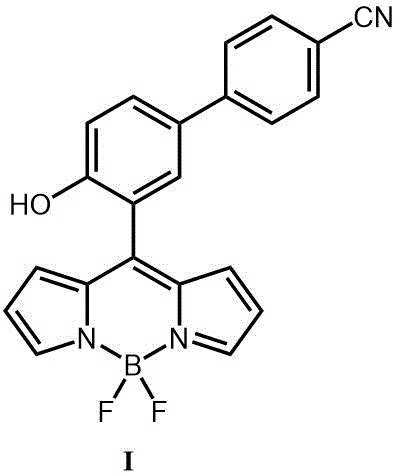
Preparation and application of boron-dipyrromethene-based diethyl chlorophosphate fluorescent probe
A technology of diethyl chlorophosphate and fluorescent probes, applied in the field of fluorescent probes, can solve the problems of limited use of fluorescent probes, high detection limit of fluorescent probes, low selectivity of fluorescent probes, etc., and achieves good application prospects, The effect of good sensitivity and selectivity, and simple synthesis steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Embodiment 1, the synthesis of probe molecule I
[0046](a) Nitrogen protection, take 6.24g (about 32mmol) of cyanobiphenol and 4.56g (about 48mmol) of anhydrous MgCl 2 , add 80 mL of anhydrous acetonitrile, then add 16.32 mL (about 122 mmol) of triethylamine and 12.3 g (about 440 mmol) of paraformaldehyde (excess). Mixture 70 o C for 5 hours. After the reaction was complete, it was quenched with water and acidified with 6M hydrochloric acid. Crude product with CH 2 Cl 2 Extraction, liquid separation, desolventization under reduced pressure, and separation by column chromatography gave 3.5 g of white solid III, with a yield of about 50%.
[0047] (b) Under nitrogen protection, 2.00 g (8.96 mmol) of compound III was dissolved in 50 mL of dry dichloromethane, and stirred at room temperature. Add 12.0 mL (174.4 mmol) of pyrrole and react under the catalysis of 0.03 mL of trifluoroacetic acid for 1 hour. The crude product was then purified by column chromatography to...
Embodiment 2
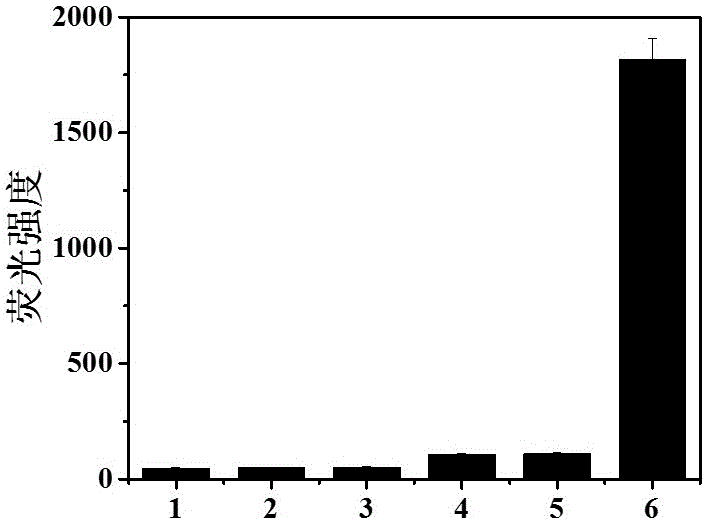
[0049] Embodiment 2, probe selectivity experiment
[0050] The fluorescent probe compound prepared in Example 1 was prepared into a DMF solution containing the probe, triethylamine (20 μM) and DMAP (10 μM) for later use.
[0051] Take the fluorescent probe DMF solution and divide it into 6 groups, 10 ml in each group. One group does not add detection species, and the remaining 5 groups are added with solutions containing HCl, TFA, triphosgene, Phosgene, DCP, so that each group of solutions contains probes The concentration of I is 10 μM, the concentration of the detection species is 100 μM, so that the molar ratio of the detection species to the probe compound is 10:1; under the excitation of light with a wavelength of 480 nm, the fluorescence intensity of the fluorescence is tested. Such as image 3 As shown, the probe solution of the present invention has no fluorescence itself. Once DCP is added, the fluorescence of the solution at 530nm increases rapidly, but the fluoresce...
Embodiment 3
[0052] Embodiment 3, probe sensitivity experiment
[0053] Take the fluorescent probe solution prepared in Example 2, and divide it into 12 groups, each with 10 milliliters, add DCP solutions of different concentrations respectively, adjust the concentration of the probe compound contained in the solution to be 10 μM, and the concentration of DCP to be 0, 5, 10, 15, 20, 25...60 μM. Under the excitation of light with a wavelength of 480nm, test its fluorescence intensity, such as Figure 4 shown. The results showed that the fluorescence of the solution increased rapidly at 530nm, and the fluorescence intensity was linearly related to the concentration. According to the calculation, the minimum detection limit of this probe compound is 1.3×10 -8 mol / L.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com