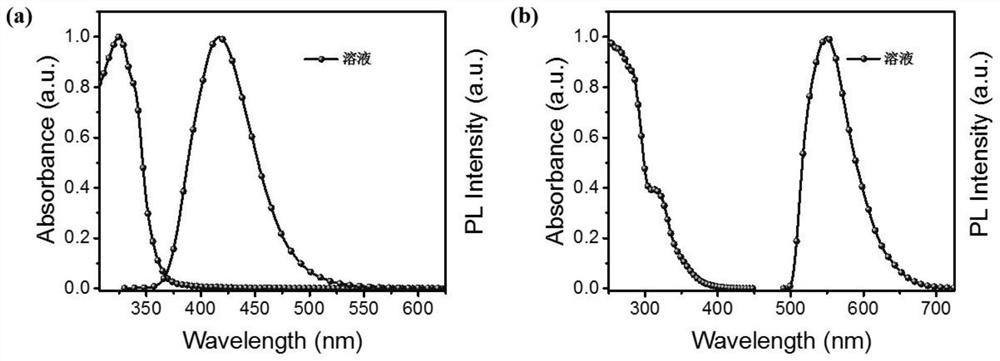
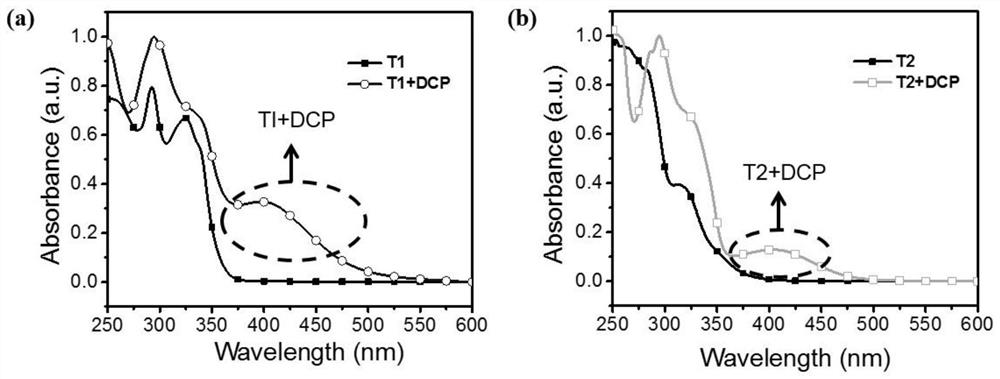
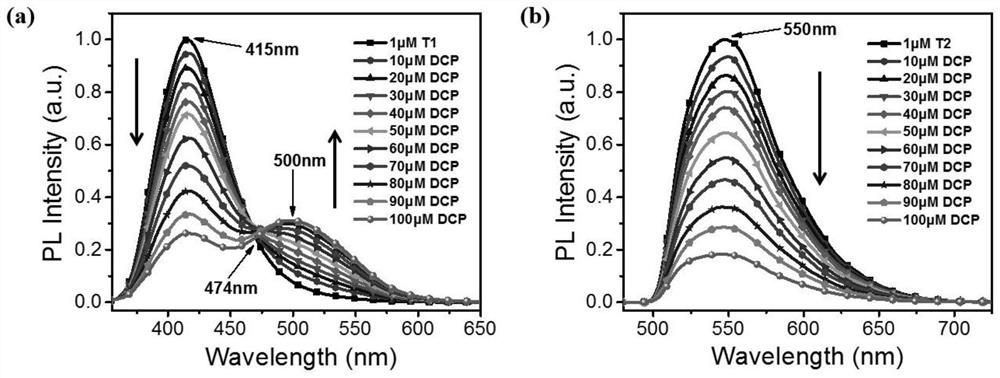
An organic fluorescent molecule and its preparation method, a fluorescent sensor and its application, and a standard fluorescent card
A fluorescent sensor and fluorescent molecular technology, applied in the field of fluorescent sensing, can solve the problems of complex material synthesis, easy response of fluorescence enhancement and fluorescence quenching to the external environment, long response time, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0052] The present invention provides a method for preparing organic fluorescent molecules described in the above technical solution, comprising the following steps:
[0053] Mixing a compound containing group A, a terpyridine compound, a catalyst, a solvent and an alkaline reagent, and performing a coupling reaction to obtain an organic fluorescent molecule having a structure shown in formula I;
[0054] The compound containing group A is naphthalene, anthracene, naphthacene, carbazole, phenothiazine, phenoxazine, phenazine, dimethylacridine, diphenylamine, triphenylamine, tetraphenylethylene, pyrene, quinone Oxaline or aniline;
[0055] The terpyridine compound has a structure shown in formula II:
[0056] Wherein, X is Cl, Br or I.
[0057] In the present invention, unless otherwise specified, the required preparation materials are commercially available products well known to those skilled in the art.
[0058] In the present invention, the compound containing group A ...
Embodiment 1
[0091] The preparation process of the organic fluorescent molecule T1 is as follows:
[0092]
[0093] Specific steps include:
[0094] Under the protection of argon, carbazole (1.67g, 0.01mmol), 4'-(4-bromophenyl)-2,2':6',2"-terpyridine (3.88 g, 0.01mol) and cesium carbonate (4.88g, 0.015mmol), stirred at room temperature, added 40mLN, N-dimethylformamide, under the condition of protective gas, the resulting mixture was frozen-vacuumized once, the The frozen reagent is liquid nitrogen, the freezing temperature is -80°C, and the time is 5 minutes. Vacuumize the system until the system pressure drops below 500 mTorr, and then add the main catalyst CuI (95mg, 0.5mmol) and the cocatalyst 1,10-ortho Phenanthroline (45 mg, 0.23 mmol), repeat the freezing-vacuum operation three times, heat to 160 ° C, carry out the coupling reaction for 8 h, cool to room temperature, and dichloromethane and water (volume ratio of dichloromethane and water: 3:1) extraction, after washing with wa...
Embodiment 2
[0098] The preparation process of the organic fluorescent molecule T2 is as follows:
[0099]
[0100] Specific steps include:
[0101] Under the protection of argon, add phenothiazine (2g, 0.01mmol), 4'-(4-bromophenyl)-2,2':6',2"-terpyridine (3.88 g, 0.01mol) and cesium carbonate (4.88g, 0.015mmol), stirred at room temperature, added 40mLN, N-dimethylformamide, under the condition of protective gas, the resulting mixture was frozen-vacuumized once, the The frozen reagent is liquid nitrogen, the freezing temperature is -80°C, and the time is 5 minutes. Vacuumize the system until the system pressure drops below 500 mTorr, and then add the main catalyst CuI (95mg, 0.5mmol) and the cocatalyst 1,10-ortho Phenanthroline (45 mg, 0.23 mmol), repeat the freezing-vacuum operation three times, heat to 160 ° C, carry out the coupling reaction for 8 h, cool to room temperature, and dichloromethane and water (volume ratio of dichloromethane and water: 3:1) extraction, after washing wi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com