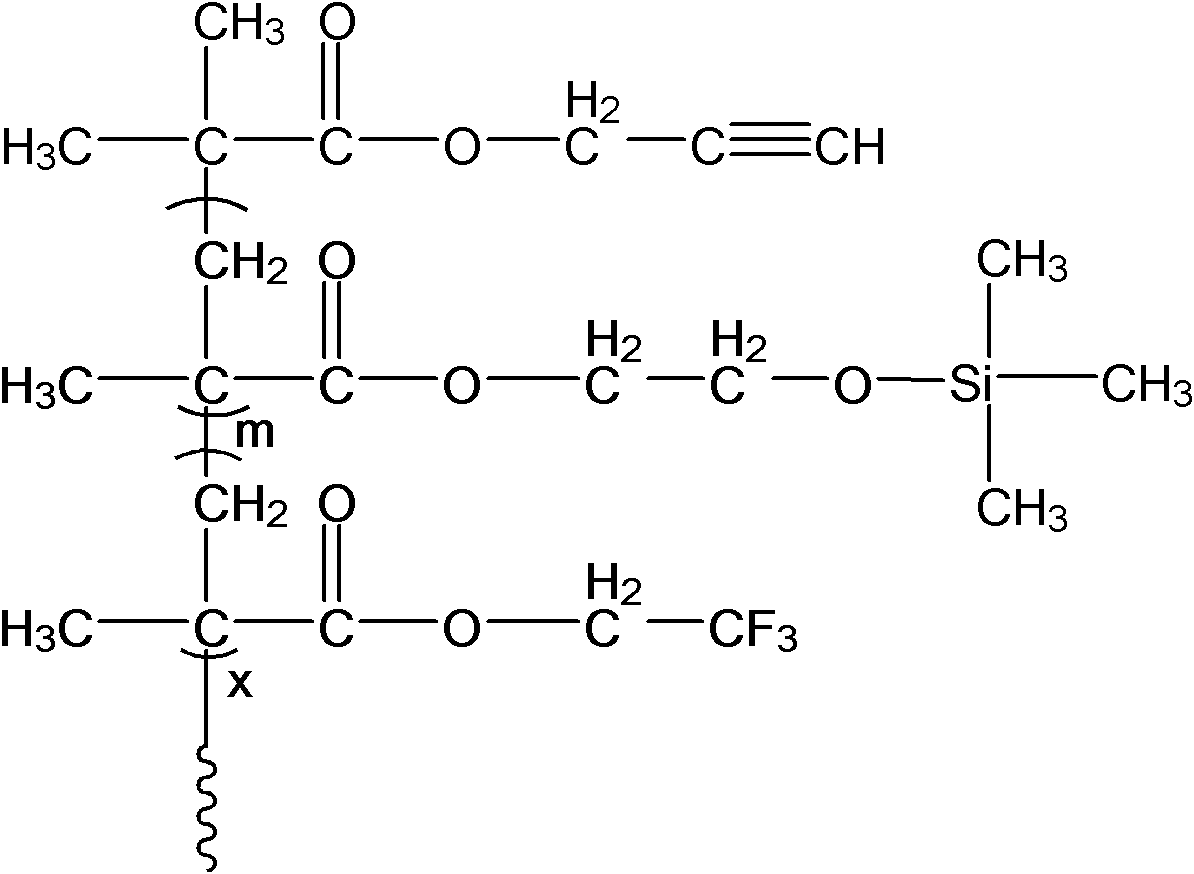
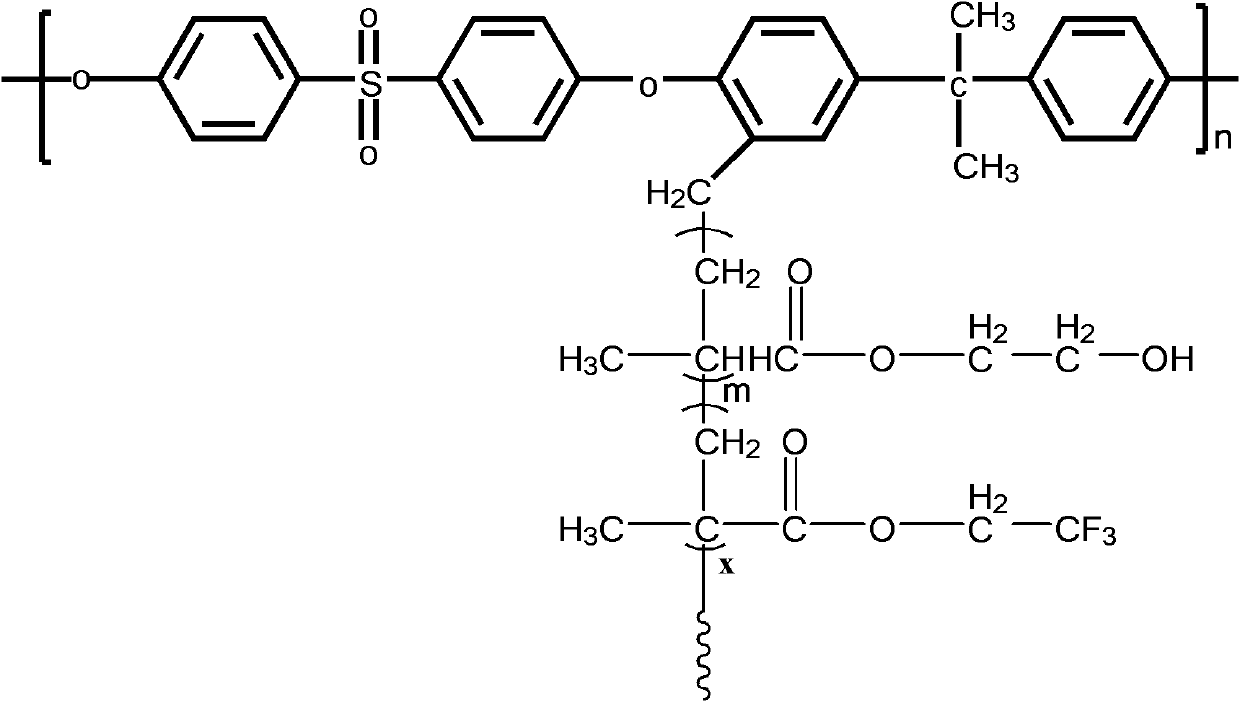
Structure controllable phosphorylated polymer composite film used for vanadium battery and preparation method thereof
InactiveCN102117925AImprove proton conductivityImprove efficiencyRegenerative fuel cellsCell component detailsPolymer dissolutionComposite film
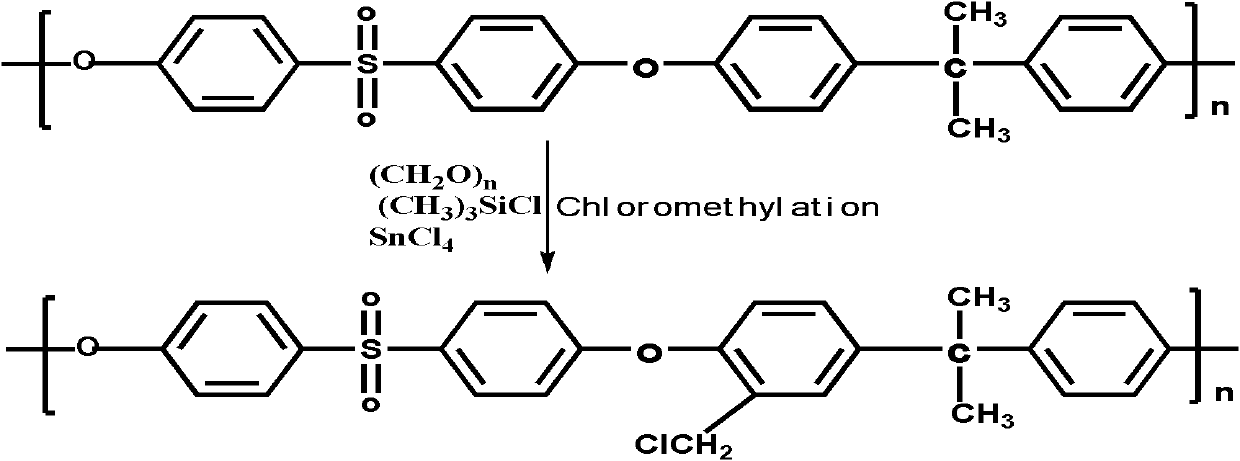
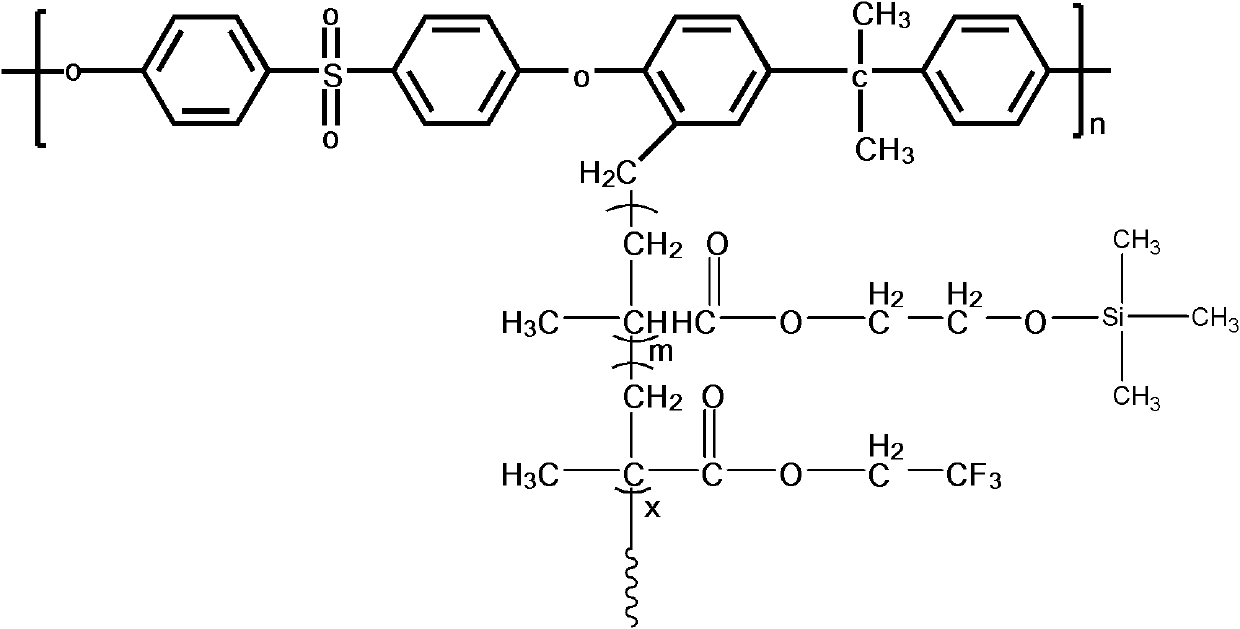
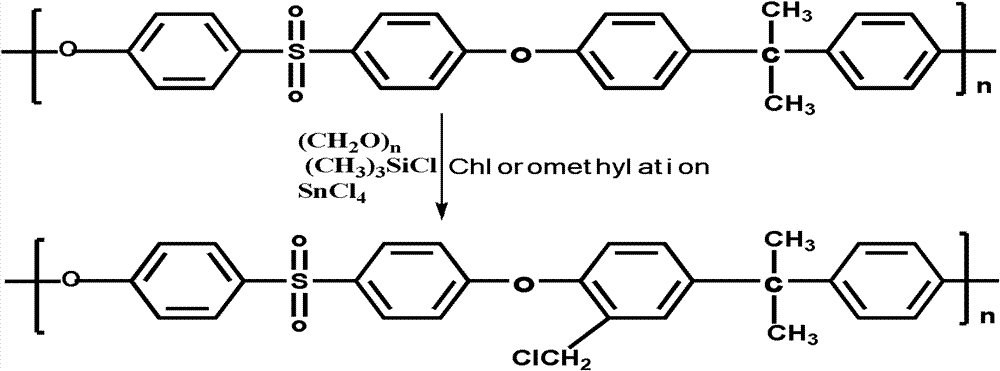
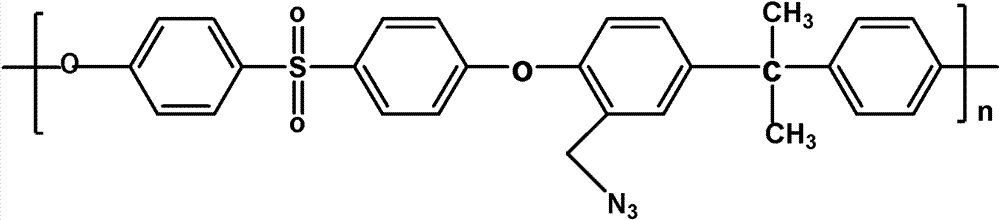
The invention discloses a structure controllable phosphorylated polymer composite film used for a vanadium battery and a preparation method thereof. The polymer composite film has the structure shown in the formula (I). The preparation method of the polymer composite film comprises the steps of: preparing a copolymer containing an alkynyl; preparing polysulfone containing an azide group; preparing a polymer; preparing a polymer containing an oxhydryl; dissolving the polymer containing the oxhydryl in dioxane, adding triethylamine and cuprous chloride, dropping diethyl chlorophosphate, filtering after reaction, adding filtrate in normal hexane to be precipitated, dissolving the precipitate in chloroform again, adding the solvent in the normal hexane to be precipitated again, and washing and drying the precipitate to obtain a phosphorus-esterificated polymer; dissolving the phosphorus-esterificated polymer in a high-boiling-point solvent to obtain a phosphorus-esterificated polymer solution; forming a film on a glass plate, and demolding in water to obtain a phosphorus-esterificated polymer film; and putting the film in concentrated hydrochloric acid for back flow and hydrolysis, taking the film out, washing and drying the film to obtain a product. The composite film has low permeability of vanadium ions, good proton conductivity, stable mechanical performance and chemical performance, and low cost.
Owner:GUANGZHOU CHEM CO LTD CHINESE ACADEMY OF SCI +1
Method for synthesizing flavone alkyl phosphate compound and application thereof in cholesterol esterase inhibitor medicine
InactiveCN101974034AHigh yieldHigh product purityOrganic active ingredientsMetabolism disorderPhosphoric Acid EstersPhosphorylation
The invention relates to a method for synthesizing a flavone alkyl phosphate compound and application thereof in a cholesterol esterase inhibitor, which overcomes the disadvantages of the traditional method that CCl4 is used as a solvent for synthesizing organic phosphate, while flavone usually has a low solubility in CCl4 and thus is difficult to carry out a phosphorylation reaction in CCl4. In the method, DMSO (Dimethylsulfoxide), DMF (Dimethyl Formamide), acetonitrile, diethyl ether, dioxane, THF (Tetrahydrofuran), and the like, which have stronger polarity are used as solvents, and flavone has high solubility in the solvents; nitrogenous bases, such as triethylamine, diethylamine, pyridine, and the like, are used as alkali reagents; ClPO (OEt)2 (Diethyl Chlorophosphate) is used as a phosphorus esterification reagent; and DMAP (Dimethylaminopyridine) is used as a catalyst. Thus, the method has the advantages of high compounding reaction speed, mild reaction conditions, high chemical selectivity, high reaction efficiency and higher yield of a flavone phosphate compound with a novel structure. The new compound has the advantages of simple separation and purification method, high currency and low production cost, and the conditions required in the whole process from synthesis to separation and purification are suitable for condition requirements on production in a large scale. The new synthesized compound can be applied to preparing the cholesterol esterase inhibitor with a high activity, and the active IC50 of the cholesterol esterase inhibitor is within 3.76-390nM and shows a very obvious structure-activity relationship.
Owner:HUNAN AGRICULTURAL UNIV
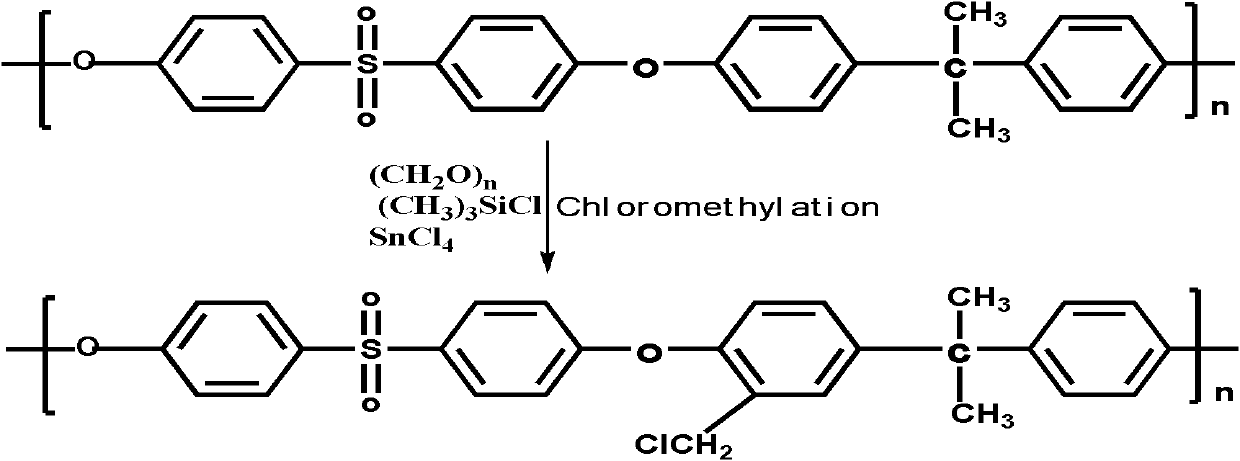
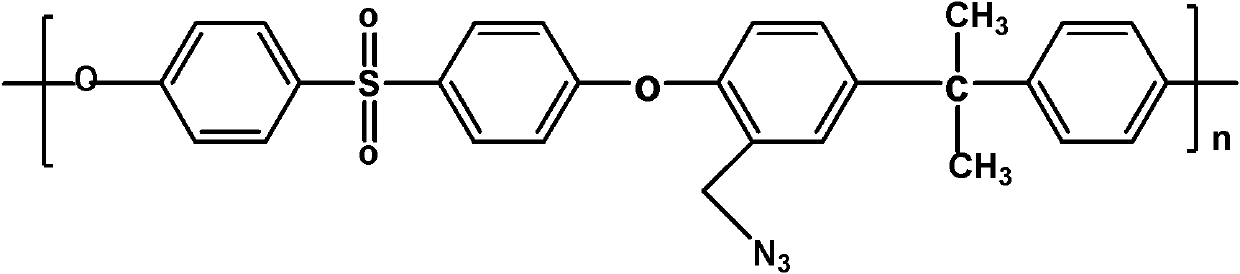
Phosphorylated polymer composite film for full vanadium flow battery and preparation method thereof
InactiveCN102167785AImprove proton conductivityImprove efficiencyCell component detailsFuel cell detailsPolymer dissolutionPolymer science
The invention discloses a phosphorylated polymer composite film for a full vanadium flow battery and a preparation method thereof. The polymer composite film has a structure shown as a formula (I). The preparation method comprises the following steps of: mixing a monomer mixture, chloromethylate polysulfone, cuprous chloride, genin and a solvent; performing deoxidization; perfomring polymerization; dissolving the obtained polymer into dioxane; adding triethylamine and the cuprous chloride; adding diethyl chlorophosphate dropwise; reacting and filtering; adding filtrate into norml hexane to precipitate; redissolving the precipitate into chloroform; adding the solution into the norml hexane again to precipitate; washing the precipitate; drying to obtain the phosphorylated polymer; dissolving the phosphorylated polymer into a high-boiling-point solvent to obtain phosphorylated polymer solution; forming a film on a glass plate; demoulding in water to obtain a phosphorylated polymer film; placing the phosphorylated polymer film into concentrated hydrochloric acid to perform reflux and hydrolysis; taking out the phosphorylated polymer film to wash with water; and drying to obtain the product. The phosphorylated polymer composite film has low vanadium ion permeability, high proton conductivity, stable mechanical and chemical properties and low cost.
Owner:GUANGZHOU CHEM CO LTD CHINESE ACADEMY OF SCI +1
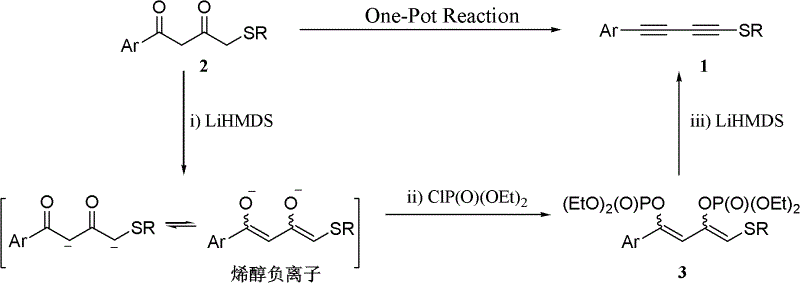
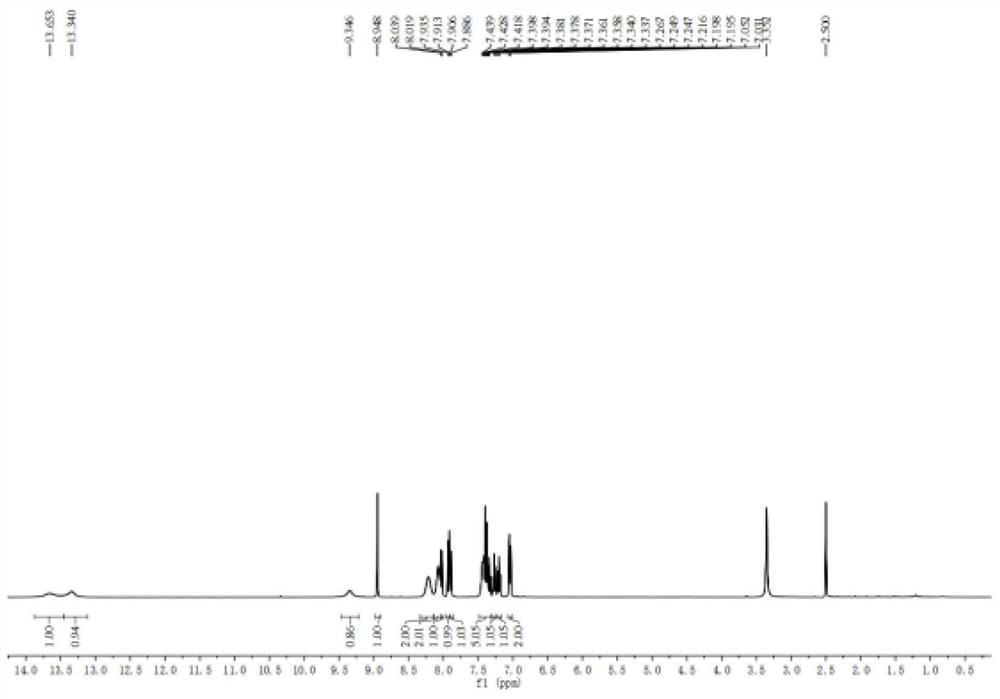
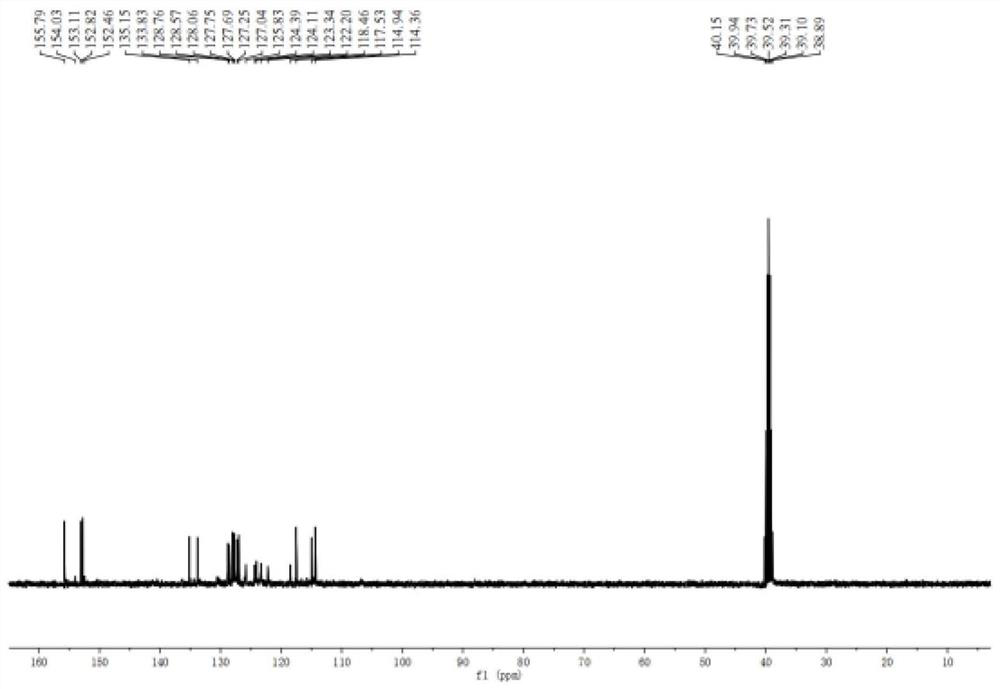
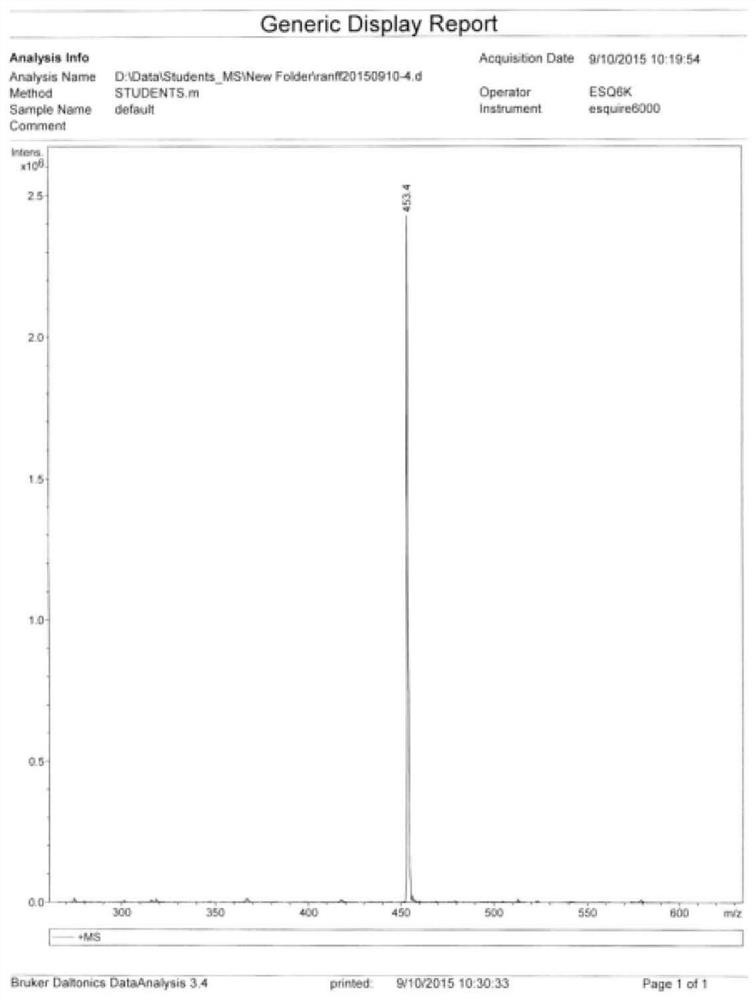
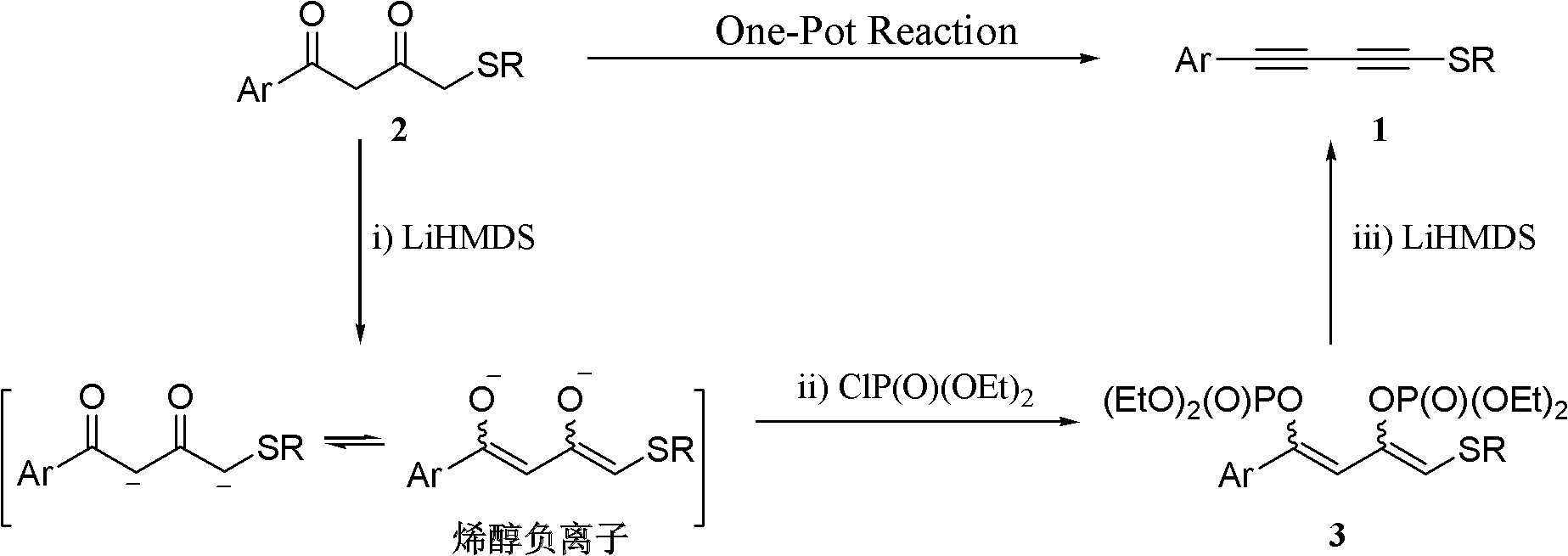
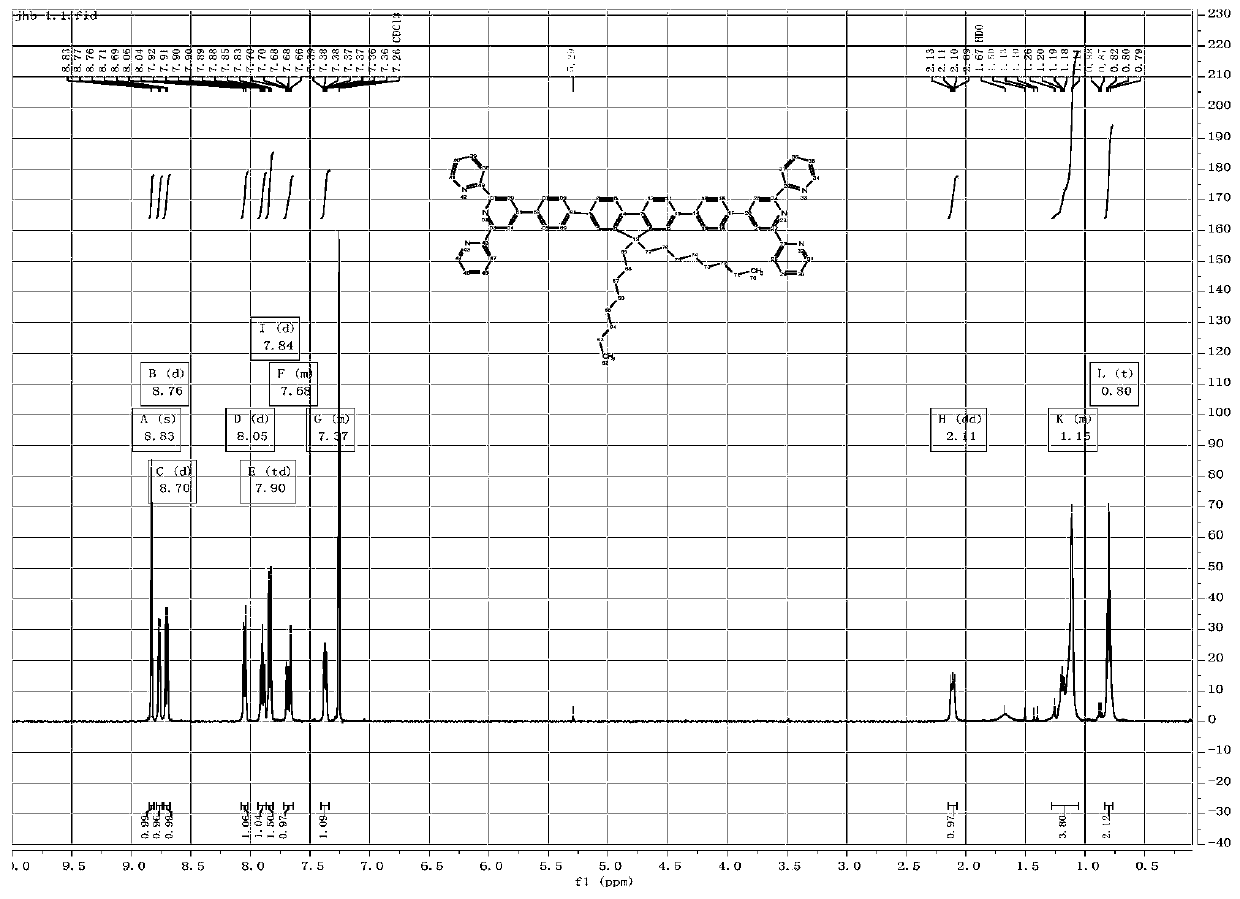
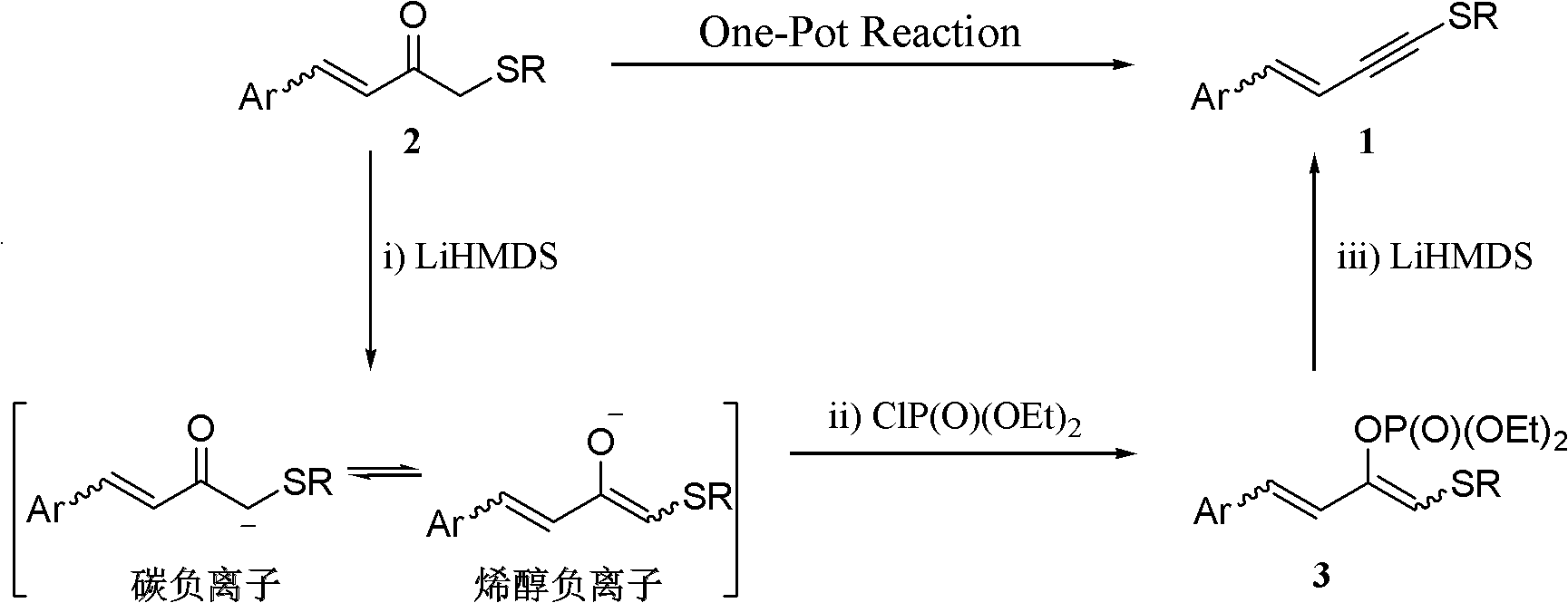
One-pot synthesis method of alkyne thioether
The invention provides a one-pot synthesis method of alkyne thioether 1 based on 2-hydrocarbon sulfenyl-1- aryl ethanone substrate. The method includes feeding Lithium Hexamethyldisilazide (LiHMDS) to tetrahydrofuran (THF) solution of the 2-hydrocarbon sulfenyl-1- aryl ethanone and stirring for reaction under protection of low-temperature nitrogen; adding [C1P(O) (OEt)2] dropwisely to the reaction system, increasing temperature to a room temperature and continuing to stir for reaction; after the reaction system cools to a low temperature again, adding the lithium hexamethyldisilazide (LiHMDS) dropwisely to the reaction system and keeping stirring for 1-3 hours under the temperature; and obtaining the arylethynes from the reaction mixture after normal aftertreatment and column chromatographic separation. The method is a convenient and economical processing method for the alkyne thioether.
Owner:HUNAN UNIV
Anti-flaming organic glass and preparation method thereof
InactiveCN106589753AEvenly dispersedIncrease flame retardancy and environmental protectionFiberOXALIC ACID DIHYDRATE
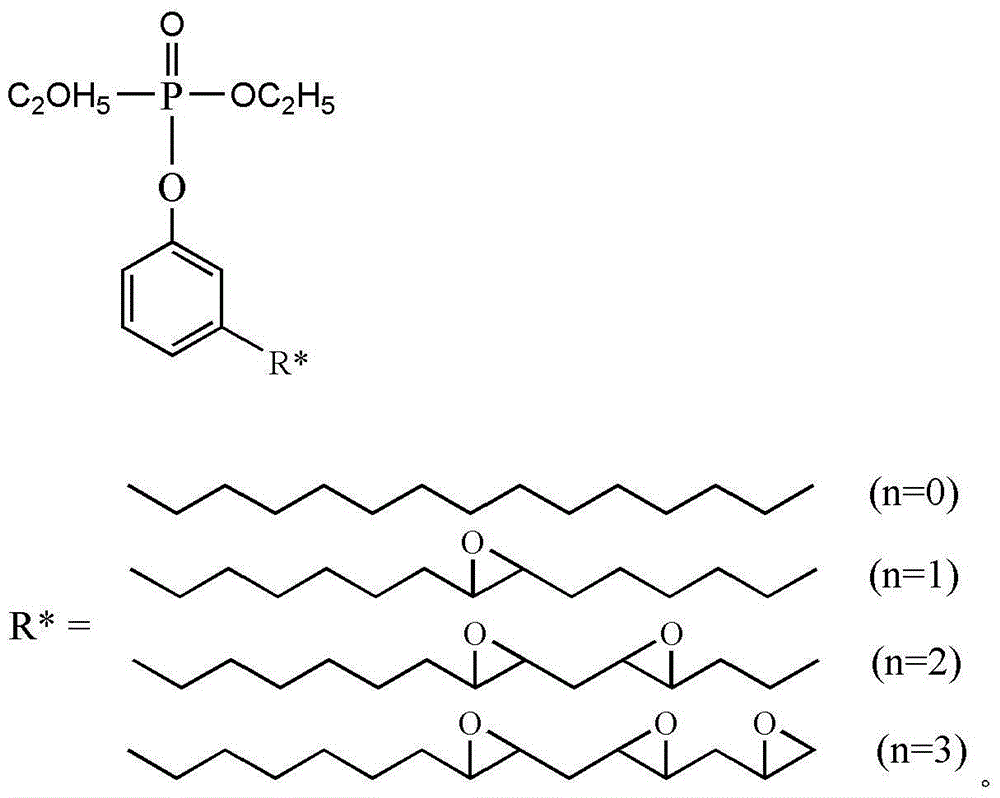
The invention discloses anti-flaming organic glass which comprises the following raw materials in parts by weight: 100-120 parts of methyl methacrylate, 5-10 parts of composite flame retardant, 10-12 parts of oxalic acid acrylate, 1-3 parts of trioctyl acetylcitrate, 13-17 parts of rubber particles, 2-4 parts of carboxymethylcellulose, 2-4 parts of modified carbon fibers and 0.5-1 part of lauroyl peroxide. The preparation process of the composite flame retardant comprises the following steps: uniformly mixing diethyl chlorophosphate with tetrahydrofuran; regulating the temperature; adding triethylamine and uniformly mixing; dropwise adding hydroxyethyl acrylate into the tetrahydrofuran solution and stirring under the heat preservation mode; increasing the temperature to the room temperature; stirring under the heat preservation mode and then purifying, thereby acquiring a material A; mixing the material A with expansible graphite and ammonium polyphosphate, thereby acquiring the composite flame retardant. The invention also discloses a preparation method for the anti-flaming organic glass. The anti-flaming organic glass is high in fire resistance and toughness.
Owner:安徽亚克力实业有限公司
Preparation method of N-alkynyl benzimidazole derivatives
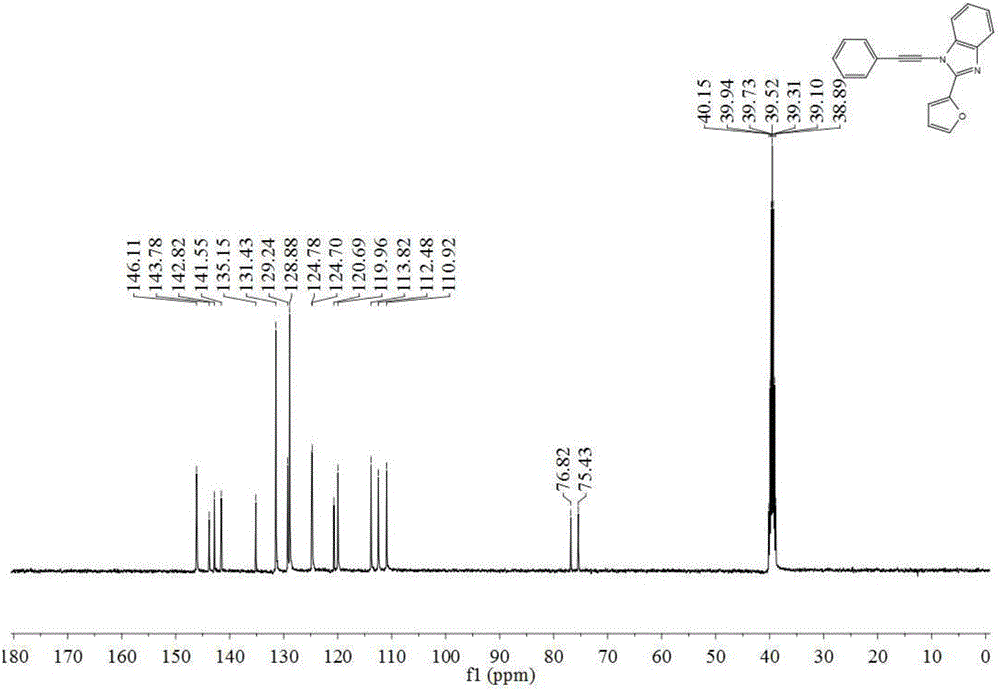
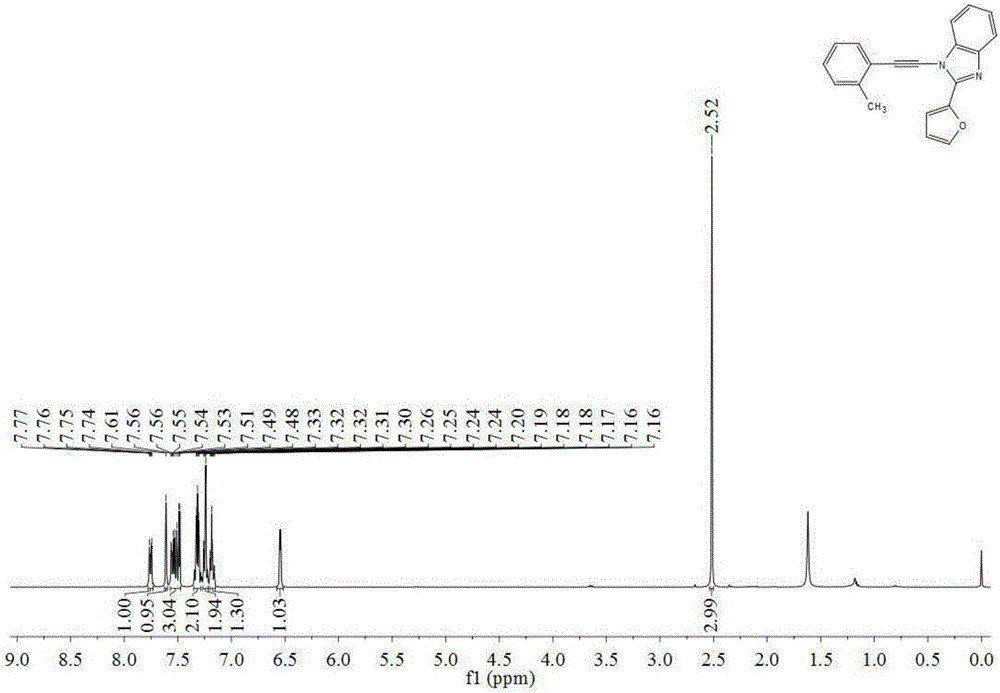
The invention discloses a preparation method of N-alkynyl benzimidazole derivatives, which comprises the following step: under the atmosphere of protective gas, sequentially adding LiHMDS, diethyl chlorophosphate and LiHMDS into an anhydrous solvent of 1-R1 formyl-methyl 2-R2 benzimidazole as shown in the general formula 2 so as to react to obtain an N-alkynyl benzimidazole derivative as shown in the general formula 1, wherein when LiHMDS is added for reacting, the temperature is controlled at -78-0 DEG C, and after ClP(O)(OEt)2 is added, the system temperature rises to 10-30 DEG C so as to react. The preparation method of the N-alkynyl benzimidazole derivatives has the advantages that (1) substrates can be conveniently prepared through cheap raw materials; (2) the universality is good, and preparation of benzimidazole alkyne amine derivatives containing different substituent combinations can be easily realized; (3) the operation is simple and convenient, and the separation of intermediates is not needed; and (4) target compounds are easy to separate and purify, and the yield is relatively high.
Owner:HUNAN UNIV
Functional pyrophyllite powder and application of functional pyrophyllite powder in reinforced and toughened PC-ABS alloy
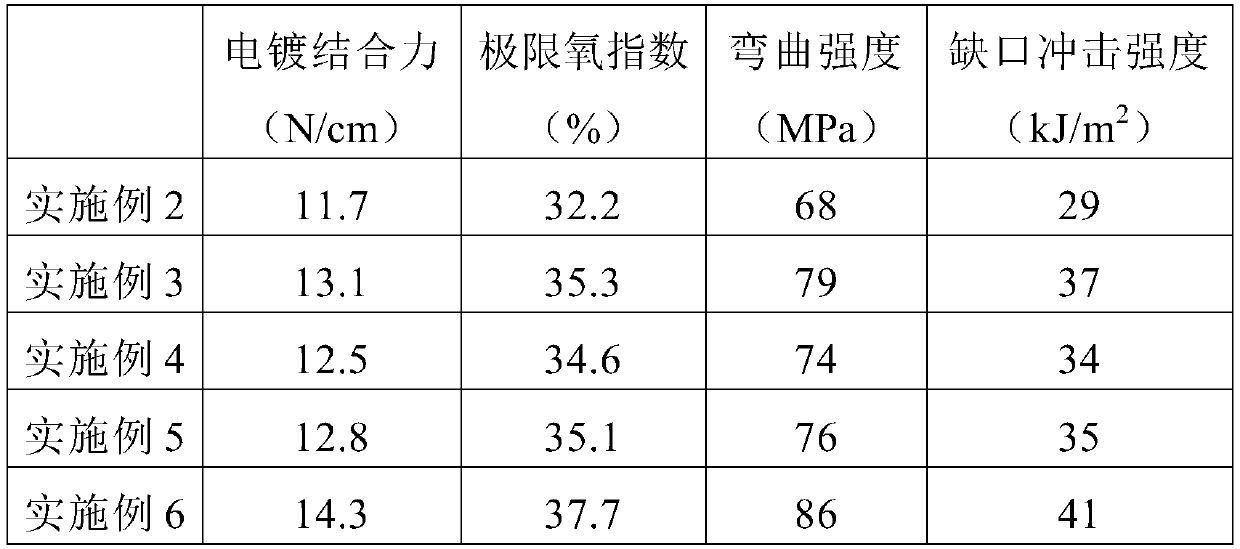
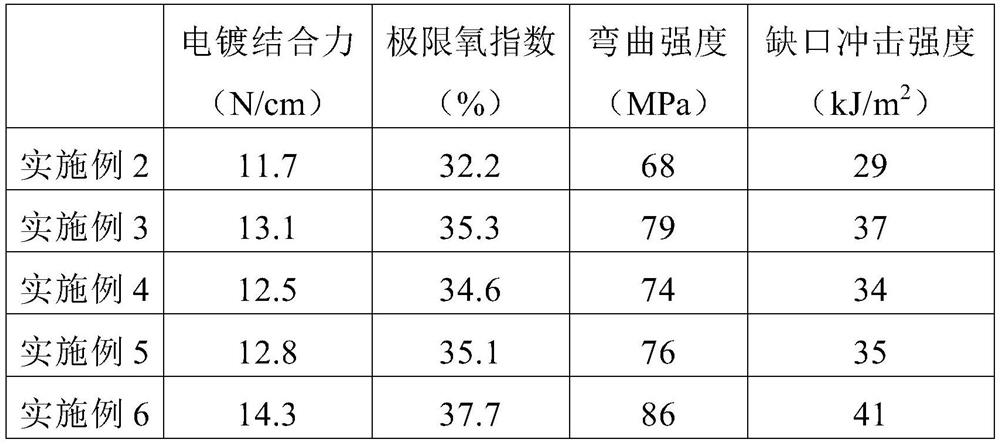
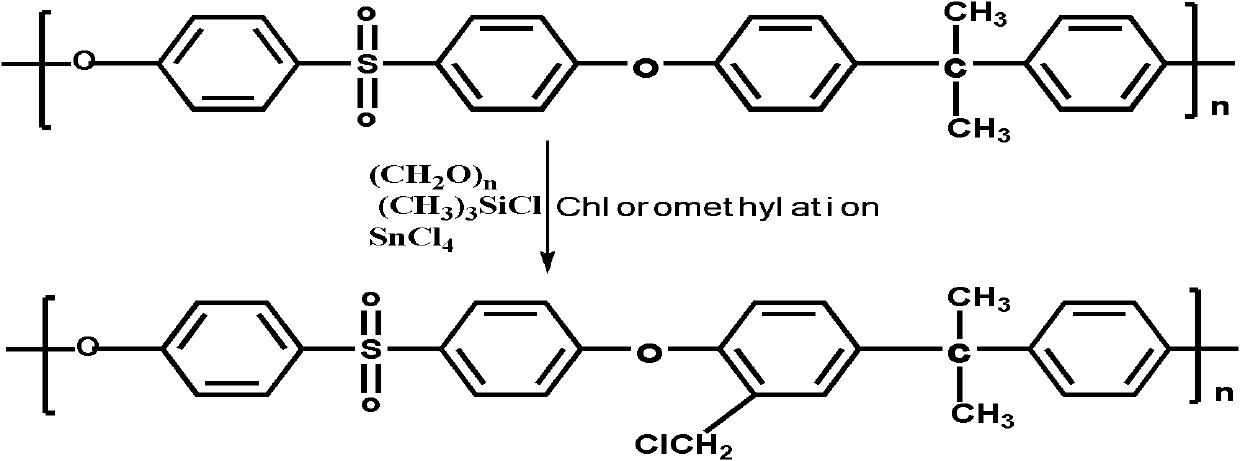
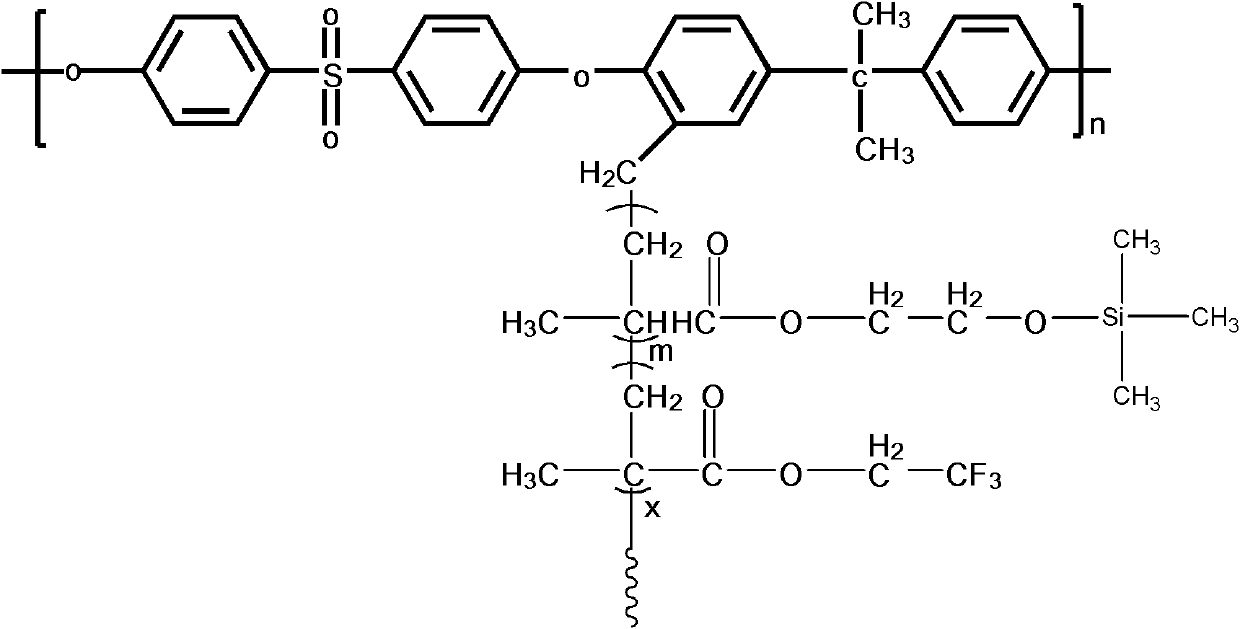
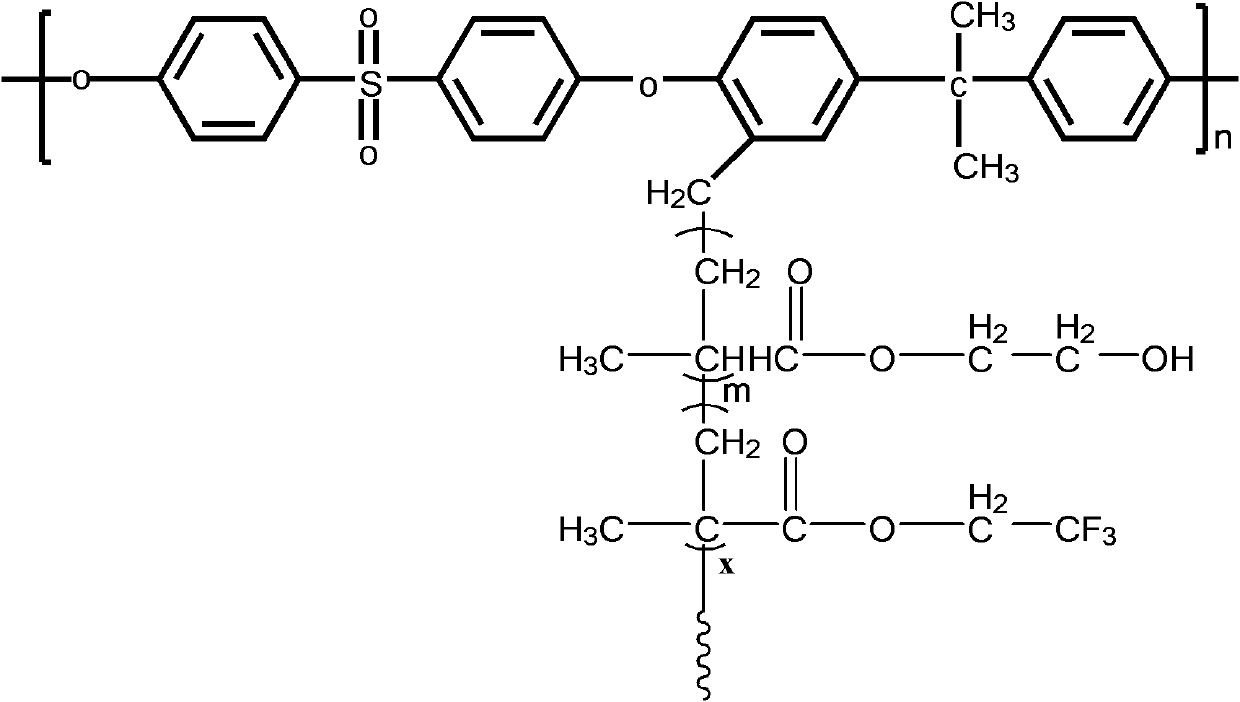
The invention discloses functional pyrophyllite powder and an application of the functional pyrophyllite powder in a reinforced and toughened PC-ABS (polycarbonate-acrylonitrile butadiene styrene) alloy. The functional pyrophyllite powder is prepared by performing acid treatment and alkali treatment on pyrophyllite powder, and then adding methacrylamide, silicane, triphenyl phosphate and diethyl chlorophosphate for functional modification. Compared with the prior art, the functional pyrophyllite powder is applied to preparation of the toughened and reinforced electroplated PC-ABS alloy, and aprepared toughened and reinforced electroplated PC-ABS alloy material has good electroplating performance, and higher tensile strength, bending strength, impact toughness and flame retardance, is extensive in application scope, and can be used for the field of forming various products that have requirements of higher comprehensive performance and good flame resistance on materials and higher surface requirements and are required to be electroplated.
Owner:江阴超润高分子材料有限公司
Method for producing redox shuttles
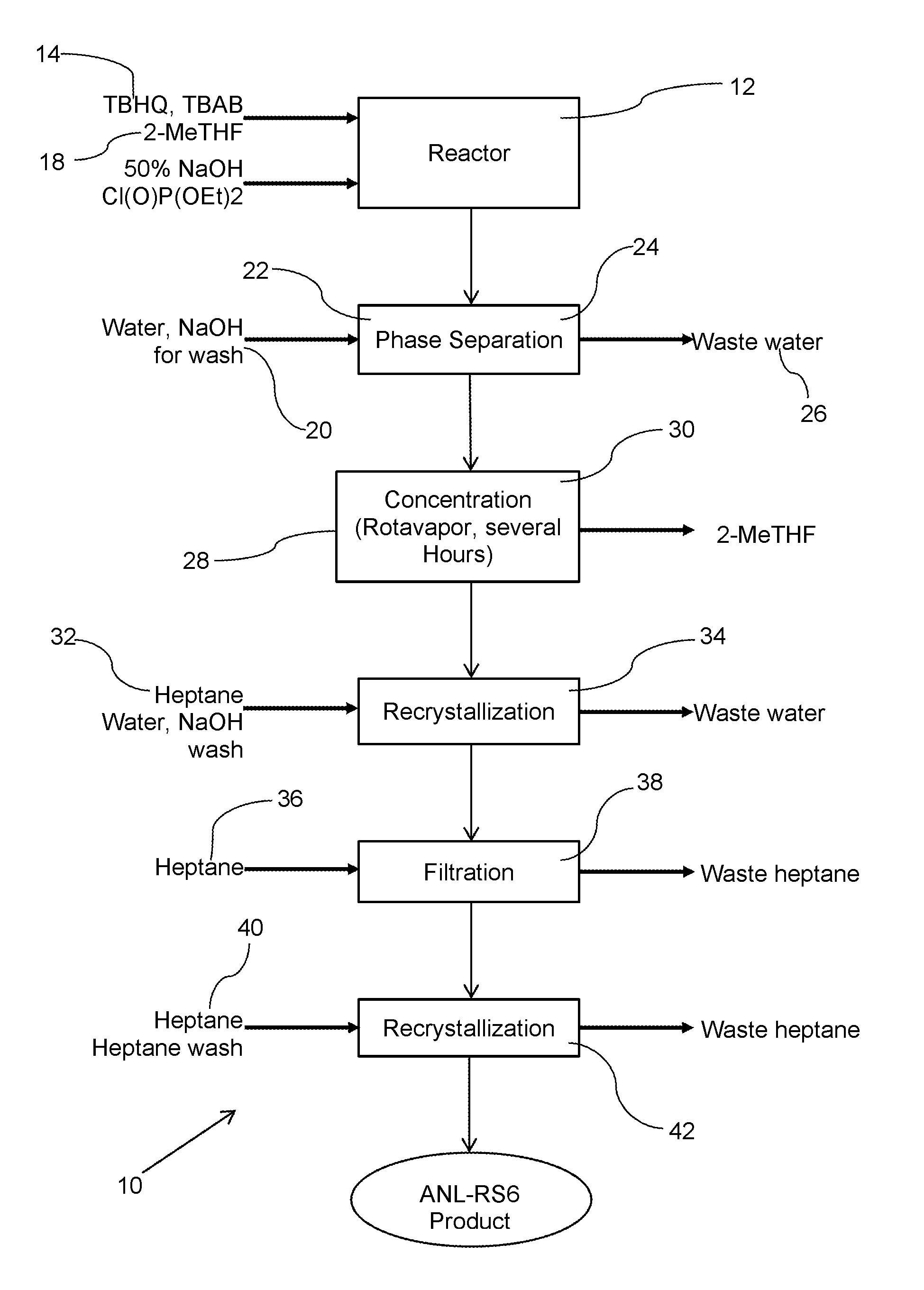
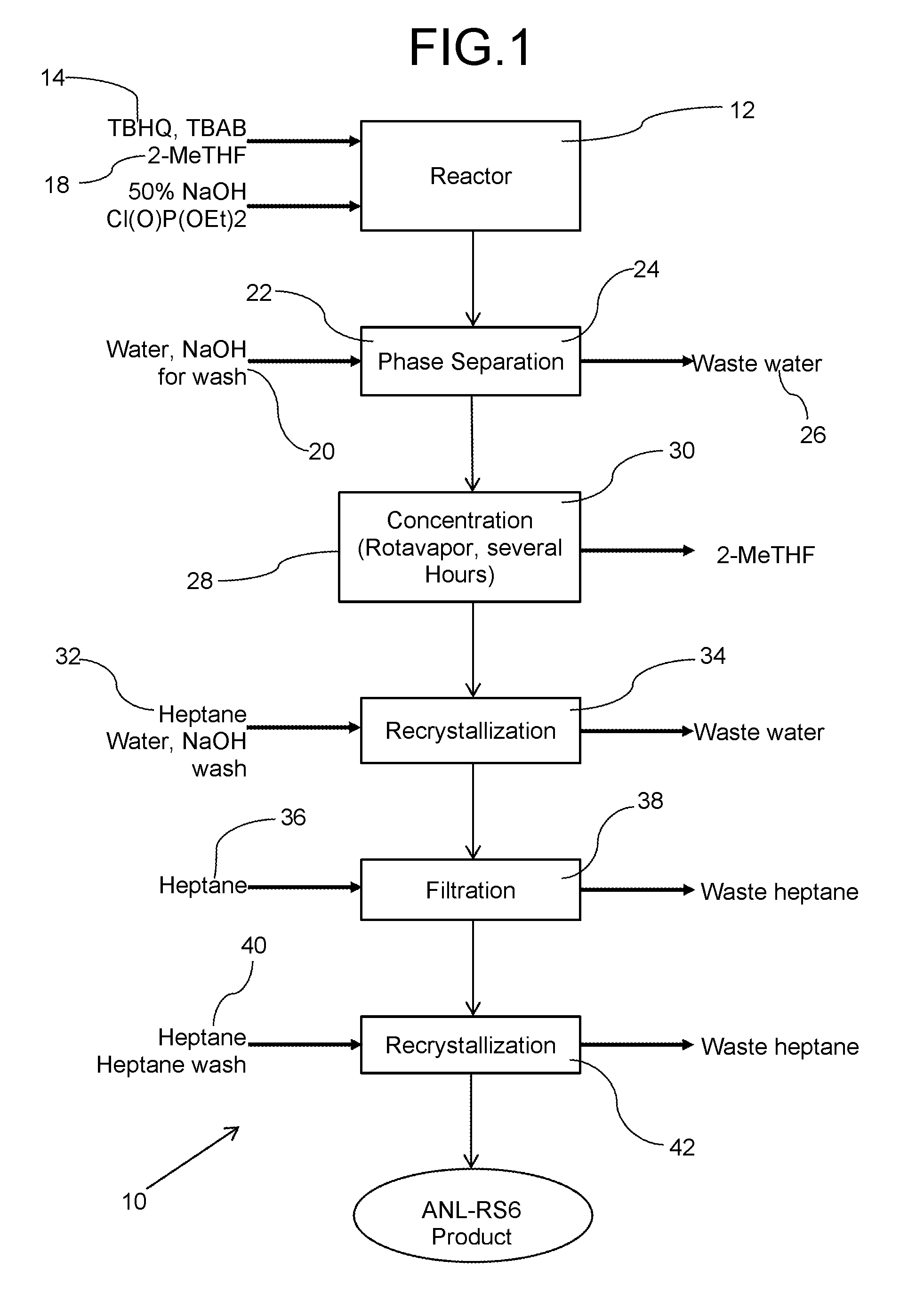
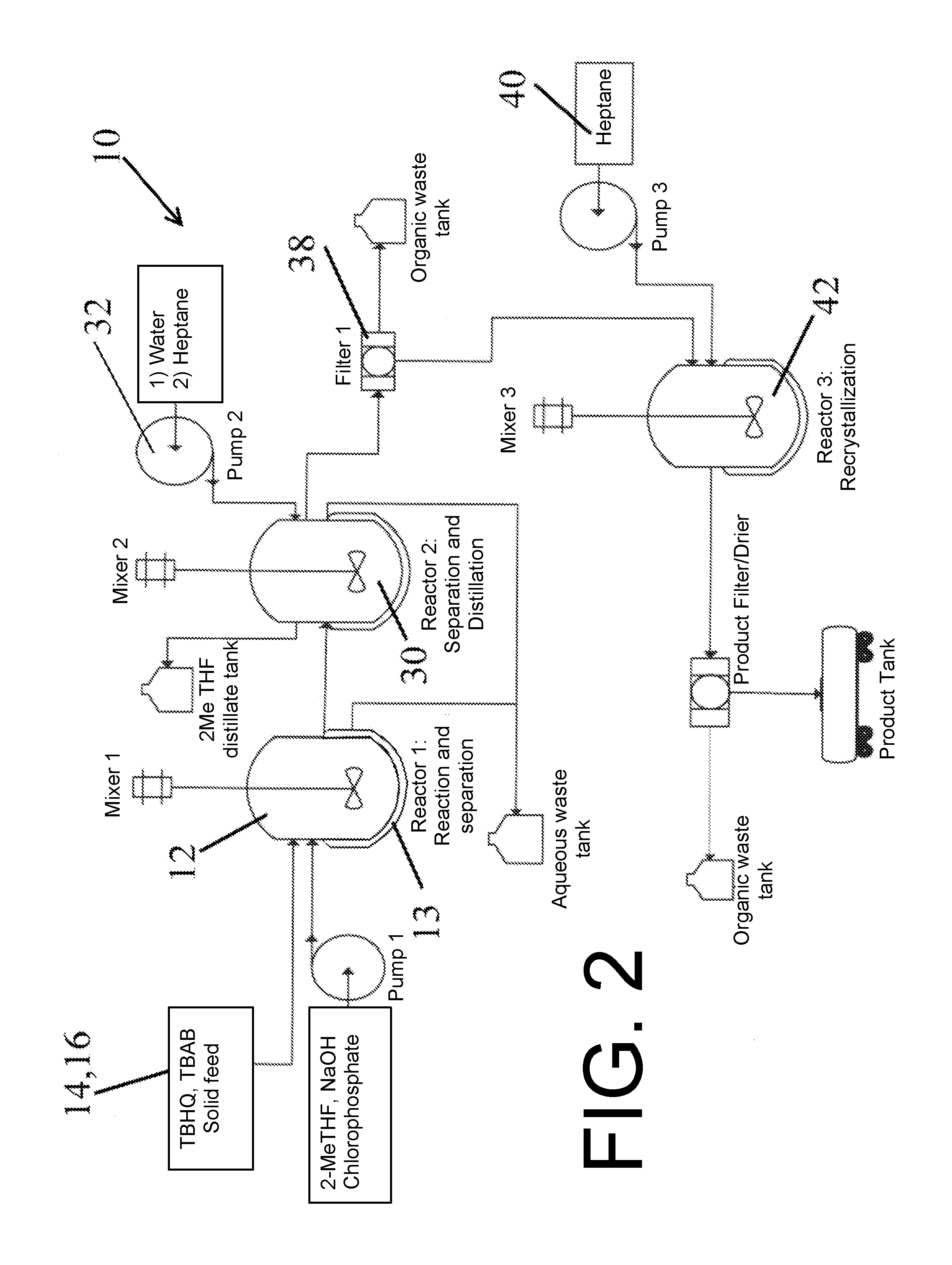
A single step method for producing a redox shuttle having the formula 2,5-di-tert-butyl-1,4-phenylene tetraethyl bis(phosphate) is provided, the method comprising phosphorylating tert butyl hydroquinone with a phosphate-containing reagent. Also provided is method for producing 2,5-di-tert-butyl-1,4-phenylene tetraethyl bis(phosphate), the method comprising solubilizing tert-butyl hydroquinone and tetrabutylammonium bromide with methyltetrahydrofuran to create a mixture; heating the mixture while adding base to the mixture in an amount to turn the mixture orange; and adding diethyl chlorophosphate to the orange mixture in an amount to phosphorylate the hydroquinone.
Owner:UCHICAGO ARGONNE LLC
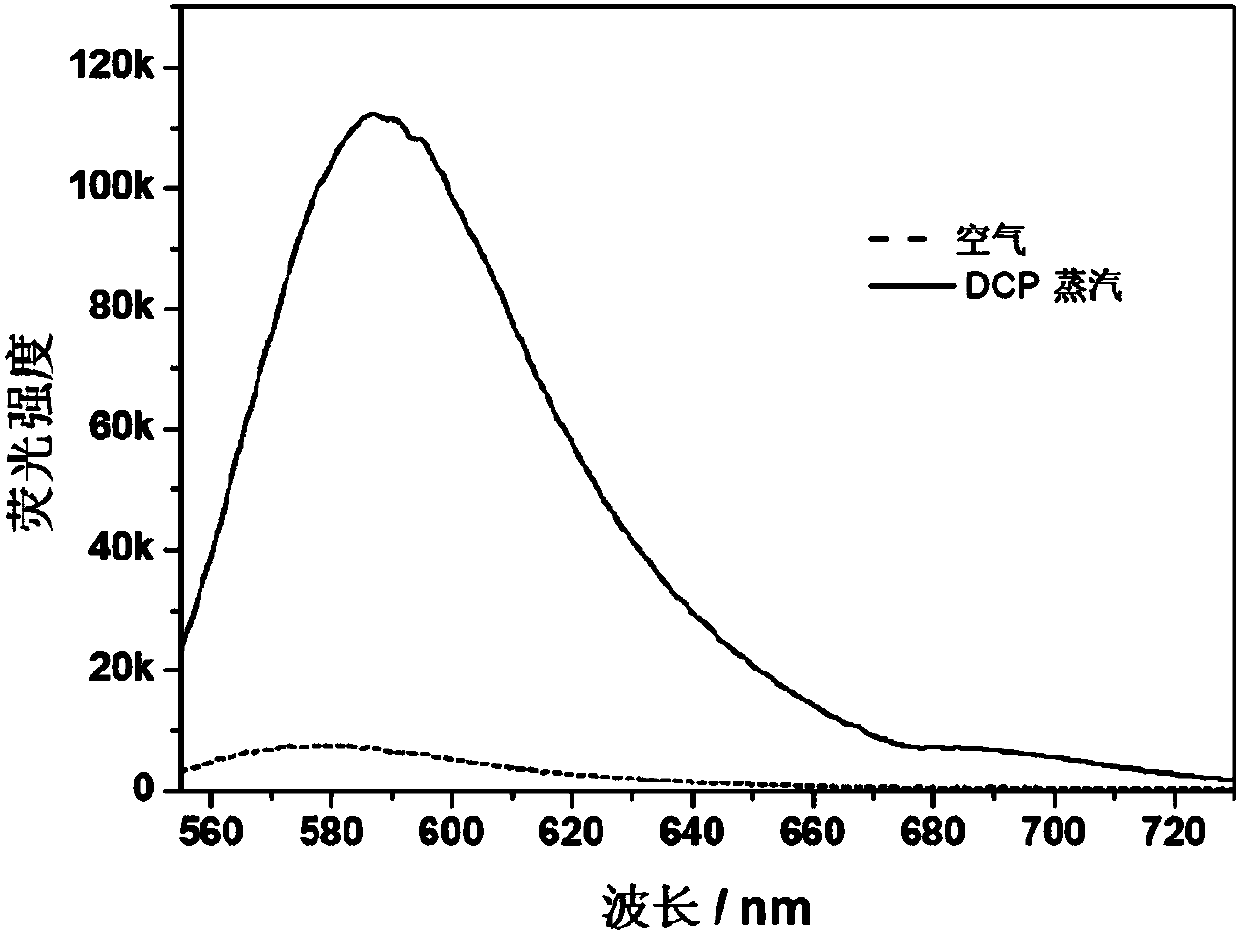
Method for detecting diethyl chlorophosphate gas and/or sarin toxic gas based on charge transfer complex
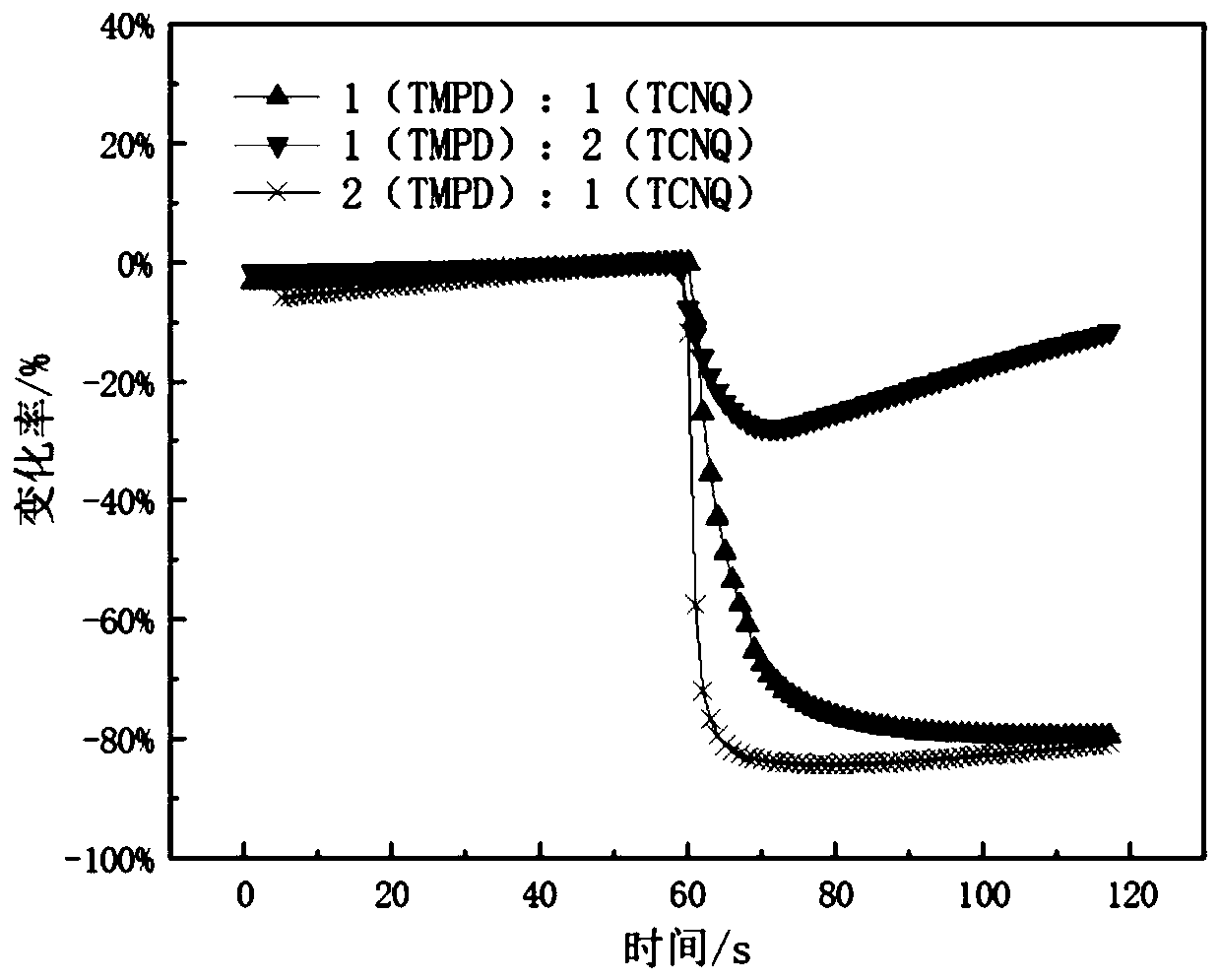
ActiveCN109752417AImprove conductivityGood film formingMaterial resistanceCharge-transfer complexElectrochemistry
The invention relates to a method for detecting diethyl chlorophosphate gas and / or sarin toxic gas based on a charge transfer complex. The method comprises the steps that a first compound containing aN,N dimethyl aromatic amine structure and a second compound containing a tetracyanoquinodimethane structure interacts through intermolecular charge transfer in a solvent to obtain a solution of the charge transfer complex; a sensing material is loaded on the surface of an electrode to form a sensor piece; and the sensor piece is put into the atmosphere containing the diethyl chlorophosphate gas and / or the sarin toxic gas to be detected. According to the method, through the charge transfer complex formed by mixing of the first compound and the second compound in the solvent, the high electronmobility and high hole mobility are achieved, the independent chemical physical properties of the first compound and the second compound are maintained while complexing is conducted, the charge transfer complex can serves as an electrochemical sensing probe to detect the sarin toxic gas, and the problems that in the prior art, detection sensitivity of the sarin toxic gas is low, specificity is low, the means is complex, and the cost is high are solved.
Owner:SHANGHAI INST OF MICROSYSTEM & INFORMATION TECH CHINESE ACAD OF SCI
Organic fluorescent molecule and preparation method thereof, fluorescent sensor and application thereof, and standard fluorescent card
ActiveCN112062752AExtended conjugation propertiesExcellent fluorescence performanceOrganic chemistryFluorescence/phosphorescenceFluid phaseBipyridine
The invention provides an organic fluorescent molecule and a preparation method thereof, a fluorescent sensor and application thereof, and a standard fluorescent card, and belongs to the technical field of fluorescent sensing. A skeleton of the organic fluorescent molecule provided by the invention is composed of 2,2':6',2''-terpyridyl (TPY) and an electron donating group, the TPY group is used asan electron acceptor group, and after the electron donating group (i.e., a group A in a formula I) is introduced, an intramolecular charge transfer state (CT) can be formed to construct a donor-acceptor type organic fluorescent material; and meanwhile, the TPY group can also be used as a recognition site of sensing reaction to detect electrophilic nerve poison molecules. After the organic fluorescent molecule is used to prepare the sensor, efficient and rapid fluorescence / chromogenic dual-channel detection of liquid-phase and gas-phase trace sarin poisoning agents (Sarin) and / or diethyl chlorophosphate (DCP) can be realized, and the organic fluorescent molecule is high in response speed, good in selectivity, high in sensitivity and reusable.
Owner:JILIN UNIV
Fluorescent probe capable of detecting nerve poison in ratio multi-time visualization manner as well as preparation and application
ActiveCN111606896AReduce cost inputEasy post-processingOrganic chemistryFluorescence/phosphorescenceFluoProbesPhosphoric acid
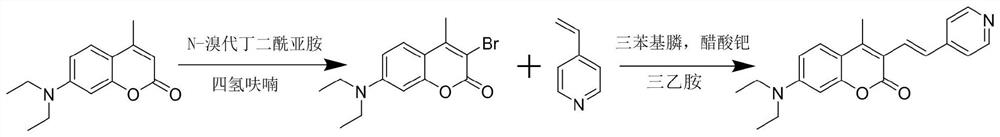
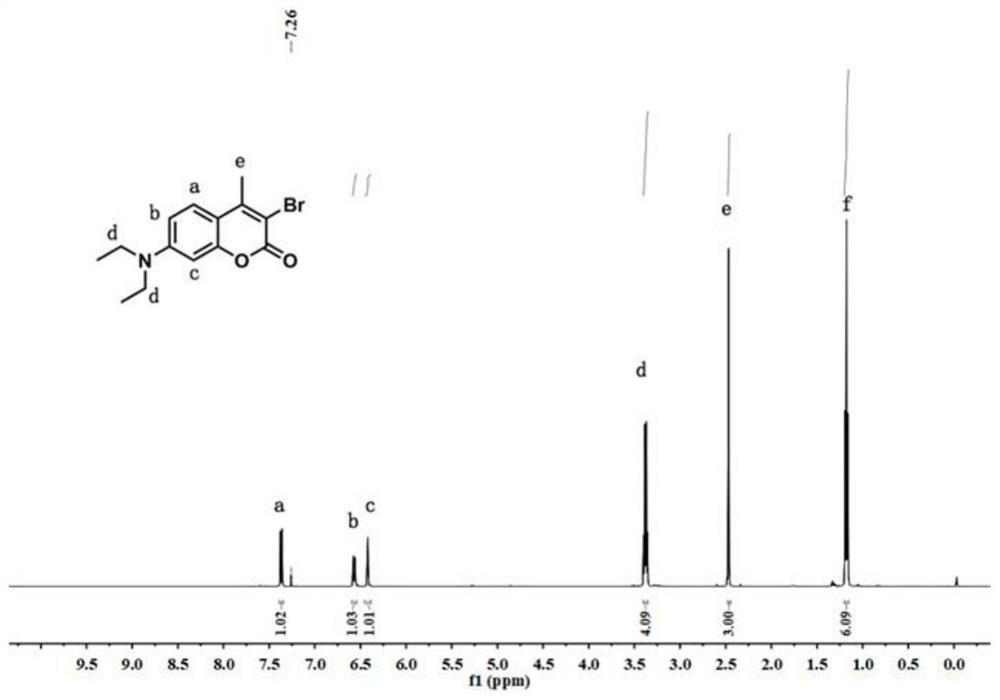
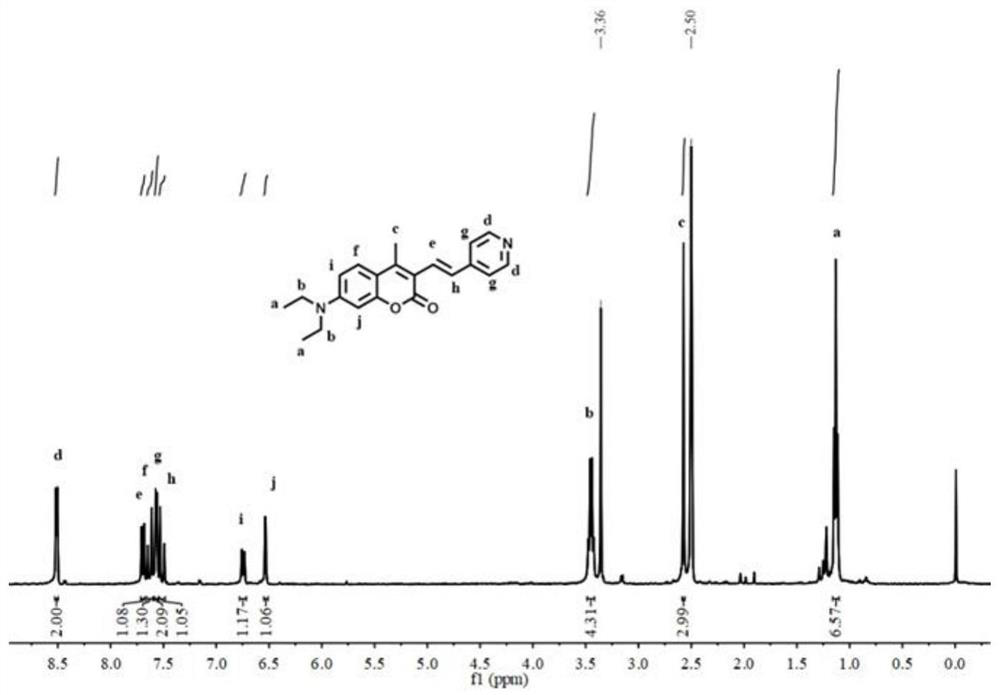
The invention discloses a fluorescent probe capable of detecting a nerve poison in a ratio mult-time visualization manner as well as preparation and application. The probe is a ratio type fluorescentprobe prepared from 7-diethylamino-4-methylcoumarin, N-bromosuccinimide and p-nitrobenzyl bromide as raw materials. The probe can realize high-sensitivity ratio fluorescence detection on diethyl chlorophosphate. Compared with an existing fluorescence detection technology, the obtained fluorescence probe has double-ratio detection and repeated use functions, is high in fluorescence, simple in synthetic route, simple and convenient in detection method and suitable for amplification synthesis and actual production application, and has huge application prospects in the technical fields of analytical chemistry, life science, environmental science and the like.
Owner:HUNAN UNIV OF SCI & TECH
One-pot synthesis method of methylthio aryl alkynye
The invention provides a new method of the one-pot synthesis of methylthio aryl alkynye 1 based on 4-sulfenyl-1-aryl-1,3-butanedione substrates. lithium hexamethyldisilazide (LiHMDS) is added in tetrahydro furan (THF) solution containing 4-sulfenyl-1-aryl-1, 3-diacetyl under the protection of low temperature nitrogen, and the stirring is carried out; in addition, diethyl chlorophosphate [ClP(O)(OEt)2] is dripped into the above-mentioned reaction system, the temperature rises to reach the room temperature naturally after the dripping is finished, and stirring is carried out continuously. The reaction system is cooled down to get low temperature again, then LiHMDS is dripped into the reaction system, and stirring is carried out continuously for reaction under the temperature. Reaction mixtures are processed through conventional post-processing and column chromatography separation, and then methylthio aryl alkynye is obtained. The new method is simple, convenient and economical to prepare methylthio aryl alkynye.
Owner:HUNAN UNIV
Synthesis and application of fluorescent probe for detecting diethyl chlorophosphate
PendingCN111808130ALow costCorrosion resistanceSilicon organic compoundsFluorescence/phosphorescenceDiethyl phosphateFluoProbes
The invention discloses synthesis and application of a fluorescent probe for detecting diethyl chlorophosphate. A 1, 1 '-binaphthol structure based silicon compound fluorescent probe is synthesized. The silicon-containing compound fluorescent probe can realize specific detection of diethyl chlorophosphate serving as a war poison stimulant; the response speed is high, the detection limit is low; the light-emitting wavelength is long; the commercial degree is high; the cost required by probe synthesis is greatly reduced; the invention further discloses an application method of the fluorescent probe for detecting diethyl chlorophosphate based on normalization processing data. A miniature spectrum analyzer, an excitation light source and a computer are adopted to carry out signal transmissionthrough a signal transmission optical fiber, unique advantage of electromagnetic interference resistance is achieved, the optical fiber probe has the advantage of corrosion resistance, due to the accuracy of optical signals, the sensor is different from conventional naked eye observation, and the sensor has the advantage of being high in diethyl chlorophosphate steam detection accuracy.
Owner:SOUTHWEST JIAOTONG UNIV
Functionalized pyrophyllite powder and its application in strengthening and toughening pc-abs alloy
The invention discloses functional pyrophyllite powder and an application of the functional pyrophyllite powder in a reinforced and toughened PC-ABS (polycarbonate-acrylonitrile butadiene styrene) alloy. The functional pyrophyllite powder is prepared by performing acid treatment and alkali treatment on pyrophyllite powder, and then adding methacrylamide, silicane, triphenyl phosphate and diethyl chlorophosphate for functional modification. Compared with the prior art, the functional pyrophyllite powder is applied to preparation of the toughened and reinforced electroplated PC-ABS alloy, and aprepared toughened and reinforced electroplated PC-ABS alloy material has good electroplating performance, and higher tensile strength, bending strength, impact toughness and flame retardance, is extensive in application scope, and can be used for the field of forming various products that have requirements of higher comprehensive performance and good flame resistance on materials and higher surface requirements and are required to be electroplated.
Owner:江阴超润高分子材料有限公司
Phosphorylated polymer composite film for full vanadium flow battery and preparation method thereof
InactiveCN102167785BImprove proton conductivityImprove efficiencyCell component detailsFuel cell detailsPolymer dissolutionPolymer science
The invention discloses a phosphorylated polymer composite film for a full vanadium flow battery and a preparation method thereof. The polymer composite film has a structure shown as a formula (I). The preparation method comprises the following steps of: mixing a monomer mixture, chloromethylate polysulfone, cuprous chloride, genin and a solvent; performing deoxidization; perfomring polymerization; dissolving the obtained polymer into dioxane; adding triethylamine and the cuprous chloride; adding diethyl chlorophosphate dropwise; reacting and filtering; adding filtrate into norml hexane to precipitate; redissolving the precipitate into chloroform; adding the solution into the norml hexane again to precipitate; washing the precipitate; drying to obtain the phosphorylated polymer; dissolving the phosphorylated polymer into a high-boiling-point solvent to obtain phosphorylated polymer solution; forming a film on a glass plate; demoulding in water to obtain a phosphorylated polymer film; placing the phosphorylated polymer film into concentrated hydrochloric acid to perform reflux and hydrolysis; taking out the phosphorylated polymer film to wash with water; and drying to obtain the product. The phosphorylated polymer composite film has low vanadium ion permeability, high proton conductivity, stable mechanical and chemical properties and low cost.
Owner:GUANGZHOU CHEM CO LTD CHINESE ACADEMY OF SCI +1
A kind of chitosan oligosaccharide derivative containing thiourea and diethoxyphosphoramide structure and preparation method thereof
ActiveCN109021033BImprove biological activityLow reaction temperatureSugar derivativesFungicidesPolymer scienceThiourea
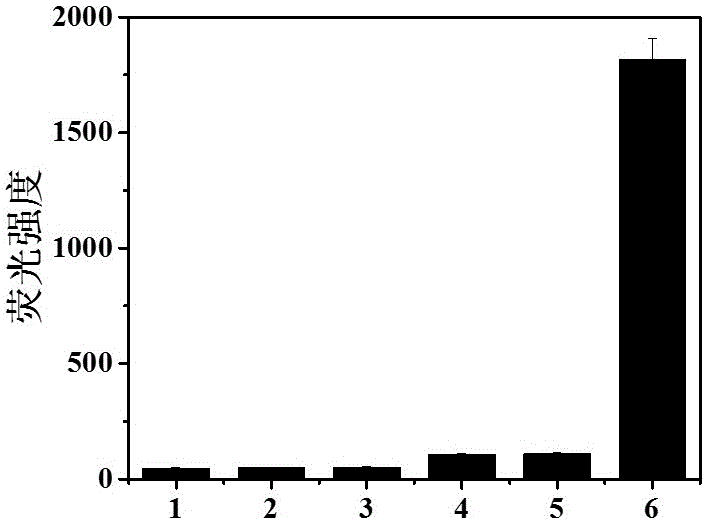
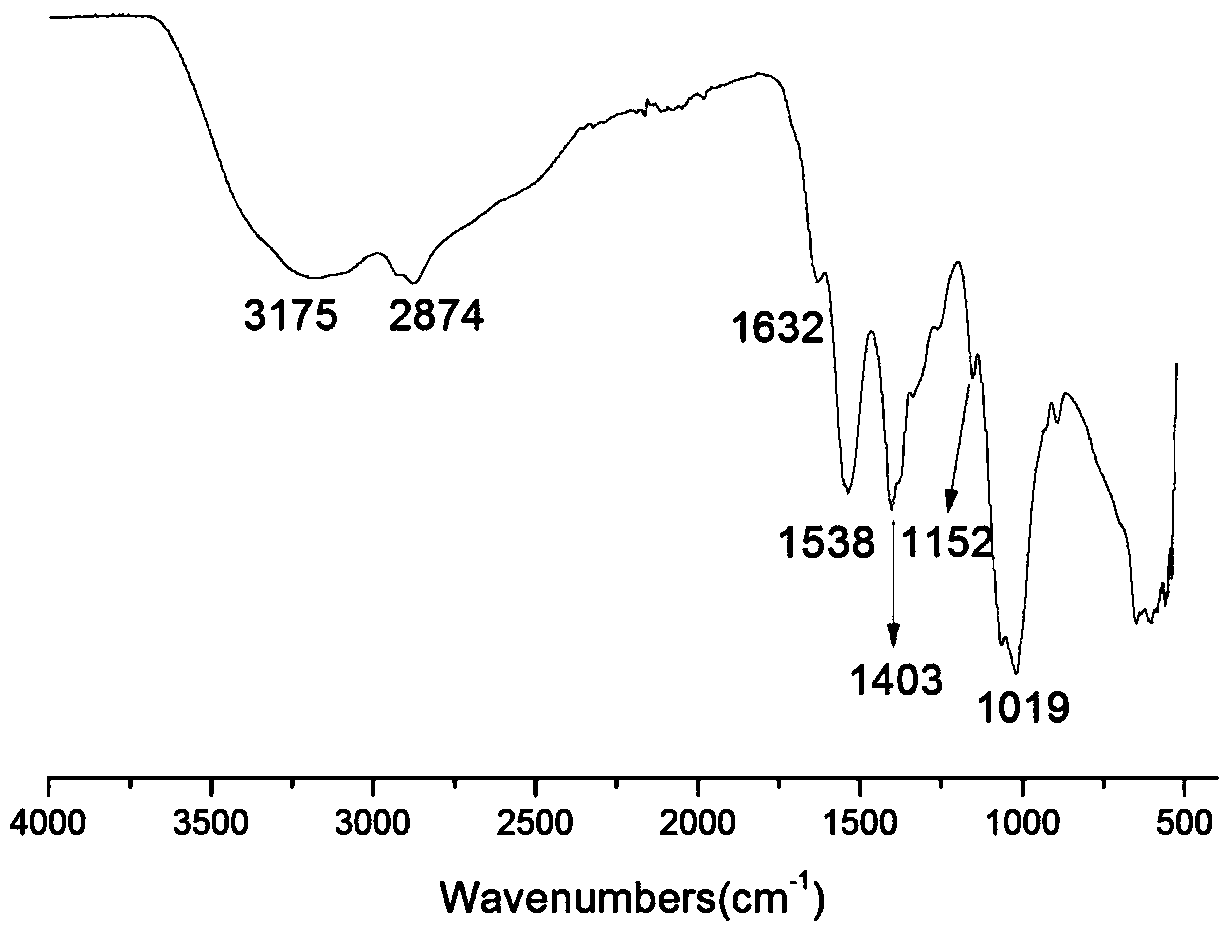
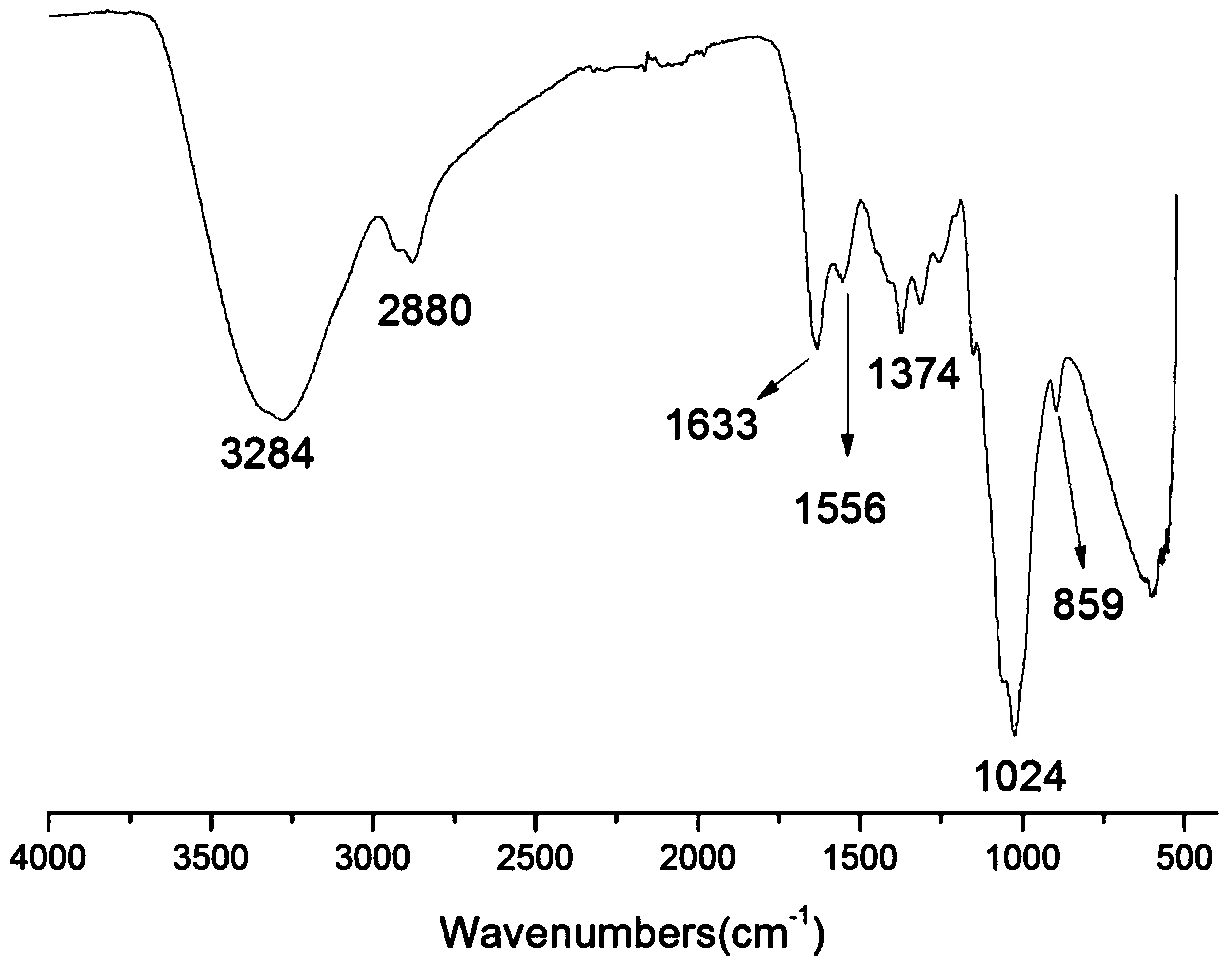
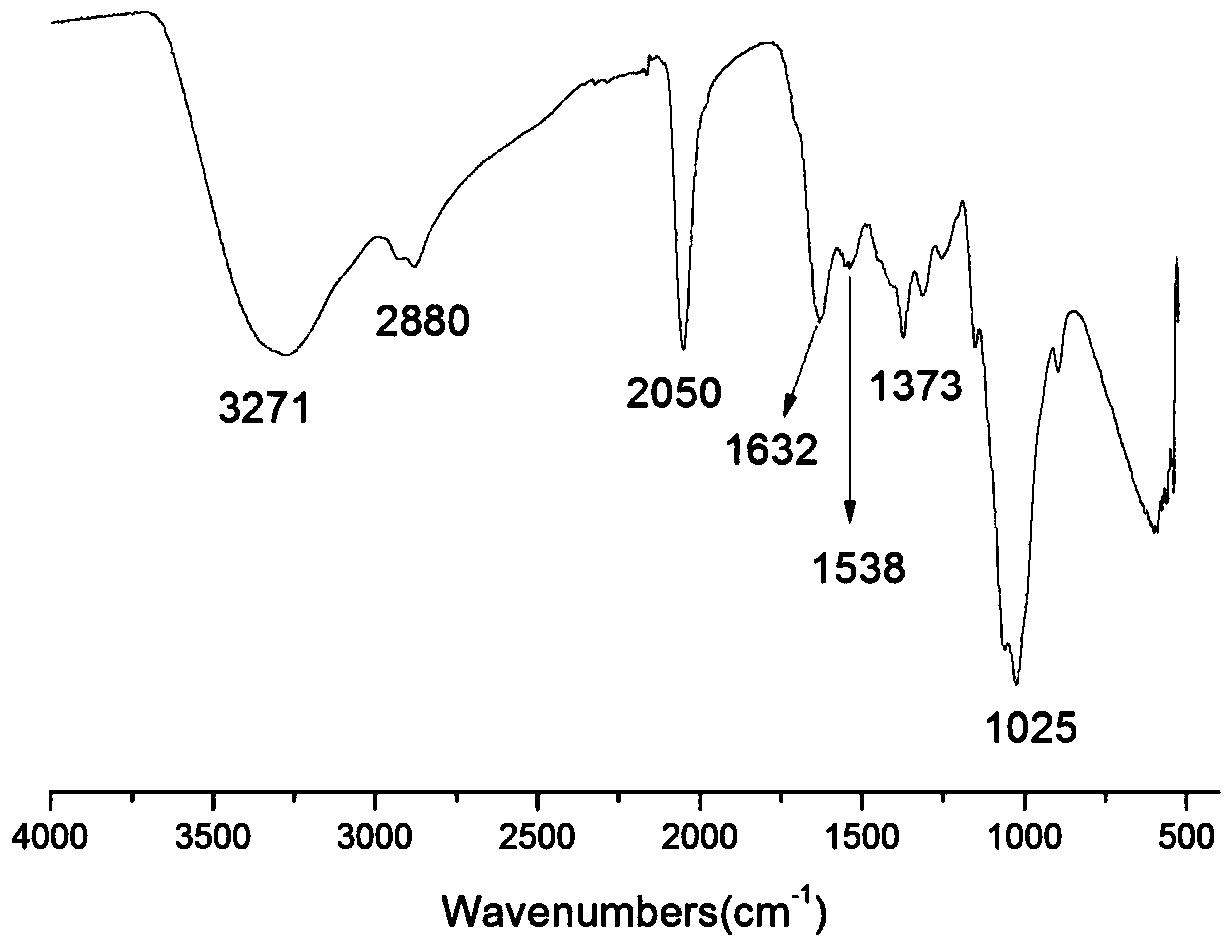
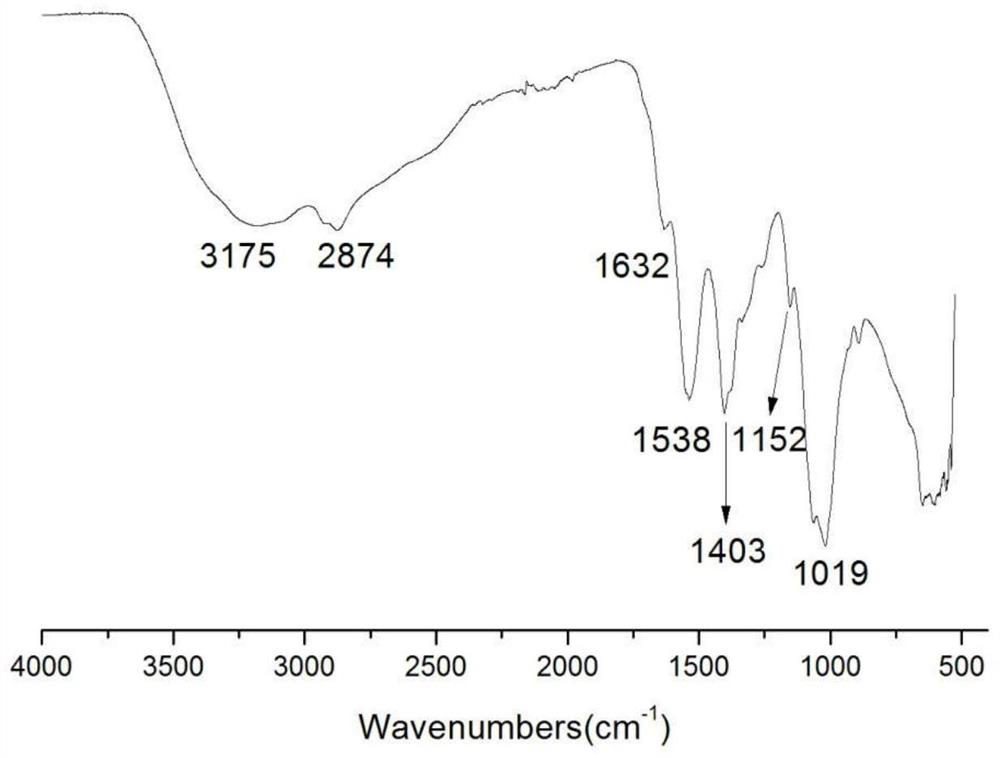
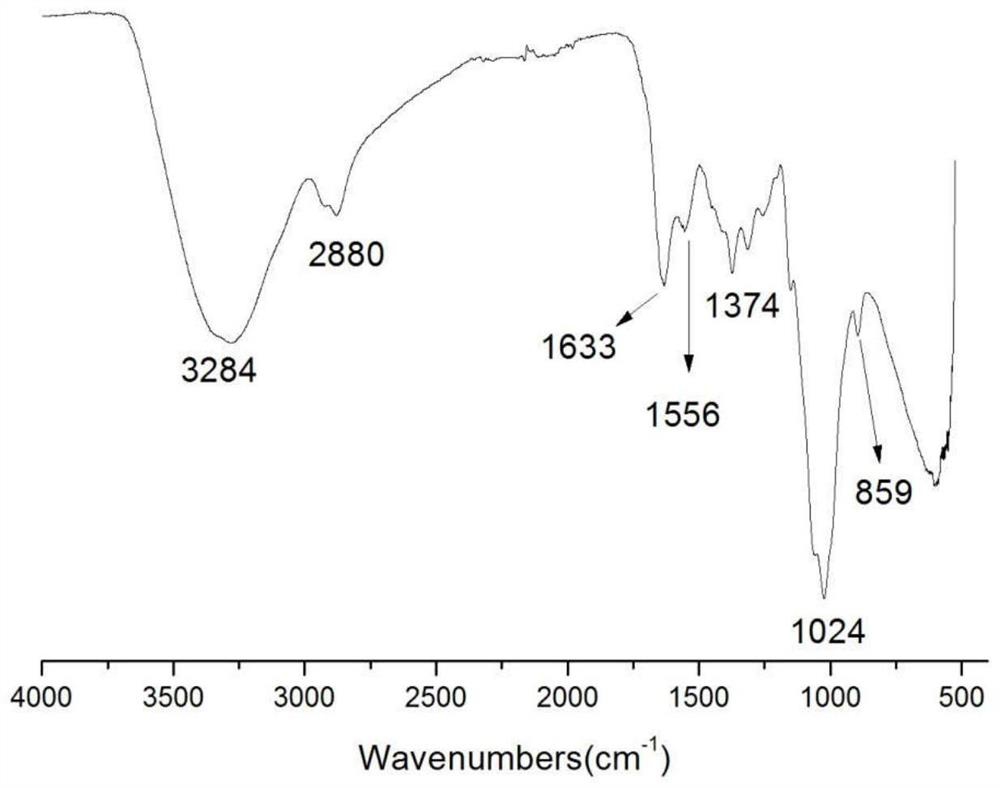
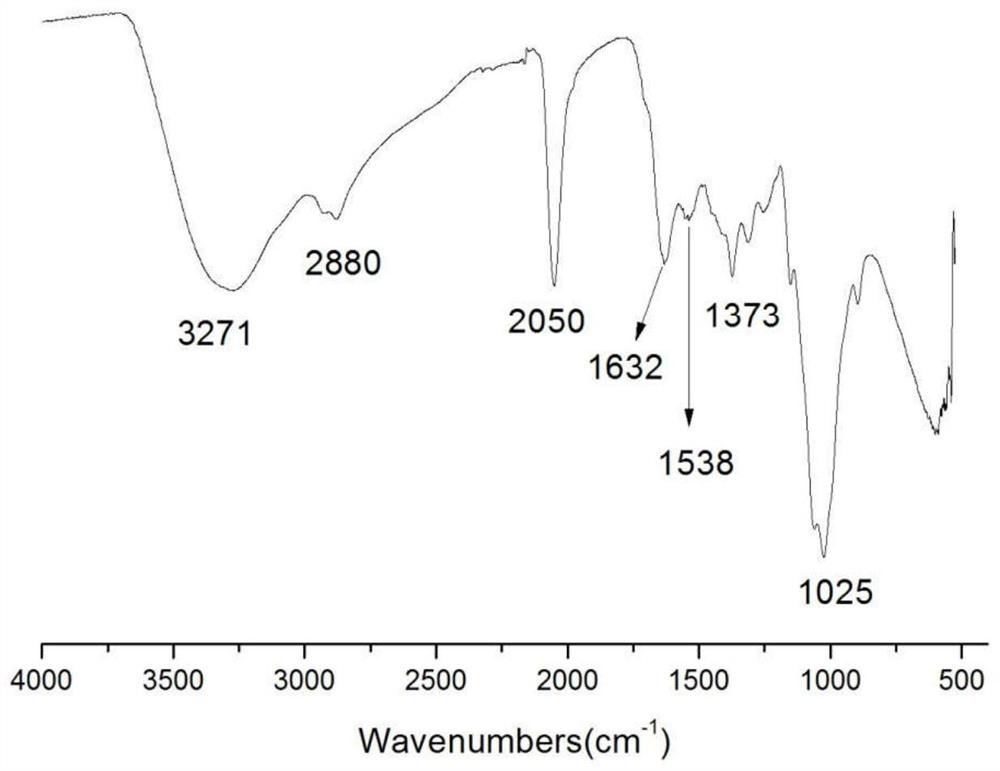
The invention belongs to marine chemical engineering technology, in particular to a chitosan oligosaccharide derivative containing thiourea and diethoxyphosphoramide structure and a preparation method thereof. The chitosan oligosaccharide derivative containing thiourea and diethoxyphosphorhydrazide structure is shown in formula I, wherein, n=2‑20. In the present invention, at first by chitosan oligosaccharide and 1,2-dibromoethane reaction generates bromoethyl chitosan derivative, then adds ammonium thiocyanate, reacts with bromoethyl to produce isothiocyanate ethyl shell Oligosaccharide derivatives, the isothiocyanate group is further reacted with hydrazine hydrate to obtain aminothiourea ethyl chitooligosaccharide derivatives containing thiourea groups, and finally reacted with diethyl chlorophosphate to obtain thiourea and chitosan oligosaccharide derivatives with diethoxyphosphoramide structure. The resulting derivatives were analyzed by infrared spectroscopy to determine their structure. Chitooligosaccharides were effectively combined with the inserted groups to generate chitosan oligosaccharide derivatives containing thiourea and diethoxyphosphoramide structures, which improved the biological activity of chitosan oligosaccharides. .
Owner:INST OF OCEANOLOGY - CHINESE ACAD OF SCI
One-pot synthesis method of methylthio aryl alkynye
The invention provides a new method of the one-pot synthesis of methylthio aryl alkynye 1 based on 4-sulfenyl-1-aryl-1,3-butanedione substrates. lithium hexamethyldisilazide (LiHMDS) is added in tetrahydro furan (THF) solution containing 4-sulfenyl-1-aryl-1, 3-diacetyl under the protection of low temperature nitrogen, and the stirring is carried out; in addition, diethyl chlorophosphate [ClP(O)(OEt)2] is dripped into the above-mentioned reaction system, the temperature rises to reach the room temperature naturally after the dripping is finished, and stirring is carried out continuously. The reaction system is cooled down to get low temperature again, then LiHMDS is dripped into the reaction system, and stirring is carried out continuously for reaction under the temperature. Reaction mixtures are processed through conventional post-processing and column chromatography separation, and then methylthio aryl alkynye is obtained. The new method is simple, convenient and economical to prepare methylthio aryl alkynye.
Owner:HUNAN UNIV
A fluorescent probe for detection of sarin poison and its simulant, its synthesis method and application
ActiveCN106748976BThe synthesis method is simpleOptimize detection conditionsOrganic chemistryFluorescence/phosphorescenceFluoProbesChemical compound
The invention relates to a fluorescent probe for detecting sarin and an analogue thereof. The fluorescent probe comprises a compound which is loaded on a substrate and has a structure shown as a formula I, wherein the formula I is shown in the description. The invention further provides a synthesis method and application of the fluorescent probe for detecting the sarin and the analogue thereof. According to the application provided by the invention, when a compound with the structure shown as the formula I in the fluorescent probe is in contact with the sarin or the DCP (Diethyl Chlorophosphate) in air, the fluorescence intensity and the emission wavelength are both changed, and gas-phase detection of the sarin and the DCP can be realized. The compound with the structure shown as the formula I can have one or more terpyridines; when the sarin or the DCP reacts with one certain pyridine in one certain terpyridine in a molecule, charge transferring from the fluorescent probe to the sarin or the DCP occurs; meanwhile, energy transferring from a polymer core to a peripheral radical occurs, and fluorescent quenching of the whole molecule is caused, so that the fluorescent probe has excellent sensing performance.
Owner:SHANGHAI INST OF MICROSYSTEM & INFORMATION TECH CHINESE ACAD OF SCI
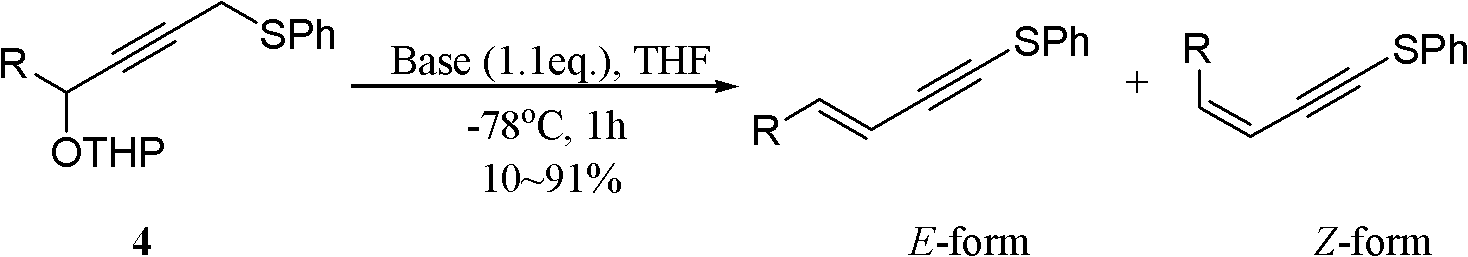
One-pot synthesis method of conjugate eneyne thioether
ActiveCN102617419BEasy to prepareImprove versatilitySulfide preparationChromatographic separationPhosphoric acid
The invention provides a one-pot synthesis method of conjugate eneyne thioether based on 1-hydrocarbon sulfenyl-4-aryl-butene-2-ketone substrate. The method includes feeding Lithium Hexamethyldisilazide (LiHMDS) to a tetrahydrofuran (THF) solution of 1-hydrocarbon sulfenyl-4-aryl-butene-2-ketone under protection of low-temperature nitrogen; stirring; adding [C1P(O) (OEt)2] dropwisely to the reaction system, increasing the temperature to a room temperature after the addition and continuing to stir; after the reaction system cools again to a low temperature, dripping the LiHMDS to the reaction system and stirring for reaction under the temperature; and obtaining the conjugate eneyne thioether from the reaction mixture after normal aftertreatment and column chromatographic separation. The method is a convenient and economical processing method for the conjugate eneyne thioether.
Owner:HUNAN UNIV
Structure controllable phosphorylated polymer composite film used for vanadium battery and preparation method thereof
InactiveCN102117925BImprove proton conductivityImprove efficiencyRegenerative fuel cellsCell component detailsPolymer dissolutionPolymer science
Owner:GUANGZHOU CHEM CO LTD CHINESE ACADEMY OF SCI +1
A kind of intumescent flame retardant functionalized hydrotalcite flame retardant and preparation method thereof
The invention provides an intumescent flame retardant functionalized hydrotalcite flame retardant. The flame retardant is good in flame retardant effect, and adopts a halogen-free system, so that secondary harm during combustion of materials is reduced. The intumescent flame retardant functionalized hydrotalcite flame retardant is prepared through the steps as follows: a silane coupling agent containing amine groups and hydrotalcite powder particles react with each other to prepare silane coupling agent modified hydrotalcite, and then, the silane coupling agent modified hydrotalcite and diethyl chlorophosphate react with each other. The flame retardant is wide in application range, good in flame resistant effect, and stable in performance; the preparation process of the flame retardant is simple and convenient in operation, the cost is low, the solvent recycling is convenient, and obtained products are easy to separate, so that the industrial production is facilitated.
Owner:TAIZHOU UNIV
An organic fluorescent molecule and its preparation method, a fluorescent sensor and its application, and a standard fluorescent card
ActiveCN112062752BEfficient detectionQuick responseOrganic chemistryFluorescence/phosphorescenceBipyridineTerpyridine
Owner:JILIN UNIV
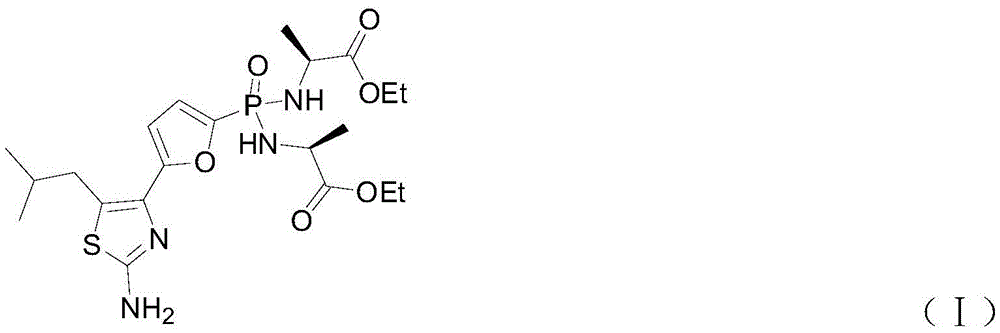
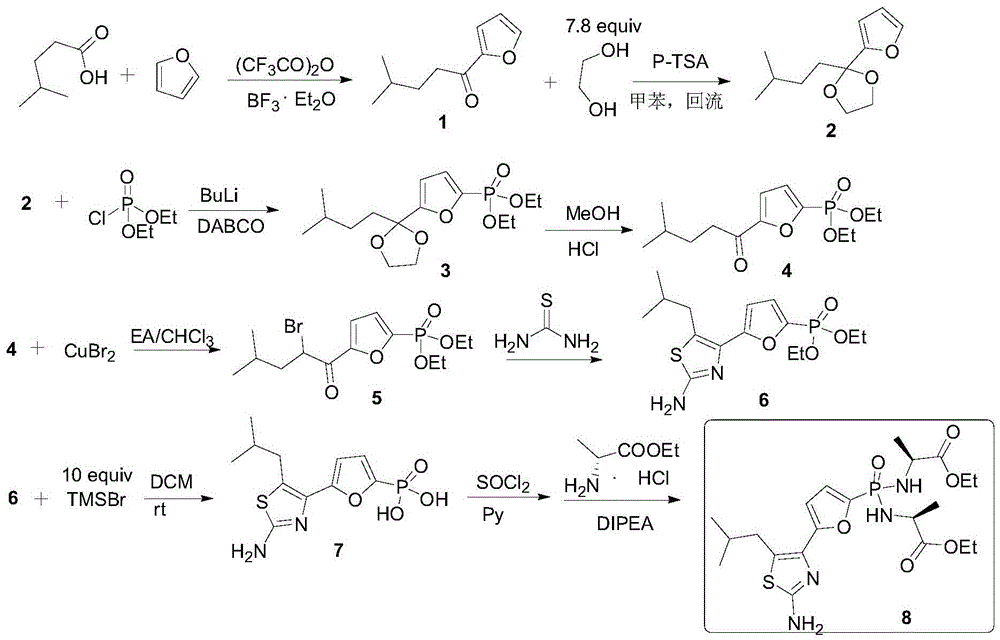
Preparation method of Managlinat Dialanetil
The invention discloses a preparation method of Managlinat Dialanetil. The method comprises the following steps: 1, adding ethylene glycol and triethyl orthoformate to 2-(4-methylvaleryl)-furan, and carrying out a ketalization reaction under the action of an acid catalyst to obtain a ketal mixture; 2, carrying out a phosphorylation reaction on the ketal mixture and diethyl chlorophosphate, and carrying out a deprotection reaction after the phosphorylation reaction is completed to obtain a phosphoryl lipid intermediate; 3, carrying out a bromination reaction on the phosphoryl lipid intermediate to obtain a bromide; 4, carrying out a ring closure reaction on the bromide and thiourea to obtain a thiazole intermediate; and 5, carrying out a one-pot reaction on the thiazole intermediate, TMSBr and L-alanine ethyl ester hydrochloride under the action of triphenylphosphine and dithiodipyridine, and carrying out post-treatment after the reaction is completed to obtain the Managlinat Dialanetil. The preparation method simplifies the reaction operation through optimizing step 1 and step 5, reduces the dosage of reagents and improves the reaction yield.
Owner:ZHEJIANG UNIV
Anilino-containing amphiphilic 4-difuoro-4-borata-3a-azonia-4a-aza-s-indacene derivative as well as preparation method and application thereof
ActiveCN107936046AEasy to prepareMild reaction conditionsGroup 3/13 element organic compoundsFluorescence/phosphorescenceAnilineStructural formula
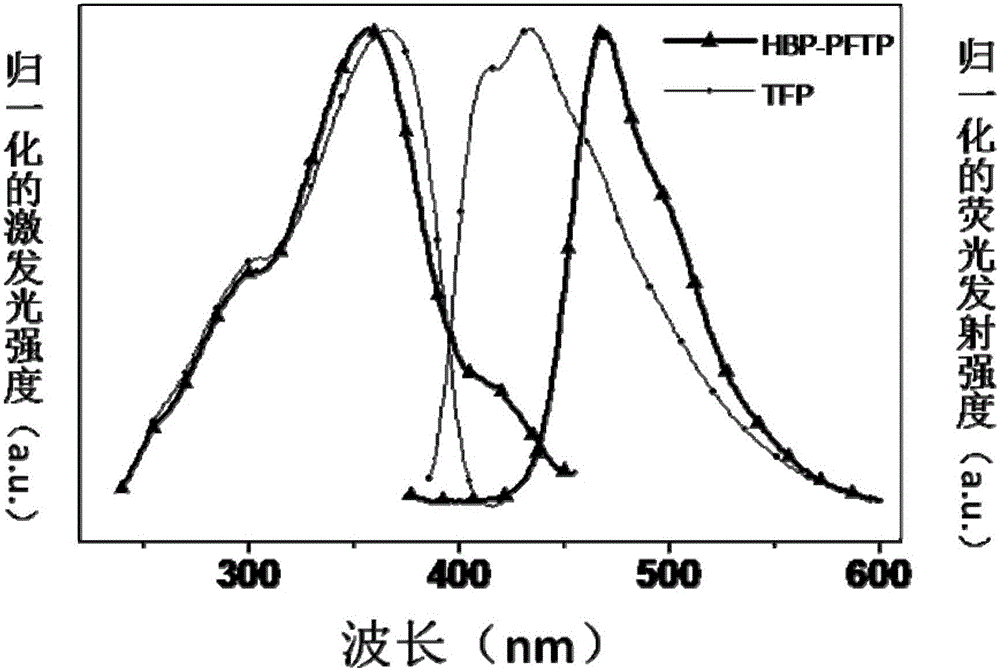
The invention discloses an aniline-containing amphiphilic 4-difuoro-4-borata-3a-azonia-4a-aza-s-indacene derivative as well as a preparation method and application thereof. A structural formula of thederivative is shown in the description; in the formula, n is an integer of 3 to 5; R1, R2, R3 and R4 are independently H or CH3; the derivative takes an anilino-containing 4-difuoro-4-borata-3a-azonia-4a-aza-s-indacene main body molecule as an amphiphilic fluorescent molecule with a hydrophobic head group and an ether-oxygen chain as a hydrophobic tail. The preparation method of the aniline-containing amphiphilic 4-difuoro-4-borata-3a-azonia-4a-aza-s-indacene derivative has the advantages of simplicity, mild reaction conditions, high yield and self assembly characteristics; the aniline-containing amphiphilic 4-difuoro-4-borata-3a-azonia-4a-aza-s-indacene derivative can be self assembled on a PEG200 gas-liquid interface to form a monomolecular layer fluorescent sensing film; the fluorescent sensing film can be used for quick high-selectivity trace detection of diethyl chlorophosphate (DCP) gas and realizing reversible detection.
Owner:SHAANXI NORMAL UNIV
Synthesis method of 1, 4-bis [4-(di-p-toluene amino) styryl] benzene
InactiveCN102503839BShort reaction timeHigh reaction yieldAmino preparation from aminesFiltrationSynthesis methods
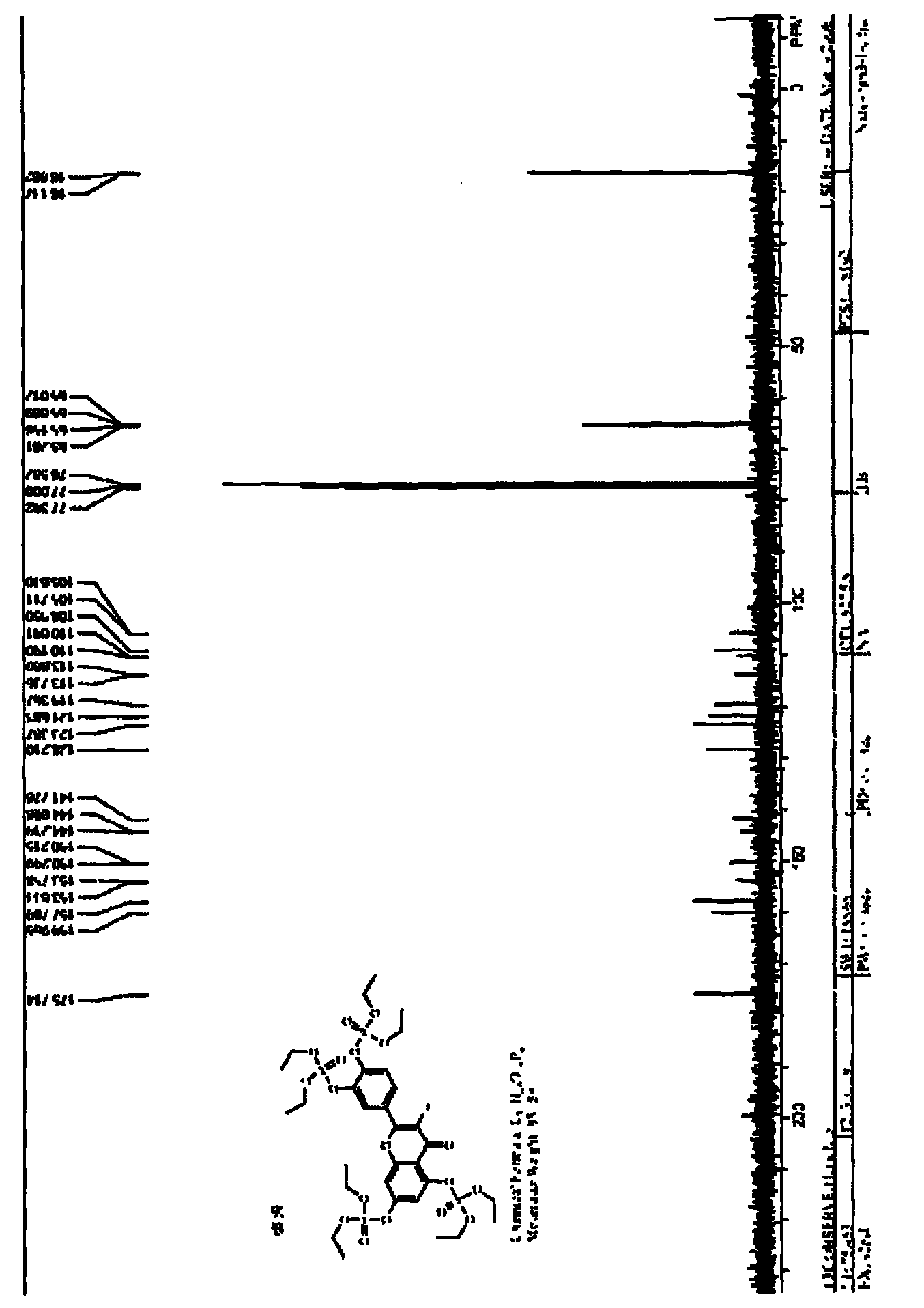
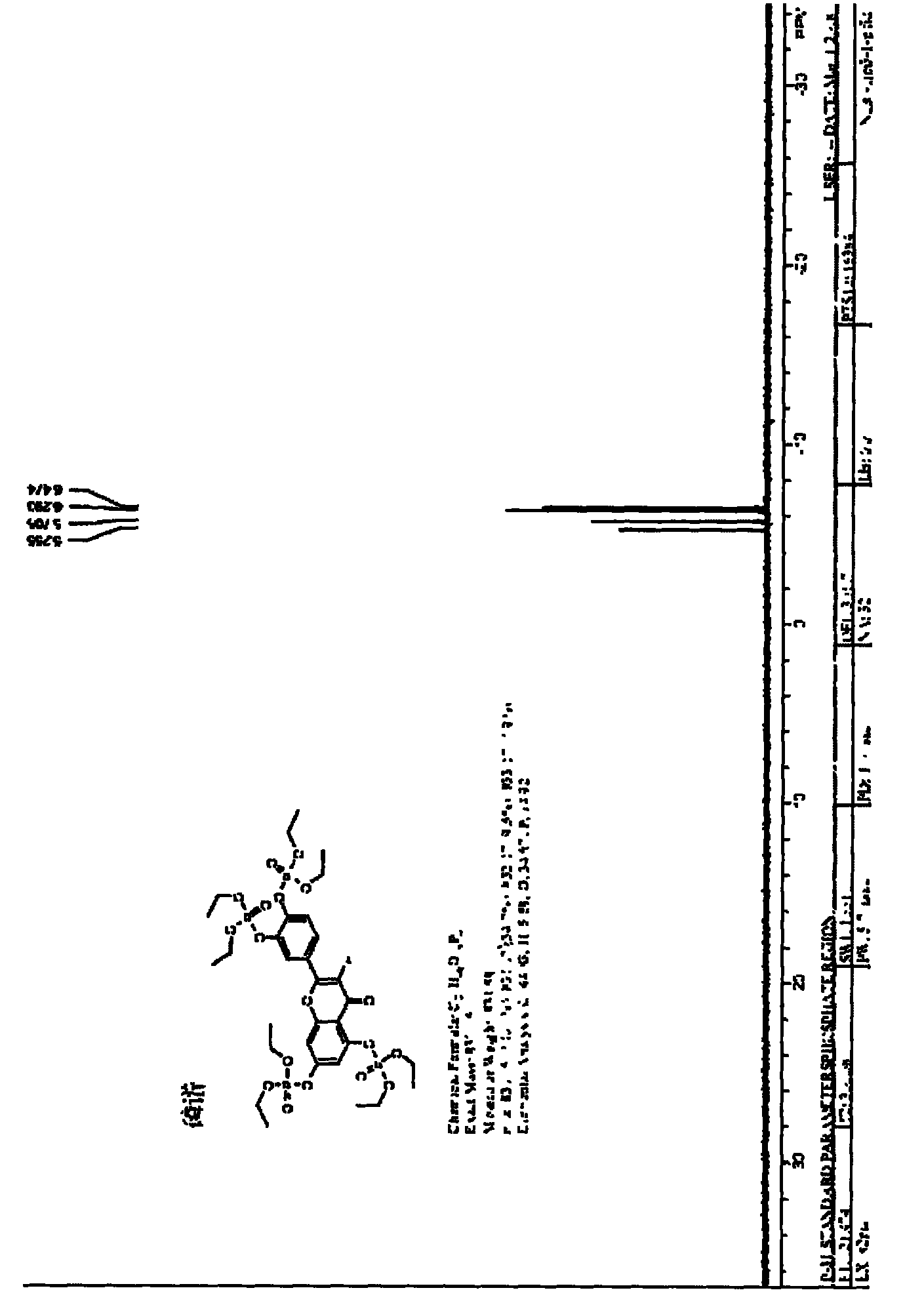
The invention discloses a synthesis method of 1, 4-bis [4-(di-p-toluene amino) styryl] benzene. The method comprises the following steps that: 4-(di-p-toluene) benzaldehyde, p-dibenzyl benzene diethyl chlorophosphate, sodium tert-butoxide and N, N-dimethyl acetamide are added into a reaction bottle at room temperature, after the materials are dissolved through stirring, the reaction bottle is placed into a microwave chemical reactor, the reaction lasts 3 to 10 minutes at the microwave radiation power being 800W, the reaction liquid is poured into 100mL methanol solution, filtration is carried out, and the 1, 4-bis [4-(di-p-toluene amino) styryl] benzene is obtained after the methylbenzene recrystallization. The synthesis method is mainly used for preparing the 1, 4-bis [4-(di-p-toluene amino) styryl] benzene.
Owner:XIAN MODERN CHEM RES INST
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
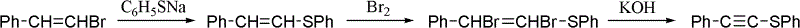
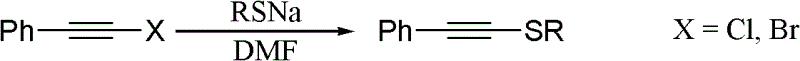


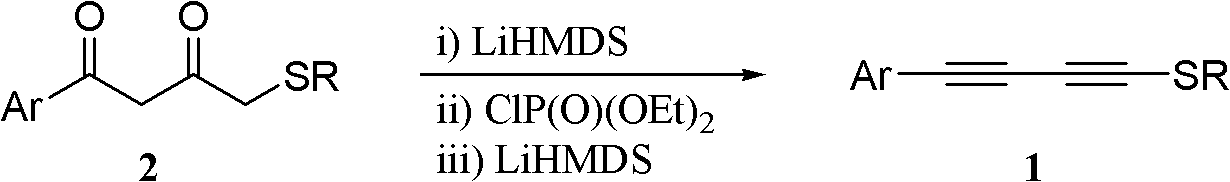
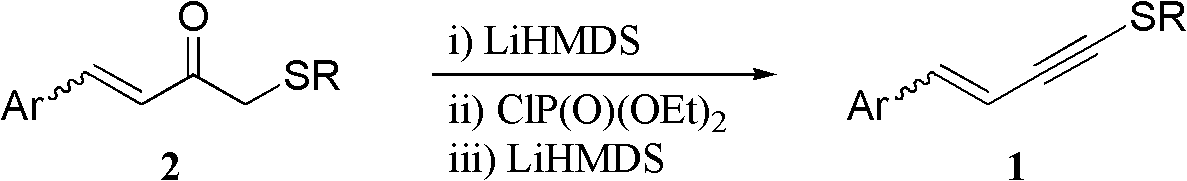
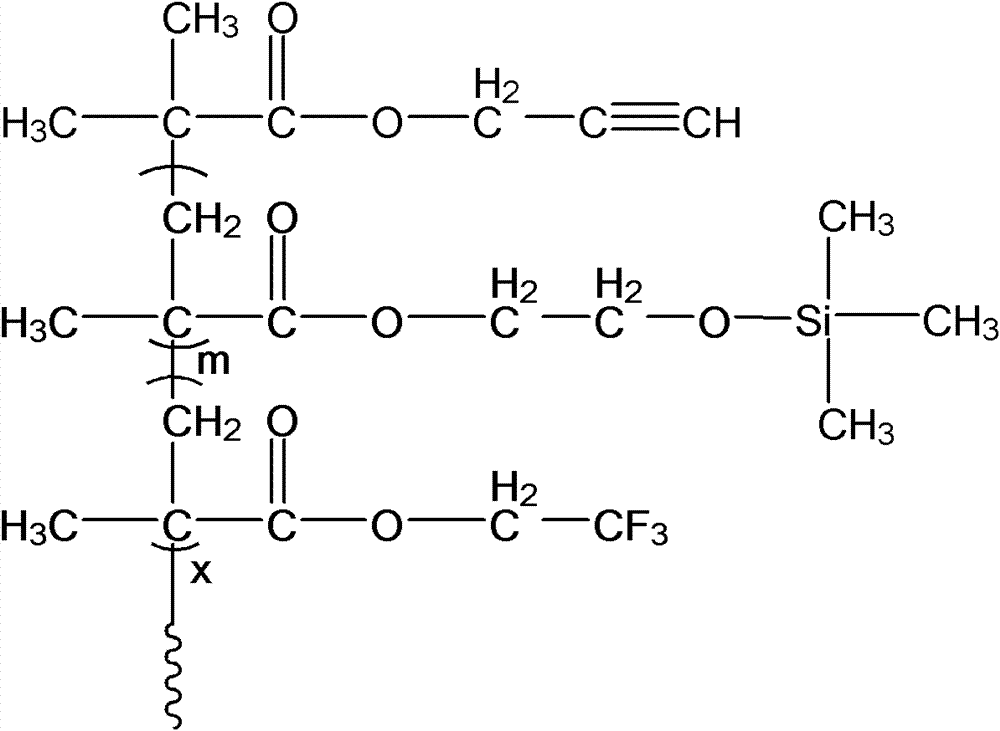

![Synthesis method of 1, 4-bis [4-(di-p-toluene amino) styryl] benzene Synthesis method of 1, 4-bis [4-(di-p-toluene amino) styryl] benzene](https://images-eureka.patsnap.com/patent_img/b52a5f36-1d95-49d1-9783-3f4bb1f133d1/BSA00000620949600011.PNG)
![Synthesis method of 1, 4-bis [4-(di-p-toluene amino) styryl] benzene Synthesis method of 1, 4-bis [4-(di-p-toluene amino) styryl] benzene](https://images-eureka.patsnap.com/patent_img/b52a5f36-1d95-49d1-9783-3f4bb1f133d1/BSA00000620949600021.PNG)
![Synthesis method of 1, 4-bis [4-(di-p-toluene amino) styryl] benzene Synthesis method of 1, 4-bis [4-(di-p-toluene amino) styryl] benzene](https://images-eureka.patsnap.com/patent_img/b52a5f36-1d95-49d1-9783-3f4bb1f133d1/BSA00000620949600022.PNG)