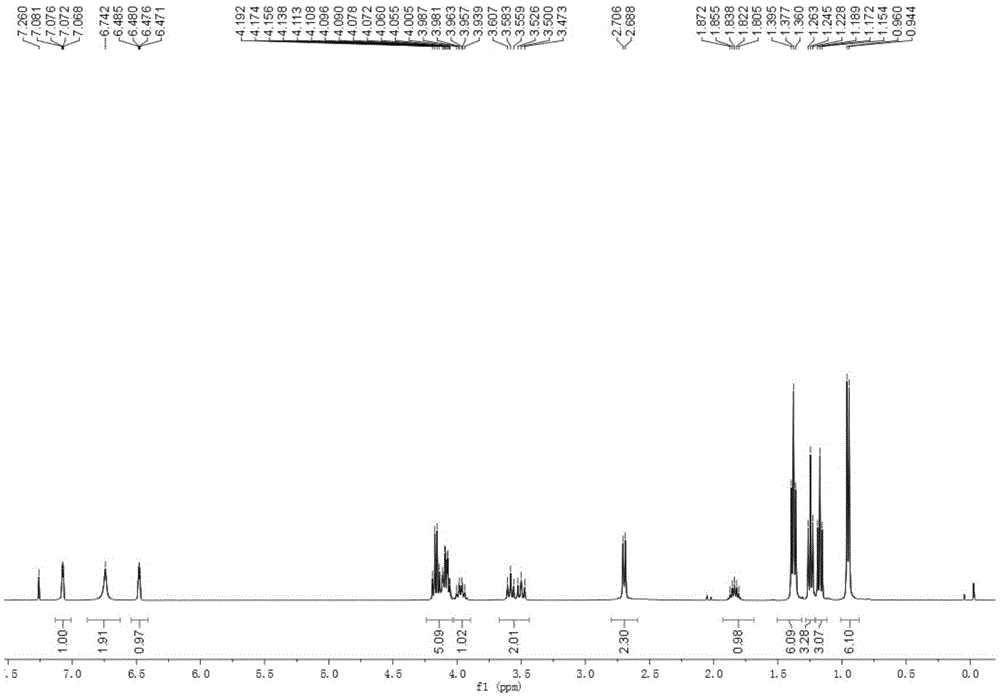
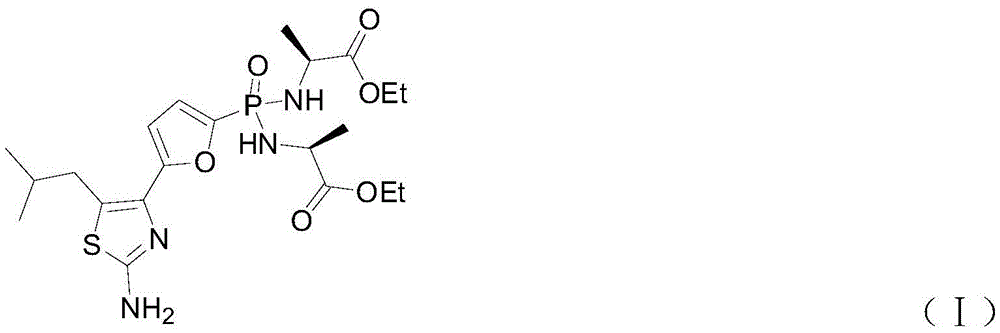
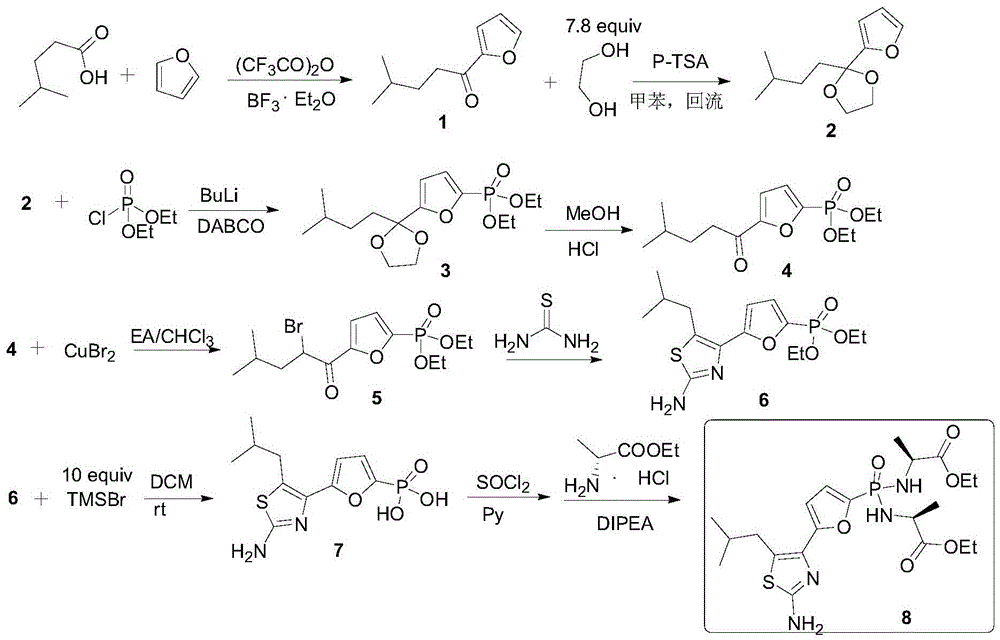
Preparation method of Managlinat Dialanetil
A technology of reactions and intermediates, applied in the field of preparation of demagreglide, which can solve the problems of large amount of TMSBr, reduced yield, low total yield, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] In a 500mL three-necked reaction flask, add a suitable magnetic stirring bar, install a thermometer and a tee with an argon balloon. Heat the reaction flask with an electric blower while vacuuming it, and replace it with argon three times. Inject 220 mL of anhydrous toluene through a syringe. Furan 24 mL (335 mmol, 1.3 equiv) and 4-methylpentanoic acid (33 mL, 258 mmol, 1.0 equiv) were added in sequence. 44 mL (1.1 equiv) of trifluoroacetic anhydride and 3.3 mL (10 mmol%) of boron trifluoride diethyl ether were added under stirring at room temperature. Raise the temperature to keep the internal temperature at 50-55°C for 2h. The reaction flask was placed in an ice-water bath, and 150 mL of Na with a mass fraction of 21% was added in batches. 2 CO 3 Solution neutralization. The liquid was separated, and the organic phase was filtered through diatomaceous earth and dried over anhydrous sodium sulfate. Solvent was removed as much as possible by distillation under red...
Embodiment 2
[0044] Embodiment 2 synthetic intermediate 2 and 2 '
[0045] Ketone 1 (20g, 120mmol, 1.0equiv) was weighed into a 250mL double-necked reaction flask, vacuumed and replaced with argon, 150mL of benzene was injected, 7mL of ethylene glycol (1.1equiv) was added, and 16mL of trimethyl orthoformate (1.2 equiv), remove the three-way valve and quickly add 0.34 g (1.5% mmol) of p-toluenesulfonic acid, and stir at room temperature for 2 h. Add 10 mL (0.8 equiv) of trimethyl orthoformate, react for 30 min, then add 13.2 mL (1.0 equiv) of trimethyl orthoformate, and stir overnight. Pour the reaction solution into saturated NaHCO 3 , extracted with ethyl acetate. NaHCO for organic phase 3 Wash with aqueous solution. Underpressure distillation obtains the about 18g of the mixture of product 2 and 2', yield 71%.
Embodiment 3
[0049] Ketal (16.5 g, 78 mmol, 1.0 equiv) was weighed into a 100 mL three-neck reaction flask, vacuumed under argon protection, 30 mL of anhydrous THF was added, DABCO (8.74 g, 1.0 equiv) was added, and the temperature was lowered to -42°C. Add n-butyllithium (49mL, 1.6M, 1.0equiv) dropwise, keep the temperature at -43~-40°C, raise to 0°C and stir for 1h after dropping, then cool down to -8°C. In another 250 mL three-neck reaction flask, inject 30 mL THF in an argon atmosphere, add diethyl chlorophosphate (12.5 mL, 1.1 equiv), and cool to -45°C. Add the solution in the 100mL reaction flask into the 250mL three-neck reaction flask through a syringe, and keep the temperature at -44~-40°C. After the addition, the reaction bottle was moved to room temperature for 2 h. Remove the solvent tetrahydrofuran under reduced pressure as much as possible, add 100mL ethyl acetate and 100mL water, separate the layers, and extract the aqueous phase with ethyl acetate. The organic phases were...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com