Chitosan oligosaccharide derivative containing thiourea and diethoxyphosphamide structure and preparation method thereof
A technology of diethoxyphosphoramide and aminothiourea ethyl chitosan oligosaccharide, which is applied in the field of chitosan oligosaccharide derivatives and its preparation, can solve problems such as environmental pollution and component residues, and achieve accelerated reaction progress and improved The effect of biological activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
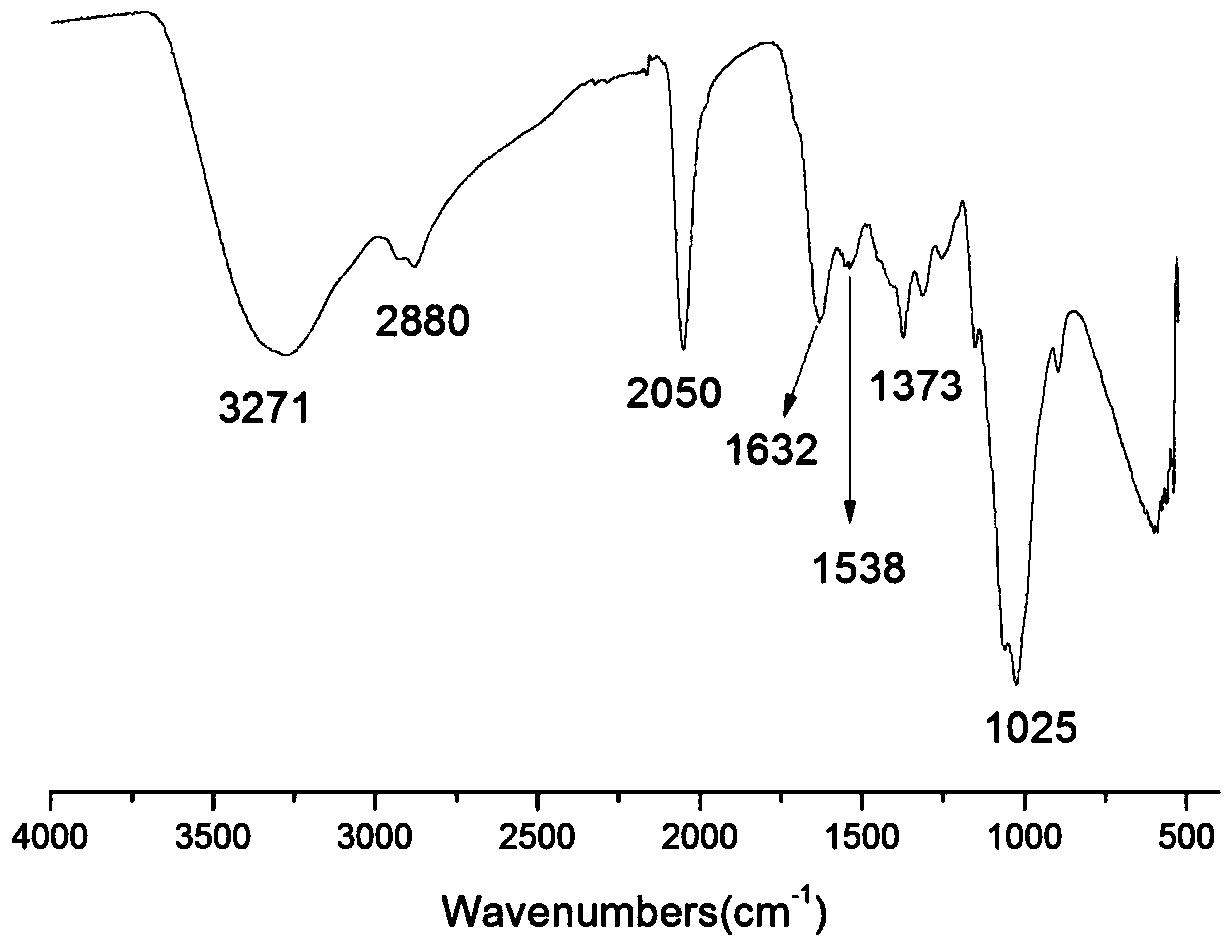
Image
Examples
Embodiment 1
[0026] 1) Preparation of bromoethyl chitosan oligosaccharide derivatives
[0027] Swell 3.0g chitosan oligosaccharide in 30mL N,N-dimethylformamide, add 3mL triethylamine, stir and swell for 12h, add 3mL 1,2-dibromoethane, heat up to 50°C, stir and reflux for 12h , filtered with suction, the filter cake was rinsed with absolute ethanol, and dried at 60°C to obtain a tan solid, which was a bromoethyl chitosan oligosaccharide derivative.
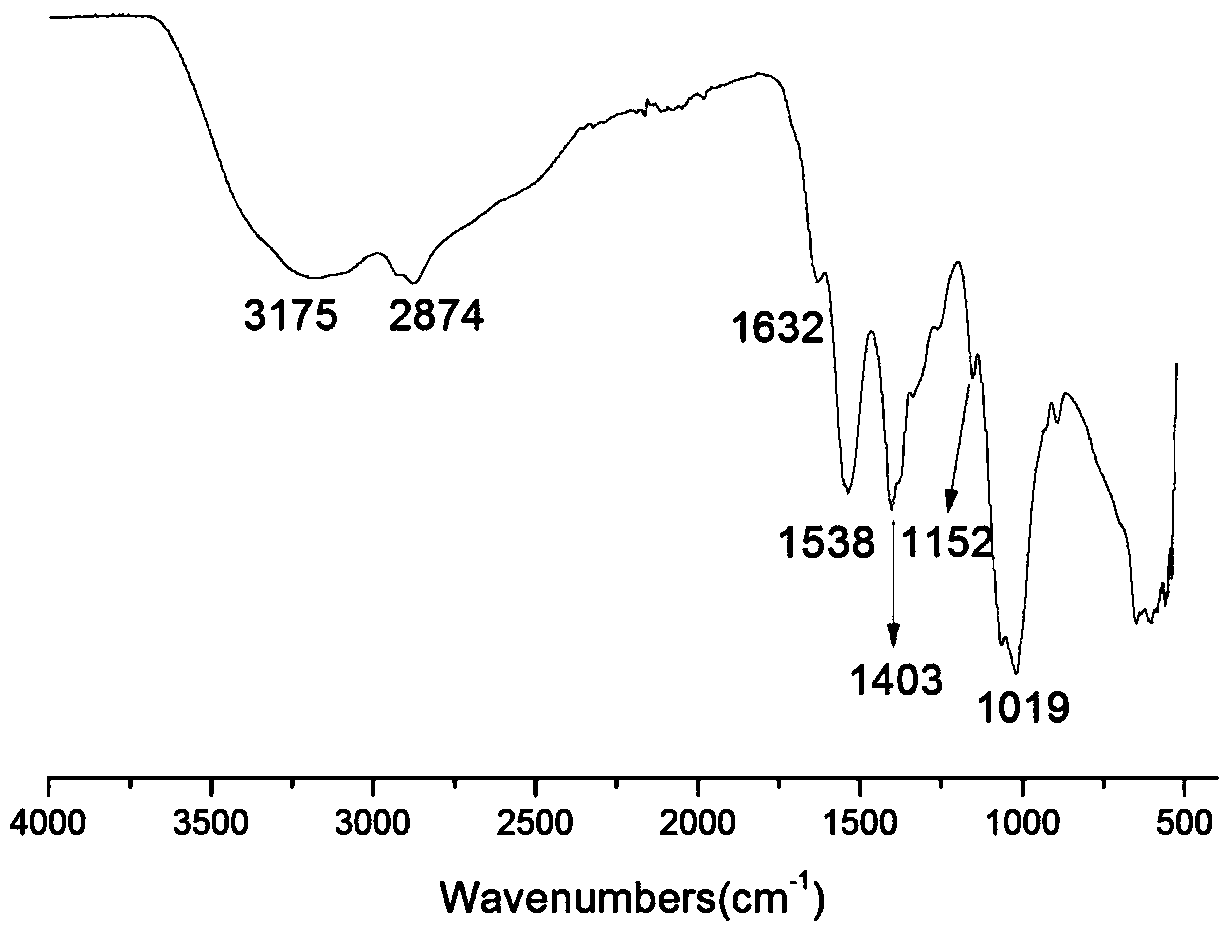
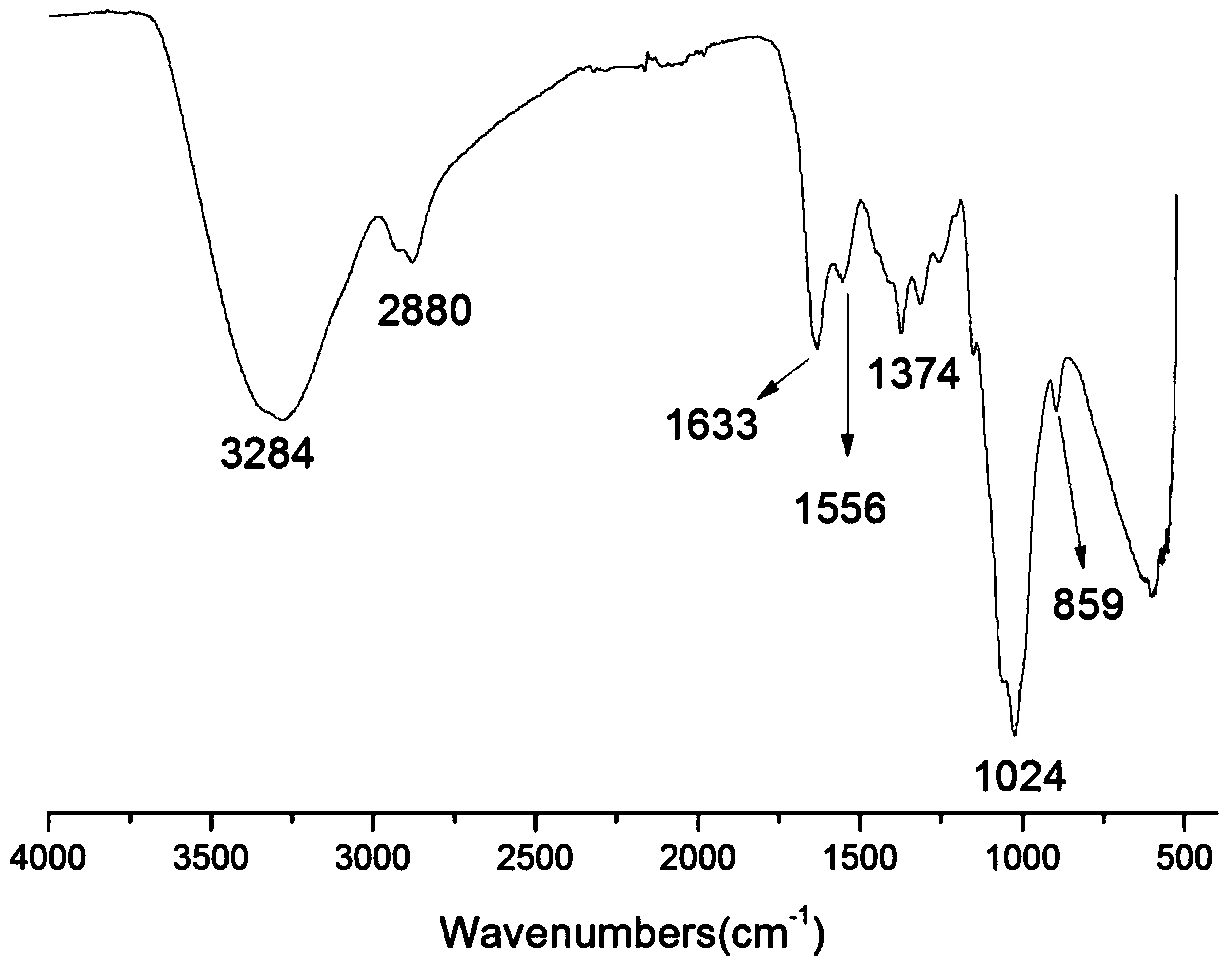
[0028] Infrared spectrum shows: the infrared spectrogram of bromoethyl chitosan oligosaccharide derivative ( figure 2 ) and infrared spectrum of chitosan oligosaccharide ( figure 1 ) compared to -NH 2 The absorption peak from 1538cm -1 Transfer to 1556cm -1 , and significantly weakened, indicating that -NH 2 Reacted, 1024cm -1 The C-O absorption peak shifted to 1019cm -1 , and the peak shape becomes sharper, indicating that a reaction has occurred on the C-OH, at 1374cm -1 The absorption peak of methylene C-H appears at , which proves...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com